Significance
The ability to exert control over our behavior is fundamental to human cognition, and is impaired in many neuropsychiatric disorders. Here, we show evidence for the neural mechanisms of adaptive control that distinguish healthy people from people who have schizophrenia. We found that the noninvasive electrical stimulation phase aligns low-frequency brain rhythms and enhances functional connectivity. This brain stimulation modulated the temporal structure of low-frequency oscillations and synchrony, improving adaptive control. Moreover, we found that causal changes in the low-frequency oscillations improved behavioral responses to errors and long-range connectivity at the single-trial level. These results implicate theories of executive control and cortical dysconnectivity, and point to the possible development of nonpharmacological treatment alternatives for neuropsychiatric conditions.
Keywords: oscillations, neural synchrony, adaptive control, schizophrenia, transcranial direct current stimulation
Abstract
Executive control and flexible adjustment of behavior following errors are essential to adaptive functioning. Loss of adaptive control may be a biomarker of a wide range of neuropsychiatric disorders, particularly in the schizophrenia spectrum. Here, we provide support for the view that oscillatory activity in the frontal cortex underlies adaptive adjustments in cognitive processing following errors. Compared with healthy subjects, patients with schizophrenia exhibited low frequency oscillations with abnormal temporal structure and an absence of synchrony over medial-frontal and lateral-prefrontal cortex following errors. To demonstrate that these abnormal oscillations were the origin of the impaired adaptive control in patients with schizophrenia, we applied noninvasive dc electrical stimulation over the medial-frontal cortex. This noninvasive stimulation descrambled the phase of the low-frequency neural oscillations that synchronize activity across cortical regions. Following stimulation, the behavioral index of adaptive control was improved such that patients were indistinguishable from healthy control subjects. These results provide unique causal evidence for theories of executive control and cortical dysconnectivity in schizophrenia.
Networks involving frontal cortex allow us to adapt our actions to dynamic environments and adjust information processing following errors (1). This adaptive control is a hallmark of healthy goal-directed behavior, but it is dysfunctional in a variety of psychiatric and neurological disorders (2–4). In particular, the adaptive-control deficits that are a central feature of schizophrenia are highly predictive of poor functioning in daily life (5). In the laboratory, a canonical signature of adaptive control is the magnitude of posterror slowing of reaction time (RT), in which healthy subjects respond more slowly after making an error (6, 7). Patients with schizophrenia show an impaired ability to slow down their responses after errors (4, 8–13, but also 14, 15), providing a laboratory index that captures the rigid, perseverative, and maladaptive behavior that is characteristic of the disorder (8, 16).
Adaptive control in the healthy brain is hypothesized to depend partly on the low-frequency EEG oscillations measured over medial-frontal cortex. The low-frequency oscillations are thought to reflect coordinated activity across the diverse set of brain areas recruited to perform a task (1, 17–22). In addition, medial-frontal theta (4–8 Hz) oscillations appear to signal the need for adaptive control across a variety of tasks and situations. Situations that call for adaptive control include stimulus novelty, response conflict, negative feedback, and behavioral errors, with all of these situations sharing a common medial-frontal spectral signature in the theta band (21). However, the functional significance of medial-frontal theta may be much broader than simply functioning as an alarm for the adaptive-control system. Theta oscillations have been hypothesized to serve as the temporal code that coordinates neuronal populations involved in implementing control (1, 19–21), with medial-frontal cortex working in concert with dorsolateral prefrontal areas to support flexible, adaptive behavior (1, 23–26). For example, when an error occurs, network-level oscillations allow executive mechanisms to adjust subordinate cognitive mechanisms (e.g., perceptual attention, response-selection thresholds). In the present study, we examined whether the executive-control deficits in patients with schizophrenia arise from communication and coordination failures among the cognitive subsystems flexibly linked through low-frequency oscillatory activity (3, 27, 28).
We recorded EEG oscillations from outpatients with schizophrenia and demographically matched healthy controls (Table S1) while they performed a two-alternative forced-choice target discrimination task with response deadlines and interleaved stop-signal trials sufficient to produce errors (similar to a go/no-go task) (Fig. 1A). We reasoned that if temporal structured medial-frontal theta activity underlies normal adaptive control, the patients should exhibit abnormal medial-frontal theta provided that they show abnormal posterror slowing.
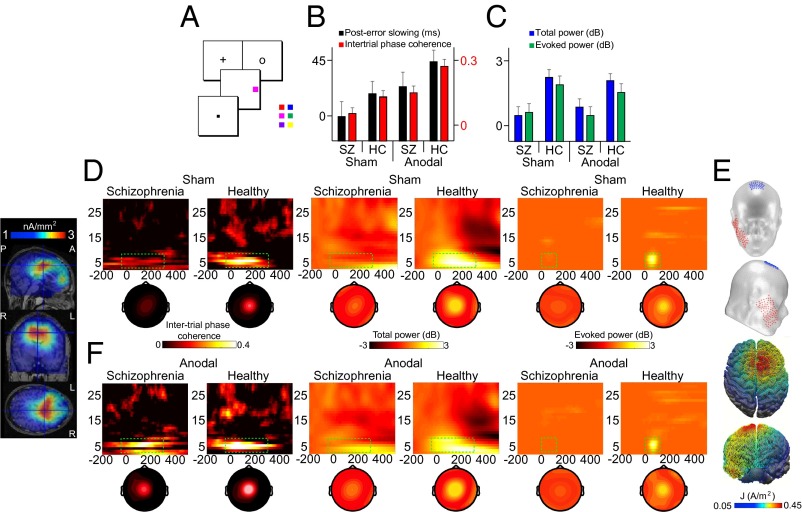
Fig. 1.
