Although most of us are more familiar with the spectacular psychotic symptoms, such as hallucinations and delusions, that characterize schizophrenia, it is the cognitive deficits that drive much of the disability seen in this illness, serving as a “glass ceiling” that limits social and occupational functioning in patients with the illness (1). Impaired cognition is largely unresponsive to antipsychotic treatment, and despite a very significant investment by the pharmaceutical industry for over a decade, no proven pharmacological treatments have been found for this disabling aspect of the illness. The study by Reinhart et al. (2) in PNAS takes a cognitive neuroscience approach to pursue an entirely new avenue for the treatment of impaired cognition and associated functional disability in schizophrenia.
Reinhart et al.’s (2) work is informed by an extensive literature on the neural mechanisms that underlie dynamic cognitive control (the moment-to-moment adjustments in attention that characterize flexible cognitive functioning) that have been shown to be disrupted in schizophrenia (3). Studies using EEG and functional MRI in healthy individuals have shown that this aspect of cognitive control depends upon interactions between the medial prefrontal cortex (PFC, specifically the dorsal anterior cingulate cortex and adjacent presupplementary motor area), which becomes active during brain states such as errors, conflict, or losses when increased cognitive control is necessary for successful goal-directed performance, and lateral prefrontal systems, which then become more engaged to enhance the level of cognitive control during subsequent performance (4). Phase coherence of synchronously firing population neuronal activity in the theta (4–8 Hz) range has been proposed as a mechanism for detecting the need for increased control, as well as for linking medial to lateral prefrontal functioning to dynamically adjust performance (5). As one aspect of their cognitive control deficit, individuals with schizophrenia show impaired dynamic control as indexed by reduced error and conflict-related activity in the medial PFC during errors, along with altered behavioral adjustments in some but not all studies (6). Reinhart et al. (2) apply very low doses of electric current to the scalp in the region overlying the medial PFC to test the hypothesis that they can enhance the function of the adaptive control system in the brain in patients with schizophrenia, predicting that the effects will be mediated by increased theta synchrony measured using EEG (Fig. 1). The authors have been remarkably successful, and their findings have considerable significance both for our understanding of the dynamics of human cognitive control and how we may treat schizophrenia and other brain disorders in the future.
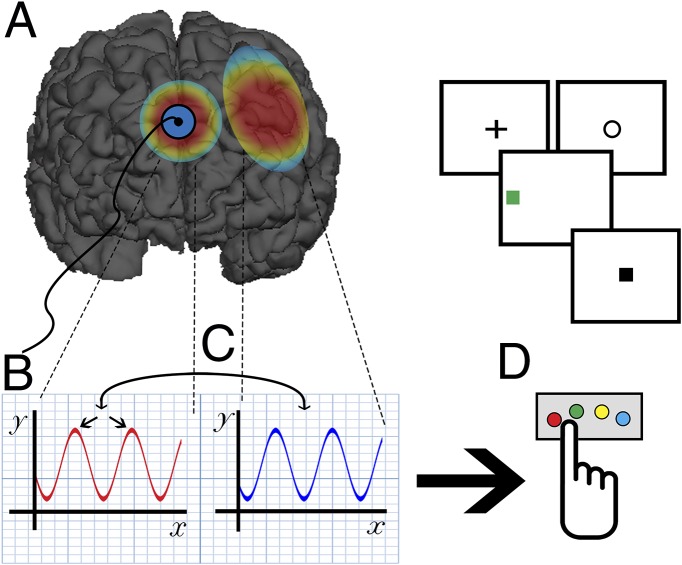
Fig. 1.
Reinhart et al. (2) apply low-dose (1.5 mAmp) direct-current electrical stimulation of the medial PFC (which includes the dorsal anterior cingulate cortex and presupplementary motor area), an area of the brain that is active and generates theta (4–8 Hz) neuronal oscillatory activity under conditions in which there is a need for increased cognitive control. The stimulation (A) leads to (B) increased intertrial phase coupling in neuronal ensembles in this region during error commission, along with (C) phase coupling between the medial PFC and lateral PFC. This activity is associated with (D) enhanced performance on a cognitive control task and increased slowing of reaction times on trials that follow the error (“adaptive cognitive control”). Effects are seen in both patients with schizophrenia (who during sham stimulation show decreased medial PFC error-related theta and an absence of adaptive control) and healthy comparison subjects. This study provides novel causal data on the role of the medial PFC in mediating dynamic cognitive control, and the results are consistent with the model of adaptive control initially proposed by Botvinick et al. (7) in 2001 and further elaborated by Cavanaugh and Frank (5) in 2014. The findings also have important clinical implications, suggesting the potential use of this form of neurostimulation to remediate the disabling, treatment refractory cognitive deficits that are widely seen in individuals with schizophrenia.
