Abstract
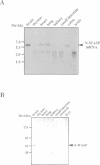
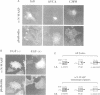
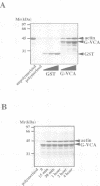
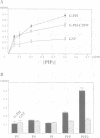
Here we identify a 65 kDa protein (N-WASP) from brain that binds the SH3 domains of Ash/Grb2. The sequence is homologous to Wiskott-Aldrich syndrome protein (WASP). N-WASP has several functional motifs, such as a pleckstrin homology (PH) domain and cofilin-homologous region, through which N-WASP depolymerizes actin filaments. When overexpressed in COS 7 cells, the wild-type N-WASP causes several surface protrusions where N-WASP co-localizes with actin filaments. Epidermal growth factor (EGF) treatment induces the complex formation of EGF receptors and N-WASP, and produces microspikes. On the other hand, two mutants, C38W (a point mutation in the PH domain) and deltaVCA (deletion of the actin binding domain), localize predominantly in the nucleus and do not cause a change in the cytoskeleton, irrespective of EGF treatment. Interestingly, the C38W PH domain binds less effectively to phosphatidylinositol 4,5-bisphosphate (PIP2) than the wild-type PH domain. These results suggest the importance of the PIP2 binding ability of the PH domain and the actin binding for retention in membranes. Collectively, we conclude that N-WASP transmits signals from tyrosine kinases to cause a polarized rearrangement of cortical actin filaments dependent on PIP2.
Full text
PDF


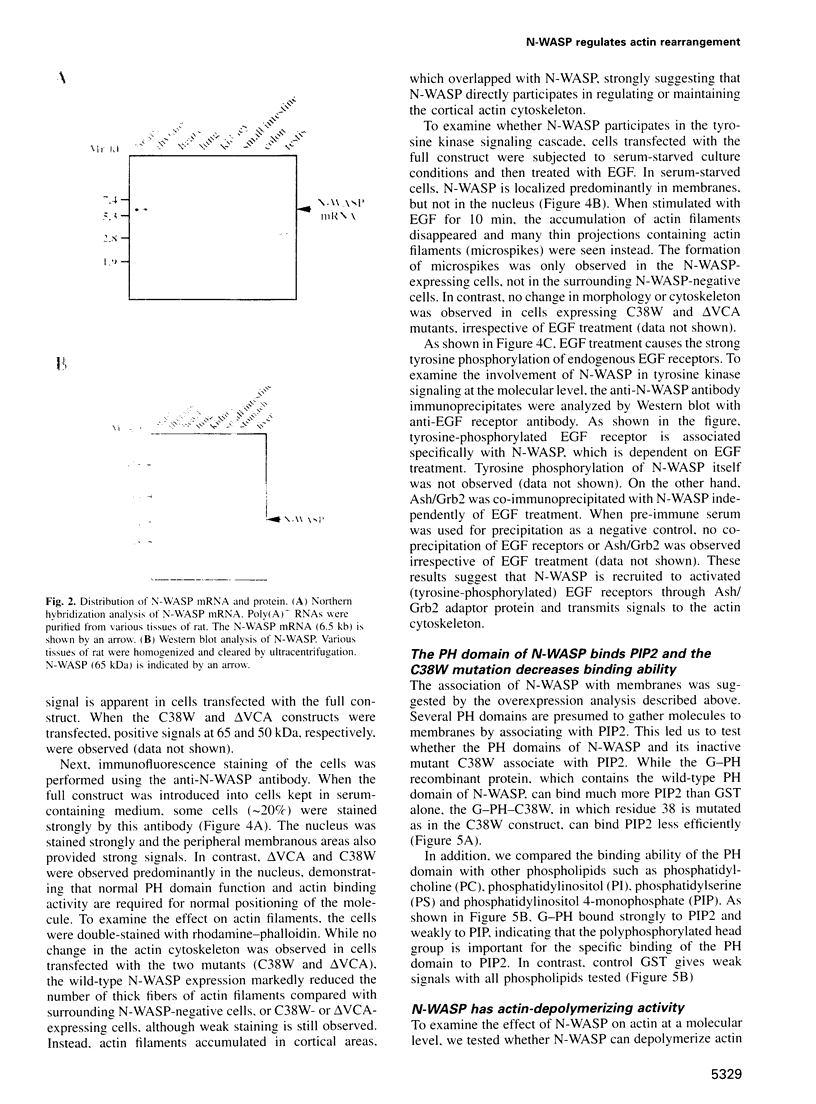

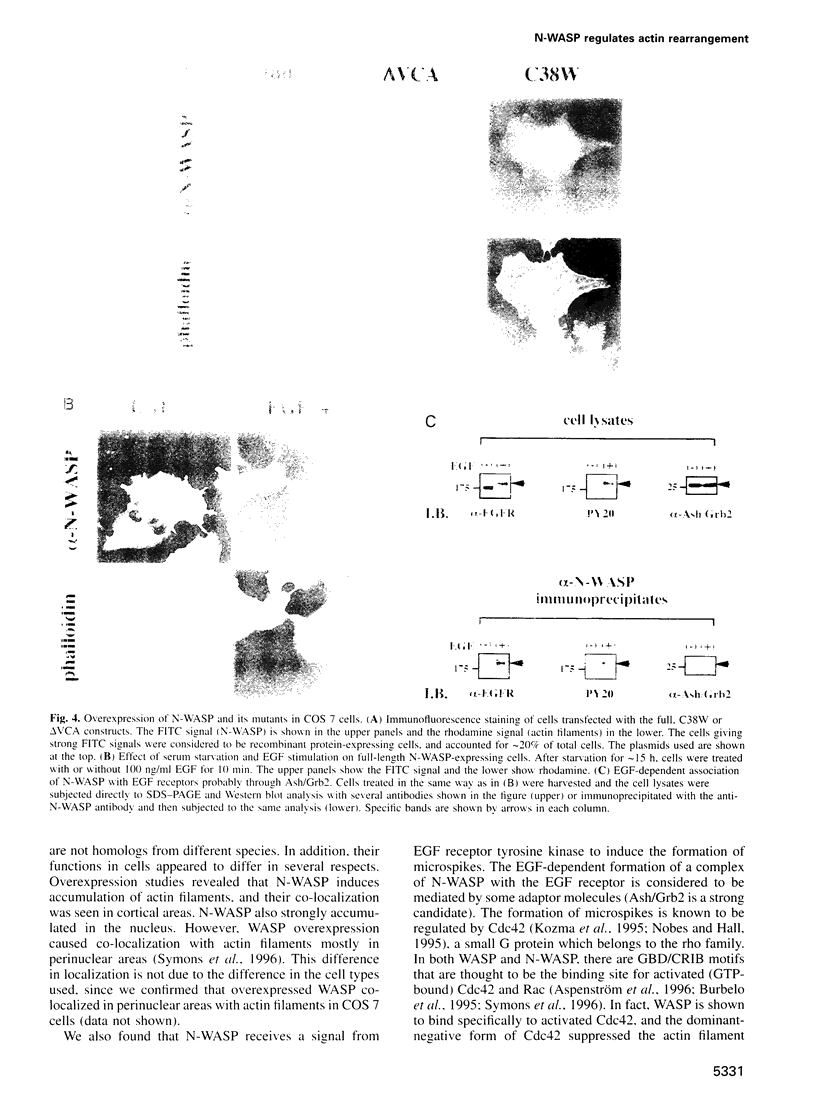
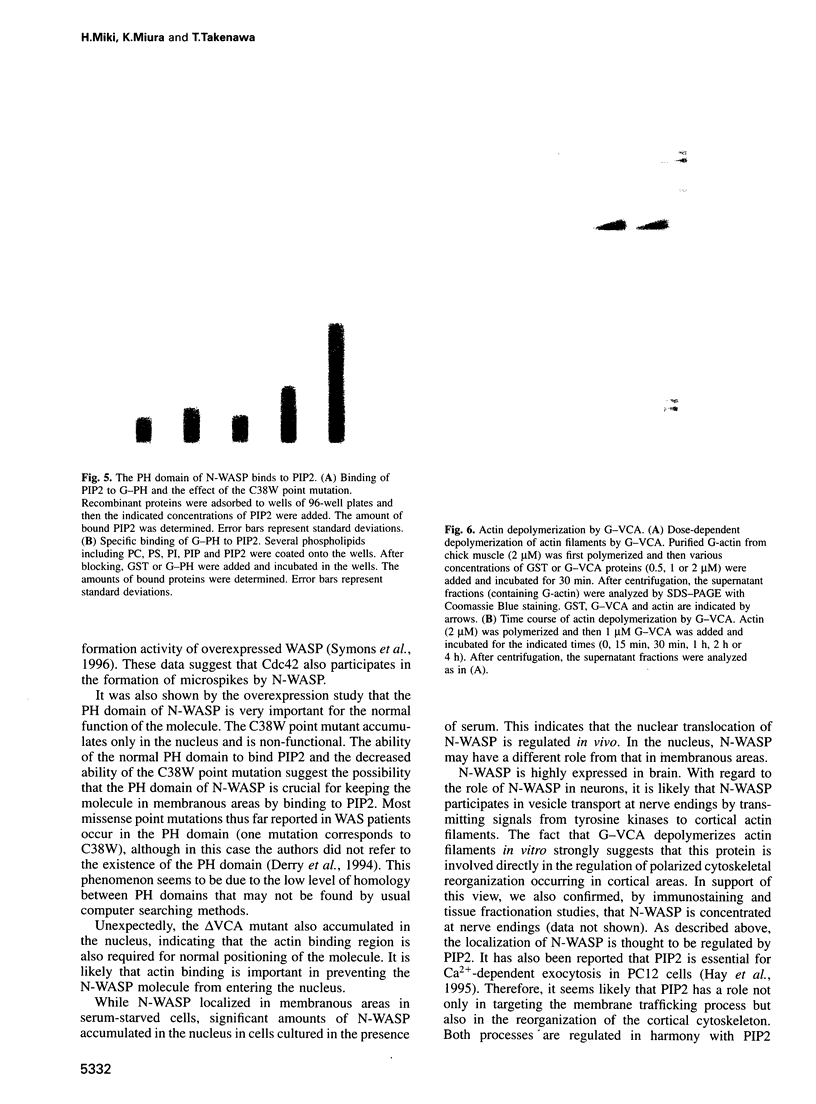



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aizawa H., Sutoh K., Yahara I. Overexpression of cofilin stimulates bundling of actin filaments, membrane ruffling, and cell movement in Dictyostelium. J Cell Biol. 1996 Feb;132(3):335–344. doi: 10.1083/jcb.132.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson A. K., Rupp E., Nånberg E., Downward J., Rönnstrand L., Wennström S., Schlessinger J., Heldin C. H., Claesson-Welsh L. Tyr-716 in the platelet-derived growth factor beta-receptor kinase insert is involved in GRB2 binding and Ras activation. Mol Cell Biol. 1994 Oct;14(10):6715–6726. doi: 10.1128/mcb.14.10.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltensperger K., Kozma L. M., Cherniack A. D., Klarlund J. K., Chawla A., Banerjee U., Czech M. P. Binding of the Ras activator son of sevenless to insulin receptor substrate-1 signaling complexes. Science. 1993 Jun 25;260(5116):1950–1952. doi: 10.1126/science.8391166. [DOI] [PubMed] [Google Scholar]