tDCS model, task, and the behavioral and spectral signatures of adaptive control. (A) Target discrimination task requiring subjects to report the color of the target (red vs. blue, magenta vs. green, or purple vs. yellow) by pressing one of two buttons on a handheld gamepad. (B) Mean posterror RT slowing and mean intertrial phase coherence shown across stimulation conditions and subject groups. HC, healthy controls; SZ, patients with schizophrenia. (C) Mean total power and mean evoked power as in B. (D) Intertrial phase coherence (Left), total power (Middle), and evoked power (Right) at Cz on error minus correct trials shown across subject groups in the sham condition. Topographies show spatial distribution from the selected time-frequency measurement windows (green rectangles). (Far Left) Source estimate of intertrial phase coherence centered on Cz peak activity for healthy subjects in the sham condition is shown across sagittal, coronal, and axial MRI slices. A, anterior; L, left; P, posterior; R, right. (E) Schematic of tDCS montage and the modeled distribution of current during active tDCS on top and front views of a 3D reconstruction of the cortical surface. (F) Response-related time-frequency representations and topographies as in D shown across subject groups in the anodal tDCS condition. The analytical window for intertrial phase coherence and total power analyses was 4–8 Hz, −50 to 300 ms periresponse. The analytical window for evoked power analyses was 4–8 Hz, 0–100 ms postresponse. Data were scalp Laplacian-transformed.
Results
Our findings were consistent with the prediction that when medial-frontal theta is disordered, the posterror slowing index of adaptive control will be impaired. We found that posterror slowing was absent in patients (i.e., not different from 0 ms: F1,17 = 0.007, P = 0.935) and reduced (F1,17 = 5.387, P = 0.033) relative to the significant posterror slowing we observed in controls (F1,17 = 6.436, P = 0.021) (Fig. 1B). Note that some studies have not found significant posterror slowing reductions in patients with schizophrenia relative to healthy controls. The reason for this discrepancy remains an open question, with possible factors including task modality (e.g., oculomotor vs. manual response), task parameters, and symptom severity. However, even the studies that do not show posterror slowing in patients report abnormal posterror behavior (14).
To test the hypothesis that theta oscillations index mechanisms related to adaptive control, we performed time-frequency decomposition of the single-trial EEGs. We directly compared the response-locked data on error trials against the response-locked data on correct trials. Consistent with the theta hypothesis of adaptive control, Fig. 1 B–D shows that the patients exhibited asynchronous and low-power central midline oscillations from 4–8 Hz. Specifically, intertrial phase coherence (main effect of group: F1,17 = 10.388, P = 0.005), total power (main effect of group: F1,17 = 28.338, P < 0.0001), and evoked power (main effect of group: F1,17 = 17.612, P = 0.001) measured from the central midline electrode (Cz) on error minus correct trials were significantly reduced in patients relative to controls (time-voltage domain analysis is provided in Fig. S1 and SI Results). Converging support from cluster-randomization analyses revealed the spatiospectral specificity of these phenomena (details are provided in SI Materials and Methods). Significant clusters were confined to the theta band and the central midline location after error relative to correct responses in controls (intertrial phase coherence: 4.5–7 Hz, P = 0.008; total power: 3.5–8.5 Hz, P = 0.004, −50 to 300 ms periresponse; evoked power: 4.5–7.5 Hz, P = 0.001, 0–100 ms postresponse), but no such clusters reached significance in patients. Although these findings are consistent with the hypothesis that impaired medial-frontal theta activity explains the adaptive-control deficits in schizophrenia, these findings are correlational in nature. We next sought to provide the first test of this hypothesis in the human brain to elucidate the potential causal mechanism.
If the medial-frontal theta oscillations index mechanisms that are necessary for the posterror slowing metric of adaptive control, a causal manipulation of medial-frontal cortex that changes the structure of the theta oscillations should change posterror slowing. To test the prediction of necessity, we delivered 20 min of electrical current over the medial-frontal cortex using transcranial direct current stimulation (tDCS) (29, 30) (Fig. 1E). Notably, the structure, connections, and activity of medial-frontal cortex are abnormal in schizophrenia (31), the hub of the adaptive-control network (1). We used anodal tDCS because this type of stimulation can enhance brain function by causing neurons’ resting membrane potential to depolarize, resulting in greater neuronal excitability (29). Each subject completed two stimulation conditions (i.e., anodal, sham baseline) on different days in randomized order, while subjects were blinded to the stimulation conditions (more details are provided in SI Materials and Methods).
We found that medial-frontal stimulation synchronized the timing of the theta oscillations across trials such that this electrophysiological activity in our patients with schizophrenia became indistinguishable from this electrophysiological activity in control subjects at baseline, effectively eliminating this component of their functional impairments. Consistent with the hypothesis that theta entrainment carries the adaptive-control signals that enable adaptive control, we found that medial-frontal stimulation resulted in normalization of patients’ posterror slowing such that their performance was identical to the performance of healthy control subjects at baseline.