Twenty minutes of anodal stimulation at 1.5 mAmps led to improvements in phase synchrony (but not power) in the response-aligned EEG over the medial PFC and restoration of posterror slowing in the schizophrenia group; these two changes were correlated at the individual subject level. Coherence between the medial and lateral PFC, reduced during the sham session, was also restored in schizophrenia. Interestingly, the pattern of performance improvements and changes in neural activity seen in patients was also paralleled in the healthy comparison group.
The demonstration that targeted neuronal activity, in the form of theta coherence within and across medial and lateral PFC circuits, can be directly manipulated through the scalp is a further demonstration that, although housed within the skull, the brain is not at all isolated and inaccessible as we might once have believed. Instead, neural activity can be modulated using noninvasive stimulation, including the more established repetitive transcranial magnetic stimulation technique, as well as the more recently and increasingly widely implemented transcranial direct current stimulation (tDCS)-based approach used in the present study (2). What distinguishes this study, and hopefully future ones that follow, is that the functional neural circuit being targeted has been extensively characterized in healthy subjects and a substantial body of evidence has suggested that reductions in its activity were associated with impaired dynamic control in schizophrenia. The same principle guided the selection of EEG-based measures of theta coherence as a neural marker of the biological impact of the intervention.
In addition to its potential significance for treating schizophrenia, the paper by Reinhart et al. (2) also has important implications for our understanding of dynamic control, because the tDCS intervention provides causal data on the role of the medial prefrontal theta in posterror dynamic adjustments in control, supporting the key role of the medial PFC in participating in a dynamic loop with the lateral PFC to support these adjustments, as initially proposed in Botvinick et al. (7) over a decade ago.
Although the results of Reinhart et al.’s (2) report have important implications for the treatment of impaired cognition in schizophrenia, as well as cognitive neuroscience in general, it also raises many important questions. First, the sample size is small and it will be important that the study be replicated, preferably in a considerably larger group of patients. Second, it is unclear to what degree tDCS stimulation would produce sustained effects on cognition in the illness, what a more prolonged course of treatment would look like, and whether booster sessions or sustained treatment would be required to have lasting positive effects. It would also be important to link changes in dynamic control to measures of social and occupational functioning, because these are the end points most relevant for reducing the functional social and economic impact of impaired cognition in schizophrenia (8). Cognitive training has also been shown to improve cognition but this has not always translated into improved functioning, which might require additional rehabilitative interventions to leverage cognitive gains into functional ones (9). Finally, it is important to note that other aspects of impaired cognition in schizophrenia, including proactive forms of cognitive control linked to a disruption of sustained activity of the dorsolateral PFC and the active maintenance of context in working memory, can be targeted using comparable methods. These cognitive and neural systems were not directly targeted in the present study (2). Impairments in the context maintenance function of the PFC have been more reliably linked to general cognitive deficits in the illness, disorganization symptoms, and functional disability in schizophrenia than the dynamic control processes that are the focus of the present study (2), so targeting these mechanisms with tDCS would also be of potential value (10).
Recent developments in neurostimulation include the use of alternating current rather than direct current, which can be modulated at frequencies targeting specific frequency bands, such as theta, and it is possible to personalize stimulation to an individual subject’s own naturally evoked frequencies (11). Future studies may examine the impact of this approach on dynamic control circuitry in health and disease.
In addition to its potential significance for treating schizophrenia, the paper by Reinhart et al. also has important implications for our understanding of dynamic control.
A number of other disorders affect the dorsomedial to dorsolateral PFC dynamic control circuitry, perhaps most notably obsessive-compulsive disorder, where this activity is pathologically enhanced. Cathodal (inhibitory) tDCS/transcranial magnetic stimulation may decrease symptoms in this disorder (12). In light of this possibility, one wonders if it would be advisable to use anodal medial frontal stimulation on a regular basis, because this was effective in enhancing activity in this circuitry in the controls in the present study (2). Could excessive medial frontal stimulation lead to obsessive-compulsive disorder or increases in other forms of anxiety in otherwise healthy individuals? With the growing popularity of “cosmetic” neurostimulation, little is known about the potential adverse effects of tDCS use in this context (13).