- Burbelo P. D., Drechsel D., Hall A. A conserved binding motif defines numerous candidate target proteins for both Cdc42 and Rac GTPases. J Biol Chem. 1995 Dec 8;270(49):29071–29074. doi: 10.1074/jbc.270.49.29071. [DOI] [PubMed] [Google Scholar]
- Clark S. G., Stern M. J., Horvitz H. R. C. elegans cell-signalling gene sem-5 encodes a protein with SH2 and SH3 domains. Nature. 1992 Mar 26;356(6367):340–344. doi: 10.1038/356340a0. [DOI] [PubMed] [Google Scholar]
- Derry J. M., Kerns J. A., Weinberg K. I., Ochs H. D., Volpini V., Estivill X., Walker A. P., Francke U. WASP gene mutations in Wiskott-Aldrich syndrome and X-linked thrombocytopenia. Hum Mol Genet. 1995 Jul;4(7):1127–1135. doi: 10.1093/hmg/4.7.1127. [DOI] [PubMed] [Google Scholar]
- Derry J. M., Ochs H. D., Francke U. Isolation of a novel gene mutated in Wiskott-Aldrich syndrome. Cell. 1994 Aug 26;78(4):635–644. doi: 10.1016/0092-8674(94)90528-2. [DOI] [PubMed] [Google Scholar]
- Donnelly S. F., Pocklington M. J., Pallotta D., Orr E. A proline-rich protein, verprolin, involved in cytoskeletal organization and cellular growth in the yeast Saccharomyces cerevisiae. Mol Microbiol. 1993 Nov;10(3):585–596. doi: 10.1111/j.1365-2958.1993.tb00930.x. [DOI] [PubMed] [Google Scholar]
- Feng S., Chen J. K., Yu H., Simon J. A., Schreiber S. L. Two binding orientations for peptides to the Src SH3 domain: development of a general model for SH3-ligand interactions. Science. 1994 Nov 18;266(5188):1241–1247. doi: 10.1126/science.7526465. [DOI] [PubMed] [Google Scholar]
- Fukami K., Endo T., Imamura M., Takenawa T. alpha-Actinin and vinculin are PIP2-binding proteins involved in signaling by tyrosine kinase. J Biol Chem. 1994 Jan 14;269(2):1518–1522. [PubMed] [Google Scholar]
- Fukami K., Furuhashi K., Inagaki M., Endo T., Hatano S., Takenawa T. Requirement of phosphatidylinositol 4,5-bisphosphate for alpha-actinin function. Nature. 1992 Sep 10;359(6391):150–152. doi: 10.1038/359150a0. [DOI] [PubMed] [Google Scholar]
- Harlan J. E., Hajduk P. J., Yoon H. S., Fesik S. W. Pleckstrin homology domains bind to phosphatidylinositol-4,5-bisphosphate. Nature. 1994 Sep 8;371(6493):168–170. doi: 10.1038/371168a0. [DOI] [PubMed] [Google Scholar]