Fig. 1F shows that after medial-frontal stimulation, the phase of theta oscillations shifted to become more highly synchronized across trials, whereas the magnitude remained relatively unaffected. This pattern of results was evidenced by an increase in intertrial phase coherence (patients: F1,17 = 12.703, P = 0.002; controls: F1,17 = 7.763, P = 0.013), but not in total power (patients: F1,17 = 0.160, P = 0.694; controls: F1,17 = 0.533, P = 0.475) or evoked power (patients: F1,17 = 0.016, P = 0.899; controls: F1,17 = 0.759, P = 0.396) after stimulation relative to sham across both groups of subjects (Fig. 1 B and C). Cluster analyses revealed that the effect of stimulation on intertrial phase coherence was specific to medial-frontal theta for both subject groups (patients: 5.5–7.5 Hz, P = 0.004; controls: 4.5–7.5 Hz, P = 0.009). Further, we found that the majority of patients (13 of 17) and controls (15 of 18) showed significantly greater intertrial phase coherence following stimulation. Thus, dc stimulation increased the phase structure of theta oscillations in a large number of our subjects without influencing the power of these oscillations.
Anodal medial-frontal tDCS elevated patients to the sham baseline level of controls in terms of the temporal consistency of their central midline theta waves across trials (F1,17 = 0.157, P = 0.697). A cortical source reconstruction of the cross-trial phase coherence estimated a potential generator in the superior frontal gyrus [i.e., cingulate gyrus, with Montreal Neurological Institute (MNI) coordinates of the gravity center (4.0, 18.1, 29.5) explaining 85% of the variance], consistent with intracranial recordings (1, 17). This phase alignment of the theta oscillations in patients paralleled the improvements in behavior, just as predicted if medial-frontal theta indexes mechanisms of adaptive control.
Fig. 1B shows that electrical stimulation over medial-frontal cortex boosted posterror slowing in patients with schizophrenia, such that the patients’ data were indistinguishable from healthy control data measured during the sham baseline condition. After stimulation, patients exhibited significant posterror slowing relative to sham (F1,17 = 5.690, P = 0.029). With this improvement, patients no longer differed from controls in their posterror behavioral adjustments (compare the middle two black bars of Fig. 1B; F1,17 = 0.126, P = 0.727). The increased posterror slowing following anodal stimulation was specific to this index of adaptive control, because neither overall mean RT (mean ± SE; patients: 521 ± 13 ms vs. 520 ± 12 ms, F1,17 = 0.009, P = 0.926; controls: 494 ± 11 ms vs. 499 ± 11 ms, F1,17 = 0.077, P = 0.785) nor the probability of responding on no-stop trials (patients: 95 ± 1.2% vs. 97 ± 2.0%, F1,17 = 1.520, P = 0.234; controls: 98 ± 0.6% vs. 98 ± 0.4%, F1,17 = 0.039, P = 0.845) changed between stimulation conditions. Patients did show mild impairment in stop signal reaction time (SSRT) (i.e., a measure of how quickly a preplanned motor response is aborted after a stop signal) relative to healthy controls (mean ± SE; patients: 250 ± 6.6 ms, controls: 232 ± 5.3 ms, F1,17 = 4.338, P = 0.053). However, no change in SSRT was observed as a function of stimulation condition (patients: 247 ± 5.0 ms, F1,17 = 0.274, P = 0.608; controls: 224 ± 2.9 ms, F1,17 = 2.335, P = 0.145), further demonstrating the specificity of the medial-frontal montage to affect processes related to posterror adjustments. Accuracy also was improved with stimulation (patients: 10.9 ± 1.9% vs. 5.9 ± 1.1%, F1,17 = 6.929, P = 0.017; controls: 5.8 ± 1.5% vs. 1.3 ± 1.1%, F1,17 = 13.366, P = 0.002), as would be expected if the posterror slowing were successfully compensating for breakdowns that result in errors. Thus, 20 min of electrical stimulation over medial-frontal cortex was sufficient to eliminate this component of the adaptive-control behavioral impairment in schizophrenia temporarily, allowing patients to adapt following errors like healthy control subjects.
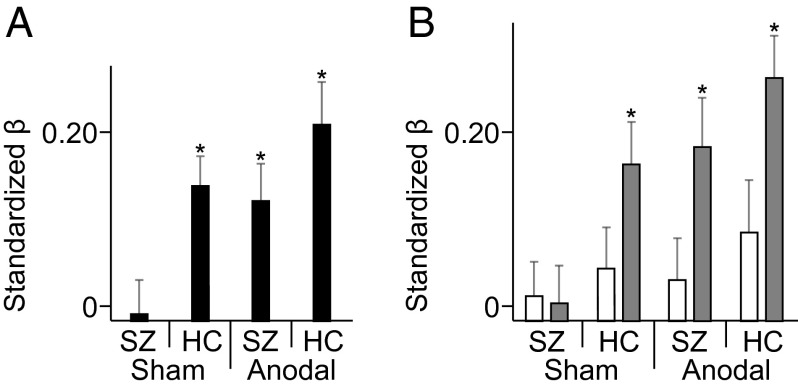
The averaged results presented in Fig. 1 provide evidence that connects the aberrant theta oscillations over medial-frontal cortex with adaptive-control deficits in schizophrenia. However, to provide more precise quantification of the theta dynamics underlying adaptive control, we performed single-trial regression analyses. We found that stimulation to medial-frontal cortex not only resulted in the emergence of temporally structured theta activity in the patients with schizophrenia who previously lacked this neural activity but also resulted in the theta dynamics being predictive of posterror slowing on a single-trial level.