Reinhart et al. (2) have shown us that direct current stimulation of the medial PFC can modulate brain activity by increasing the synchronization of neuronal ensembles that detect errors. This result is associated with improved overall task performance, as well as improvements in posterror adjustments in reaction times. This result is seen in healthy subjects, supporting a causal role for theta synchronization in both detecting errors and recruiting lateral PFC systems to enhance posterror adjustments. That these effects of tDCS also improve the function of PFC-based dynamic control systems in individuals with schizophrenia may signal an important new direction in the therapeutics of human brain disorders. The use of noninvasive stimulation to enhance impaired cognition, together with the availability of an EEG-based biomarker (theta coherence) to confirm that the targeted underlying neural system has been engaged, are both highly notable developments. Future studies will need to replicate and extend these findings, as well as address a number of issues related to the therapeutic potential of this and related approaches. These findings reinforce our growing understanding that the disordered brain is not locked away inside the skull as we once thought, but is indeed within our reach and accessible for neuromodulation. Our newfound ability to systematically stimulate and record from the intact human brain may allow us to leverage the many remarkable advances that have occurred in cognitive and affective neuroscience over the past 25 y, and develop new, safe, and effective therapies for human brain disorders.
Acknowledgments
The author thanks Lauren Zakskorn and Tyler Lesh for their assistance with preparing the figure.
Footnotes
The author declares no conflict of interest.
See companion article on page 9448.
References
- 1.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153(3):321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 2.Reinhart RMG, Zhu J, Park S, Woodman GF. Synchronizing theta oscillations with direct-current stimulation strengthens adaptive control in the human brain. Proc Natl Acad Sci USA. 2015;112:9448–9453. doi: 10.1073/pnas.1504196112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kerns JG, et al. Decreased conflict- and error-related activity in the anterior cingulate cortex in subjects with schizophrenia. Am J Psychiatry. 2005;162(10):1833–1839. doi: 10.1176/appi.ajp.162.10.1833. [DOI] [PubMed] [Google Scholar]
- 4.Kerns JG, et al. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303(5660):1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 5.Cavanagh JF, Frank MJ. Frontal theta as a mechanism for cognitive control. Trends Cogn Sci. 2014;18(8):414–421. doi: 10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minzenberg MJ, Gomes GC, Yoon JH, Swaab TY, Carter CS. Disrupted action monitoring in recent-onset psychosis patients with schizophrenia and bipolar disorder. Psychiatry Res. 2014;221(1):114–121. doi: 10.1016/j.pscychresns.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 8.Green MF, et al. Functional co-primary measures for clinical trials in schizophrenia: Results from the MATRICS Psychometric and Standardization Study. Am J Psychiatry. 2008;165(2):221–228. doi: 10.1176/appi.ajp.2007.07010089. [DOI] [PubMed] [Google Scholar]
- 9.Bowie CR, McGurk SR, Mausbach B, Patterson TL, Harvey PD. Combined cognitive remediation and functional skills training for schizophrenia: Effects on cognition, functional competence, and real-world behavior. Am J Psychiatry. 2012;169(7):710–718. doi: 10.1176/appi.ajp.2012.11091337. [DOI] [PubMed] [Google Scholar]
- 10.Lesh TA, Niendam TA, Minzenberg MJ, Carter CS. Cognitive control deficits in schizophrenia: Mechanisms and meaning. Neuropsychopharmacology. 2011;36(1):316–338. doi: 10.1038/npp.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaušovec N, Jaušovec K. Increasing working memory capacity with theta transcranial alternating current stimulation (tACS) Biol Psychol. 2014;96:42–47. doi: 10.1016/j.biopsycho.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Kuo MF, Paulus W, Nitsche MA. Therapeutic effects of non-invasive brain stimulation with direct currents (tDCS) in neuropsychiatric diseases. Neuroimage. 2014;85(Pt 3):948–960. doi: 10.1016/j.neuroimage.2013.05.117. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton R, Messing S, Chatterjee A. Rethinking the thinking cap: Ethics of neural enhancement using noninvasive brain stimulation. Neurology. 2011;76(2):187–193. doi: 10.1212/WNL.0b013e318205d50d. [DOI] [PMC free article] [PubMed] [Google Scholar]