- Haslam R. J., Koide H. B., Hemmings B. A. Pleckstrin domain homology. Nature. 1993 May 27;363(6427):309–310. doi: 10.1038/363309b0. [DOI] [PubMed] [Google Scholar]
- Hay J. C., Fisette P. L., Jenkins G. H., Fukami K., Takenawa T., Anderson R. A., Martin T. F. ATP-dependent inositide phosphorylation required for Ca(2+)-activated secretion. Nature. 1995 Mar 9;374(6518):173–177. doi: 10.1038/374173a0. [DOI] [PubMed] [Google Scholar]
- Janmey P. A., Stossel T. P. Modulation of gelsolin function by phosphatidylinositol 4,5-bisphosphate. Nature. 1987 Jan 22;325(6102):362–364. doi: 10.1038/325362a0. [DOI] [PubMed] [Google Scholar]
- Kolluri R., Shehabeldin A., Peacocke M., Lamhonwah A. M., Teichert-Kuliszewska K., Weissman S. M., Siminovitch K. A. Identification of WASP mutations in patients with Wiskott-Aldrich syndrome and isolated thrombocytopenia reveals allelic heterogeneity at the WAS locus. Hum Mol Genet. 1995 Jul;4(7):1119–1126. doi: 10.1093/hmg/4.7.1119. [DOI] [PubMed] [Google Scholar]
- Kozma R., Ahmed S., Best A., Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995 Apr;15(4):1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan S. P., Hagemann T. L., Radtke B. E., Blaese R. M., Rosen F. S. Identification of mutations in the Wiskott-Aldrich syndrome gene and characterization of a polymorphic dinucleotide repeat at DXS6940, adjacent to the disease gene. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4706–4710. doi: 10.1073/pnas.92.10.4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassing I., Lindberg U. Specific interaction between phosphatidylinositol 4,5-bisphosphate and profilactin. Nature. 1985 Apr 4;314(6010):472–474. doi: 10.1038/314472a0. [DOI] [PubMed] [Google Scholar]
- Lev S., Moreno H., Martinez R., Canoll P., Peles E., Musacchio J. M., Plowman G. D., Rudy B., Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca(2+)-induced regulation of ion channel and MAP kinase functions. Nature. 1995 Aug 31;376(6543):737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- Lowenstein E. J., Daly R. J., Batzer A. G., Li W., Margolis B., Lammers R., Ullrich A., Skolnik E. Y., Bar-Sagi D., Schlessinger J. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell. 1992 Aug 7;70(3):431–442. doi: 10.1016/0092-8674(92)90167-b. [DOI] [PubMed] [Google Scholar]
- Matuoka K., Fukami K., Nakanishi O., Kawai S., Takenawa T. Mitogenesis in response to PDGF and bombesin abolished by microinjection of antibody to PIP2. Science. 1988 Feb 5;239(4840):640–643. doi: 10.1126/science.2829356. [DOI] [PubMed] [Google Scholar]