Fig. 2A shows that theta responses during the sham baseline were tightly coupled with trial-to-trial posterror adjustments in RT, but that this coupling was only true for healthy subjects and not for patients with schizophrenia. Specifically, in controls, one-sample t tests of the individual standardized β-weights revealed that peak theta intertrial phase coherence predicted posterror slowing, with greater peak coherence predicting more slowing on the following trial (t17 = 4.010, P = 0.001). Intertrial phase coherence in patients showed no such predictive power (t17 = 0.149, P = 0.884), and their β-weights were significantly smaller than the β-weights of controls (t17 = 3.738, P = 0.002). However, after stimulation had realigned the phases of the medial-frontal theta oscillations in patients, their peak theta phase-coherence values significantly predicted single-trial fluctuations in posterror RT (t17 = 3.624, P = 0.002). Thus, by applying dc stimulation to medial-frontal cortex, we effectively brought the theta-band activity back online in patients with schizophrenia, boosting the spectral signature of adaptive control in these patients so that they were indistinguishable from healthy controls.
Fig. 2.
Single-trial analyses. (A) Aggregated individualized β-weights from bivariate regressions between posterror RT slowing and peak theta-band intertrial phase coherence at Cz. (B) Aggregated individualized β-weights between posterror RT slowing and peak phase synchrony from the Cz-F3 (white) and Cz-F4 (gray) electrode pairs. The analytical window was −50 to 300 ms periresponse from error minus correct trials. Error bars show SEM. *P < 0.05.
Our findings provide support for basic models of information processing in the brain, which propose that the theta phase provides a carrier wave for neuronal computation and communication across broad neural networks (1, 21). However, thus far, we have only assessed local oscillatory dynamics focused on the electrode nearest medial-frontal cortex. To test these ideas further, we examined long-range functional connectivity before the implementation of control in patients with schizophrenia and healthy subjects.
According to current theories of prefrontal-cortex functioning, after the occurrence of an event, such as an error, that requires a dynamic adjustment to ongoing information processing, the medial-frontal cortex interacts with the lateral-prefrontal cortex in a dynamic loop to recruit greater control and improve later performance (20, 23). If the action-monitoring and cognitive-control systems are coordinated during adaptive control via theta-band phase dynamics, we should find interregional phase synchrony between the medial-frontal and lateral-prefrontal theta oscillations following errors relative to correct responses. More importantly, if electrical stimulation to medial-frontal cortex improved adaptive control in the patients by improving this theta-band loop, we should find stimulation-induced enhancement in the phase synchrony between central midline and frontolateral sites after errors compared with correct responses.
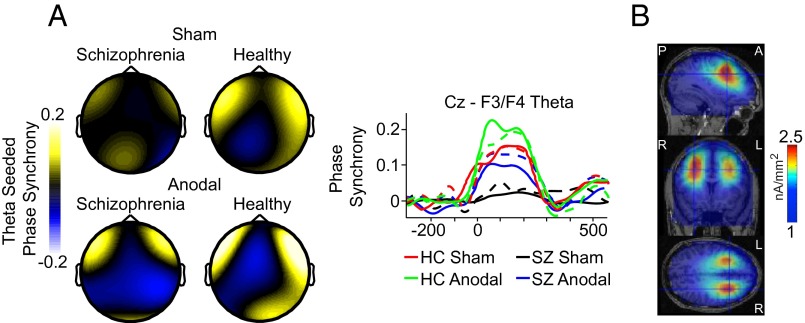
Fig. 3 shows that the predictions of the interregional phase synchrony account were supported. When we seeded our analyses with the medial-frontal theta oscillations from −50 to 300 ms periresponse, we measured intersite phase synchrony across the head and found stronger theta connectivity between medial frontal (Cz) and frontolateral sites on error relative to correct trials in healthy subjects (Cz-F3: F1,17 = 16.408, P = 0.001; Cz-F4: F1,17 = 15.167, P = 0.001). We observed striking spatial selectivity of this differential theta synchronization effect. Cluster analyses revealed significant Cz-seeded theta connectivity isolated to the frontolateral regions of the head (i.e., F3: 4.5–8 Hz, P = 0.004; F4-T4: 4–7.5 Hz, P = 0.009). Of note, our ability to capture the spatial specificity of this long-range theta effect was due, in part, to the Laplacian transform used to generate current-source density, which is known to highlight local electrical activity unique to each electrode while minimizing broadly distributed activity common to multiple electrodes (19, 20, 32) (details are provided in SI Materials and Methods). Cortical source modeling provided converging support for functional interactions between the medial-frontal and lateral-prefrontal cortices [middle frontal gyrus; MNI coordinates of the gravity center (31.3, 29.4, 21.7) accounting for 86% of the variance] (Fig. 3B). This pattern of results is exactly as predicted if the theta-band phase dynamics allow the action-monitoring system of medial-frontal cortex to communicate with the cognitive-control system of lateral-prefrontal cortex and increase decision thresholds or slow down response selection to avoid future mistakes.
Fig. 3.
Interregional phase synchrony. (A) Topographical maps of Cz-seeded theta phase synchrony at peak values between −50 and 300 ms after errors relative to correct responses shown across stimulation conditions and subject groups. Waveforms show the time course of the response-locked theta phase synchrony for Cz-F3 (dashed lines) and Cz-F4 (solid lines) electrode pairs across conditions and subject groups. (B) Source estimation of the peak theta phase synchrony, from –50 to 300-ms periresponse on error minus correct trials from the sham condition of healthy subjects shown across sagittal, coronal, and axial MRI slices. Data were scalp Laplacian-transformed.
Our manipulation of medial-frontal stimulation effectively reduced abnormal interregional functional connectivity in the patients with schizophrenia. First, consistent with faulty posterror slowing at baseline, patients exhibited no significant theta phase synchrony on error relative to correct trials (Cz-F3: F1,17 = 1.103, P = 0.308; Cz-F4: F1,17 = 0.345, P = 0.565) and weaker synchrony relative to controls in the sham condition (Cz-F3: F1,17 = 5.504, P = 0.031; Cz-F4: F1,17 = 6.351, P = 0.022) (Fig. 3A). However, after anodal tDCS over medial-frontal cortex, a similar pattern of intersite phase synchrony emerged in patients. Specifically, connectivity between medial-frontal and frontolateral sites was significantly stronger (anodal vs. sham Cz-F3: F1,17 = 5.734, P = 0.028; Cz-F4: F1,17 = 8.147, P = 0.011) and specific to this regional network (false alarm rate controlled by cluster analyses; F3: 4.5–7.5 Hz, P = 0.014; F4: 4–7.5 Hz, P = 0.010). Single-trial analyses revealed that Cz-F4 theta synchrony was predictive of posterror slowing for patients after stimulation (t17 = 4.285, P = 0.001), unlike in the sham baseline, where patients’ connectivity did not predict single-trial behavior after an error (t17 = 1.061, P = 0.303) (Fig. 2B). These results provide causal support for the hypothesis that medial-frontal and lateral-prefrontal cortices in schizophrenia fail to communicate the need for adaptive control via synchronized low-frequency oscillations. Even more striking, after 20 min of dc brain stimulation, we were able to enhance the long-range functional connectivity underlying the moment-to-moment changes in adaptive control in patients with schizophrenia.
Discussion
In summary, our findings demonstrate that the posterror slowing deficit of adaptive control in schizophrenia is governed, in part, by dysfunctional processes indexed by theta-band phase dynamics, which are abnormally decoupled from frontolateral oscillations important for the implementation of cognitive control. However, by stimulating medial-frontal cortex, considered by many to represent a fulcrum for the action-monitoring network, we were able to improve the behavioral and neural signatures of adaptive control in schizophrenia temporarily.
The present study shows that dc applied to the brain changed the nature of the oscillations that it generates. It initially seems counterintuitive that constant current stimulation can change the nature of oscillations, with a selective influence on the phase structure of low-frequency oscillations, without an accompanying change in spectral power. This study demonstrates that the unchanging dc affected a specific phase-based oscillatory mechanism in the brain. This finding is consistent with the view that the dimensions of the EEG (e.g., frequency, power, phase) reflect separable physiological mechanisms for the organization and communication of neuronal computations (33–36). For example, studies have shown that global theta synchronization, but not theta power, promotes learning and adaptation (37, 38). Also, single neurons show phase-coherent relationships with theta-field oscillations during improvements in memory (39) and adaptive behavioral control (1). The use of phase synchronization over other oscillatory features (e.g., frequency, power) may be due to a phase dependence of synaptic plasticity by low-frequency oscillations (35, 40, 41). The physiological mechanisms that allow dc stimulation to change the event-related phase dynamics of the brain will be an important topic for future work across multiple techniques (e.g., unit recordings, local field potentials, slice preparations), adding to this growing literature (42–45).
Our observation that electrical stimulation of medial-frontal areas organizes the phase of low-frequency oscillations in the brains across healthy people and patients with schizophrenia indicates that the functioning of adaptive-control mechanisms may be best conceptualized as operating along a continuum. Throughout our analyses, we found no significant group × stimulation interactions [posterror slowing (F1,17 = 0.008, P = 0.929), intertrial phase coherence (F1,17 = 1.076, P = 0.314), total power (F1,17 = 0.424, P = 0.524), evoked power (F1,17 = 0.218, P = 0.647), and intersite phase synchrony (Cz-F3: F1,17 = 1.679, P = 0.212; Cz-F4: F1,17 = 1.013, P = 0.328)]. The absence of such interactions demonstrates that patients with schizophrenia and healthy participants both benefit similarly from the medial-frontal tDCS. If the nature of the mechanism implementing adaptive control were qualitatively different in patients with schizophrenia and healthy controls, we would expect to see interactions of stimulation and group on the behavioral and electrophysiological metrics. This result does not rule out the possibility that cognitive impairments are a central feature in the pathophysiology of schizophrenia; rather, it suggests that the functioning of the adaptive-control system is best conceptualized as a continuum, consistent with the guidelines of the National Institute of Mental Health research domain criteria (RDoC) (46).
Thus, our findings inform the current debate about the nature of cognitive deficits in schizophrenia; that is, there is debate about whether symptoms of schizophrenia are categorically and qualitatively different from other forms of human behavior and experience or whether the brains of healthy people who may carry latent liability for psychosis exhibit similar characteristics, although below the threshold for diagnosis (47–50). The dimensional approach to understanding schizophrenia symptomatology is given its clearest expression in the RDoC framework, which focuses on basic dimensions of functioning across the wellness spectrum, and not on diagnoses based on heterogeneous clusters of symptoms. Our results suggest that the neural and behavioral manifestations of adaptive control may be similarly characterized as a dimension of functioning across a spectrum (or continuum), from high to low cognitive flexibility. We note that our results are based on one metric of adaptive control. Although posterror slowing is regarded as a behavioral signature of adaptive functioning, no single measure can capture the full scope of a cognitive capacity in all of its richness and complexity. Thus, future work is required to substantiate the suggestion that adaptive control may be more accurately conceptualized as a continuum, rather than having subcategories.