- Matuoka K., Shibasaki F., Shibata M., Takenawa T. Ash/Grb-2, a SH2/SH3-containing protein, couples to signaling for mitogenesis and cytoskeletal reorganization by EGF and PDGF. EMBO J. 1993 Sep;12(9):3467–3473. doi: 10.1002/j.1460-2075.1993.tb06021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuoka K., Shibata M., Yamakawa A., Takenawa T. Cloning of ASH, a ubiquitous protein composed of one Src homology region (SH) 2 and two SH3 domains, from human and rat cDNA libraries. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9015–9019. doi: 10.1073/pnas.89.19.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer B. J., Ren R., Clark K. L., Baltimore D. A putative modular domain present in diverse signaling proteins. Cell. 1993 May 21;73(4):629–630. doi: 10.1016/0092-8674(93)90244-k. [DOI] [PubMed] [Google Scholar]
- McPherson P. S., Garcia E. P., Slepnev V. I., David C., Zhang X., Grabs D., Sossin W. S., Bauerfeind R., Nemoto Y., De Camilli P. A presynaptic inositol-5-phosphatase. Nature. 1996 Jan 25;379(6563):353–357. doi: 10.1038/379353a0. [DOI] [PubMed] [Google Scholar]
- Miki H., Miura K., Matuoka K., Nakata T., Hirokawa N., Orita S., Kaibuchi K., Takai Y., Takenawa T. Association of Ash/Grb-2 with dynamin through the Src homology 3 domain. J Biol Chem. 1994 Feb 25;269(8):5489–5492. [PubMed] [Google Scholar]
- Nakanishi H., Orita S., Kaibuchi K., Miura K., Miki H., Takenawa T., Takai Y. Kinetic properties of Ash/Grb2-interacting GDP/GTP exchange protein. Biochem Biophys Res Commun. 1994 Feb 15;198(3):1255–1261. doi: 10.1006/bbrc.1994.1177. [DOI] [PubMed] [Google Scholar]
- Nishida E., Maekawa S., Sakai H. Characterization of the action of porcine brain profilin on actin polymerization. J Biochem. 1984 Feb;95(2):399–404. doi: 10.1093/oxfordjournals.jbchem.a134620. [DOI] [PubMed] [Google Scholar]
- Nishida E., Maekawa S., Sakai H. Cofilin, a protein in porcine brain that binds to actin filaments and inhibits their interactions with myosin and tropomyosin. Biochemistry. 1984 Oct 23;23(22):5307–5313. doi: 10.1021/bi00317a032. [DOI] [PubMed] [Google Scholar]