Our observation that the causal manipulation of cross-trial timing of theta oscillations governs processes related to the adaptive-control failures in schizophrenia is consistent with the dysconnectivity hypothesis of schizophrenia (3, 27, 28, 51). Specifically, low-frequency theta oscillations are thought to enable flexible connections between neural networks as task demands change or adaptive control is needed in the healthy brain, with this connectivity being disordered in the brains of patients with schizophrenia. Functional and structural connectivity problems in schizophrenia are frequently reported as abnormalities of hypoconnectivity involving the frontal cortex and present across the different stages of schizophrenia (52). Our results contribute important new knowledge to this growing body of work by showing that (i) patients with schizophrenia exhibit reduced functional connectivity, as observed in low-frequency electrophysiological activity following behavioral errors; (ii) these reductions in connectivity correspond to impaired behavioral responses to errors; and (iii) stimulation of the medial-frontal cortex can improve connectivity and normalize posterror behavior at the single-trial level in patients with schizophrenia.
Our findings also allow us to address two alternative explanations for the results. First, it is unlikely that medication of the patients with schizophrenia can explain the results. Atypical antipsychotics have been shown to produce modest but significant benefits for cognitive deficits in schizophrenia (53). Given our within-subjects design, any medication effects would apply equally across stimulation conditions. However, we observed significant neural and behavioral effects following active tDCS relative to sham in patients with schizophrenia, and we found no significant subject-wise correlations between the medication dose (i.e., chlorpromazine dose equivalent) and the primary outcome measures (r17 < 0.323, P > 0.191 across all measures). Thus, it is unlikely that the effects we observed are simply due to the presence of antipsychotic medication. However, further work is needed to better determine the specific effects of antipsychotic medications on posterror behavioral adjustments.
Second, the theta phase effects were not due to simple evoked activity. Our time-frequency analysis of the evoked activity was unlike the strong effects of stimulation on theta phase structure that we observed in the EEG activity [Fig. 1 D (Right) and F (Right), but also Fig. S1). Although the time course of the local and global synchronization effects may seem fast (∼0–300 ms posterror), 300 ms is sufficient for a single full 4-Hz (250-ms) cycle, or for multiple 6-Hz (∼167-ms) or 8-Hz (125-ms) cycles to serve as a brief excitability window for the integration of information for adaptive control. In addition to the temporal profile of the synchronization effects and the phase concentration of the effects, the long-range connectivity results argue further for an oscillatory-based explanation for how medial-frontal tDCS enhanced adaptive control following errors. However, it is possible that the relationship between low-frequency oscillatory activity and behavior may be caused by a third, undetected factor.
The present study has important implications for translating these findings from the laboratory into the real world. The treatment of cognitive deficits has traditionally been the domain of pharmacology (54); however, there are several encouraging signs that transcranial electrical stimulation may offer a safe alternative or adjunct approach. For patients with schizophrenia, atypical antipsychotic drugs (e.g., clozapine, risperidone, olanzapine) can ameliorate some aspects of cognitive deficits (55). However, there are adverse side effects, such as obesity and diabetes, and some patients develop resistance (56, 57). Therefore, there is a dire need for effective and noninvasive treatment options without the side effects. Over the past decade, tDCS has come into the spotlight, showing some promise as a drug-free intervention for neuropsychiatric illnesses, such as schizophrenia (58). Compelling rationales for using tDCS in schizophrenia include the fact that NMDA receptor dysfunction is implicated in schizophrenia pathophysiology (59) and NMDA antagonists abolish tDCS effects, whereas NMDA agonists enhance tDCS effects (60, 61). Second, schizophrenia is associated with deficits in neuroplasticity, specifically brain-derived neurotropic factor (BDNF)-dependent synaptic plasticity (62), and research has shown that dc stimulation promotes BDNF-dependent plasticity (63). Third, compared with other noninvasive stimulation methods, such as transcranial magnetic stimulation (TMS), tDCS is cost-effective, easy to use, portable, and safe, making this technique an attractive candidate as a supplementary neurointervention for people with severe neuropsychiatric conditions such as schizophrenia, which places a heavy personal and societal burden, reportedly costing more than $62.7 billion per year in the United States (64).
Materials and Methods
Materials and methods used in this study are discussed in SI Materials and Methods. Briefly, all subjects gave written informed consent approved by the Vanderbilt University Institutional Review Board and were paid. Patients or demographically matched healthy subjects were first exposed to 20 min of sham or anodal tDCS. Next, they performed a color discrimination task (Fig. 1A), requiring a two-alternative forced choice response within 700 ms, unless countermanded by a stop signal. Subjects’ EEG was continuously recorded (250-Hz sampling rate, 0.01-100–Hz bandpass filter) while they performed this task. The electrophysiological and behavioral data were analyzed offline. Each subject participated in both anodal and sham stimulation conditions on different days, with order randomized. Debriefing questions confirmed that subjects were blind to the nature of the stimulation condition.
Supplementary Material
Acknowledgments
We thank Lindsey G. McIntosh, Channing Cochran, Laura Hieber, and Jamie Michael for providing clinical operations support as well as the reviewers for their valuable comments and effort to improve the manuscript. This work was supported by the National Institutes of Health (Grants R01-EY019882, R01-MH073028, R01-EY025275, P30-EY08126, P30-HD015052, T32-EY007135, and F31-MH102042) and the National Alliance for Research on Schizophrenia and Depression.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.A.H. is a guest editor invited by the Editorial Board.
See Commentary on page 9152.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1504196112/-/DCSupplemental.
References
- 1.Narayanan NS, Cavanagh JF, Frank MJ, Laubach M. Common medial frontal mechanisms of adaptive control in humans and rodents. Nat Neurosci. 2013;16(12):1888–1895. doi: 10.1038/nn.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minzenberg MJ, Carter CS. Developing treatments for impaired cognition in schizophrenia. Trends Cogn Sci. 2012;16(1):35–42. doi: 10.1016/j.tics.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 3.Lesh TA, Niendam TA, Minzenberg MJ, Carter CS. Cognitive control deficits in schizophrenia: Mechanisms and meaning. Neuropsychopharmacology. 2011;36(1):316–338. doi: 10.1038/npp.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kerns JG, et al. Decreased conflict- and error-related activity in the anterior cingulate cortex in subjects with schizophrenia. Am J Psychiatry. 2005;162(10):1833–1839. doi: 10.1176/appi.ajp.162.10.1833. [DOI] [PubMed] [Google Scholar]
- 5.Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: Are we measuring the “right stuff”? Schizophr Bull. 2000;26(1):119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- 6.Rabbitt PMA. Error correction time without external error signals. Nature. 1966;212(5060):438. doi: 10.1038/212438a0. [DOI] [PubMed] [Google Scholar]
- 7.Ullsperger M, Danielmeier C, Jocham G. Neurophysiology of performance monitoring and adaptive behavior. Physiol Rev. 2014;94(1):35–79. doi: 10.1152/physrev.00041.2012. [DOI] [PubMed] [Google Scholar]
- 8.Velligan DI, Ritch JL, Sui D, DiCocco M, Huntzinger CD. Frontal Systems Behavior Scale in schizophrenia: Relationships with psychiatric symptomatology, cognition and adaptive function. Psychiatry Res. 2002;113(3):227–236. doi: 10.1016/s0165-1781(02)00264-0. [DOI] [PubMed] [Google Scholar]
- 9.Alain C, McNeely HE, He Y, Christensen BK, West R. Neurophysiological evidence of error-monitoring deficits in patients with schizophrenia. Cereb Cortex. 2002;12(8):840–846. doi: 10.1093/cercor/12.8.840. [DOI] [PubMed] [Google Scholar]
- 10.Carter CS, MacDonald AW, 3rd, Ross LL, Stenger VA. Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: An event-related fMRI study. Am J Psychiatry. 2001;158(9):1423–1428. doi: 10.1176/appi.ajp.158.9.1423. [DOI] [PubMed] [Google Scholar]
- 11.Malenka RC, Angel RW, Hampton B, Berger PA. Impaired central error-correcting behavior in schizophrenia. Arch Gen Psychiatry. 1982;39(1):101–107. doi: 10.1001/archpsyc.1982.04290010073013. [DOI] [PubMed] [Google Scholar]
- 12.Malenka RC, Angel RW, Thiemann S, Weitz CJ, Berger PA. Central error-correcting behavior in schizophrenia and depression. Biol Psychiatry. 1986;21(3):263–273. doi: 10.1016/0006-3223(86)90047-8. [DOI] [PubMed] [Google Scholar]
- 13.Turken AU, Vuilleumier P, Mathalon DH, Swick D, Ford JM. Are impairments of action monitoring and executive control true dissociative dysfunctions in patients with schizophrenia? Am J Psychiatry. 2003;160(10):1881–1883. doi: 10.1176/appi.ajp.160.10.1881. [DOI] [PubMed] [Google Scholar]
- 14.Thakkar KN, Schall JD, Boucher L, Logan GD, Park S. Response inhibition and response monitoring in a saccadic countermanding task in schizophrenia. Biol Psychiatry. 2011;69(1):55–62. doi: 10.1016/j.biopsych.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minzenberg MJ, Gomes GC, Yoon JH, Swaab TY, Carter CS. Disrupted action monitoring in recent-onset psychosis patients with schizophrenia and bipolar disorder. Psychiatry Res. 2014;221(1):114–121. doi: 10.1016/j.pscychresns.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corlett PR, et al. Disrupted prediction-error signal in psychosis: Evidence for an associative account of delusions. Brain. 2007;130(Pt 9):2387–2400. doi: 10.1093/brain/awm173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang C, Ulbert I, Schomer DL, Marinkovic K, Halgren E. Responses of human anterior cingulate cortex microdomains to error detection, conflict monitoring, stimulus-response mapping, familiarity, and orienting. J Neurosci. 2005;25(3):604–613. doi: 10.1523/JNEUROSCI.4151-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luu P, Tucker DM, Derryberry D, Reed M, Poulsen C. Electrophysiological responses to errors and feedback in the process of action regulation. Psychol Sci. 2003;14(1):47–53. doi: 10.1111/1467-9280.01417. [DOI] [PubMed] [Google Scholar]
- 19.van Driel J, Ridderinkhof KR, Cohen MX. Not all errors are alike: Theta and alpha EEG dynamics relate to differences in error-processing dynamics. J Neurosci. 2012;32(47):16795–16806. doi: 10.1523/JNEUROSCI.0802-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cavanagh JF, Cohen MX, Allen JJ. Prelude to and resolution of an error: EEG phase synchrony reveals cognitive control dynamics during action monitoring. J Neurosci. 2009;29(1):98–105. doi: 10.1523/JNEUROSCI.4137-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cavanagh JF, Frank MJ. Frontal theta as a mechanism for cognitive control. Trends Cogn Sci. 2014;18(8):414–421. doi: 10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anguera JA, et al. Video game training enhances cognitive control in older adults. Nature. 2013;501(7465):97–101. doi: 10.1038/nature12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306(5695):443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- 24.Narayanan NS, Laubach M. Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron. 2006;52(5):921–931. doi: 10.1016/j.neuron.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheth SA, et al. Human dorsal anterior cingulate cortex neurons mediate ongoing behavioural adaptation. Nature. 2012;488(7410):218–221. doi: 10.1038/nature11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonini F, et al. Action monitoring and medial frontal cortex: Leading role of supplementary motor area. Science. 2014;343(6173):888–891. doi: 10.1126/science.1247412. [DOI] [PubMed] [Google Scholar]