- Nobes C. D., Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995 Apr 7;81(1):53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Rozakis-Adcock M., Fernley R., Wade J., Pawson T., Bowtell D. The SH2 and SH3 domains of mammalian Grb2 couple the EGF receptor to the Ras activator mSos1. Nature. 1993 May 6;363(6424):83–85. doi: 10.1038/363083a0. [DOI] [PubMed] [Google Scholar]
- Rozakis-Adcock M., McGlade J., Mbamalu G., Pelicci G., Daly R., Li W., Batzer A., Thomas S., Brugge J., Pelicci P. G. Association of the Shc and Grb2/Sem5 SH2-containing proteins is implicated in activation of the Ras pathway by tyrosine kinases. Nature. 1992 Dec 17;360(6405):689–692. doi: 10.1038/360689a0. [DOI] [PubMed] [Google Scholar]
- Schacht J. Purification of polyphosphoinositides by chromatography on immobilized neomycin. J Lipid Res. 1978 Nov;19(8):1063–1067. [PubMed] [Google Scholar]
- Symons M., Derry J. M., Karlak B., Jiang S., Lemahieu V., Mccormick F., Francke U., Abo A. Wiskott-Aldrich syndrome protein, a novel effector for the GTPase CDC42Hs, is implicated in actin polymerization. Cell. 1996 Mar 8;84(5):723–734. doi: 10.1016/s0092-8674(00)81050-8. [DOI] [PubMed] [Google Scholar]
- Touhara K., Inglese J., Pitcher J. A., Shaw G., Lefkowitz R. J. Binding of G protein beta gamma-subunits to pleckstrin homology domains. J Biol Chem. 1994 Apr 8;269(14):10217–10220. [PubMed] [Google Scholar]
- Tsukada S., Simon M. I., Witte O. N., Katz A. Binding of beta gamma subunits of heterotrimeric G proteins to the PH domain of Bruton tyrosine kinase. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):11256–11260. doi: 10.1073/pnas.91.23.11256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L., Kawakami Y., Kawakami T. The pleckstrin homology domain of Bruton tyrosine kinase interacts with protein kinase C. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):9175–9179. doi: 10.1073/pnas.91.19.9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonezawa N., Homma Y., Yahara I., Sakai H., Nishida E. A short sequence responsible for both phosphoinositide binding and actin binding activities of cofilin. J Biol Chem. 1991 Sep 15;266(26):17218–17221. [PubMed] [Google Scholar]
- Yonezawa N., Nishida E., Sakai H. pH control of actin polymerization by cofilin. J Biol Chem. 1985 Nov 25;260(27):14410–14412. [PubMed] [Google Scholar]
- Yu F. X., Johnston P. A., Südhof T. C., Yin H. L. gCap39, a calcium ion- and polyphosphoinositide-regulated actin capping protein. Science. 1990 Dec 7;250(4986):1413–1415. doi: 10.1126/science.2255912. [DOI] [PubMed] [Google Scholar]