- 27.Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11(2):100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- 28.Ford JM, Mathalon DH. Neural synchrony in schizophrenia. Schizophr Bull. 2008;34(5):904–906. doi: 10.1093/schbul/sbn090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nitsche MA, et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimulat. 2008;1(3):206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Reinhart RMG, Woodman GF. Causal control of medial-frontal cortex governs electrophysiological and behavioral indices of performance monitoring and learning. J Neurosci. 2014;34(12):4214–4227. doi: 10.1523/JNEUROSCI.5421-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dolan RJ, et al. Dopaminergic modulation of impaired cognitive activation in the anterior cingulate cortex in schizophrenia. Nature. 1995;378(6553):180–182. doi: 10.1038/378180a0. [DOI] [PubMed] [Google Scholar]
- 32.Srinivasan R, Winter WR, Ding J, Nunez PL. EEG and MEG coherence: Measures of functional connectivity at distinct spatial scales of neocortical dynamics. J Neurosci Methods. 2007;166(1):41–52. doi: 10.1016/j.jneumeth.2007.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buzsáki G. Rhythms of the Brain. Oxford Univ Press; Oxford: 2006. [Google Scholar]
- 34.Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304(5679):1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 35.Fries P. A mechanism for cognitive dynamics: Neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9(10):474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 36.Womelsdorf T, et al. Modulation of neuronal interactions through neuronal synchronization. Science. 2007;316(5831):1609–1612. doi: 10.1126/science.1139597. [DOI] [PubMed] [Google Scholar]
- 37.Mölle M, Marshall L, Fehm HL, Born J. EEG theta synchronization conjoined with alpha desynchronization indicate intentional encoding. Eur J Neurosci. 2002;15(5):923–928. doi: 10.1046/j.1460-9568.2002.01921.x. [DOI] [PubMed] [Google Scholar]
- 38.Burke JF, et al. Synchronous and asynchronous theta and gamma activity during episodic memory formation. J Neurosci. 2013;33(1):292–304. doi: 10.1523/JNEUROSCI.2057-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rutishauser U, Ross IB, Mamelak AN, Schuman EM. Human memory strength is predicted by theta-frequency phase-locking of single neurons. Nature. 2010;464(7290):903–907. doi: 10.1038/nature08860. [DOI] [PubMed] [Google Scholar]
- 40.Fell J, Axmacher N. The role of phase synchronization in memory processes. Nat Rev Neurosci. 2011;12(2):105–118. doi: 10.1038/nrn2979. [DOI] [PubMed] [Google Scholar]
- 41.Siegel M, Donner TH, Engel AK. Spectral fingerprints of large-scale neuronal interactions. Nat Rev Neurosci. 2012;13(2):121–134. doi: 10.1038/nrn3137. [DOI] [PubMed] [Google Scholar]
- 42.Fröhlich F, McCormick DA. Endogenous electric fields may guide neocortical network activity. Neuron. 2010;67(1):129–143. doi: 10.1016/j.neuron.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reato D, et al. Transcranial electrical stimulation accelerates human sleep homeostasis. PLOS Comput Biol. 2013;9(2):e1002898. doi: 10.1371/journal.pcbi.1002898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Antal A, Varga ET, Kincses TZ, Nitsche MA, Paulus W. Oscillatory brain activity and transcranial direct current stimulation in humans. Neuroreport. 2004;15(8):1307–1310. doi: 10.1097/01.wnr.0000127460.08361.84. [DOI] [PubMed] [Google Scholar]
- 45.Polanía R, Nitsche MA, Paulus W. Modulating functional connectivity patterns and topological functional organization of the human brain with transcranial direct current stimulation. Hum Brain Mapp. 2011;32(8):1236–1249. doi: 10.1002/hbm.21104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Insel T, et al. Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 47.Raine A. Schizotypal personality: Neurodevelopmental and psychosocial trajectories. Annu Rev Clin Psychol. 2006;2:291–326. doi: 10.1146/annurev.clinpsy.2.022305.095318. [DOI] [PubMed] [Google Scholar]
- 48.Modinos G, et al. Schizotypy and brain structure: A voxel-based morphometry study. Psychol Med. 2010;40(9):1423–1431. doi: 10.1017/S0033291709991875. [DOI] [PubMed] [Google Scholar]
- 49.Woodward ND, et al. Correlation of individual differences in schizotypal personality traits with amphetamine-induced dopamine release in striatal and extrastriatal brain regions. Am J Psychiatry. 2011;168(4):418–426. doi: 10.1176/appi.ajp.2010.10020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi JS, et al. Phase-specific brain change of spatial working memory processing in genetic and ultra-high risk groups of schizophrenia. Schizophr Bull. 2012;38(6):1189–1199. doi: 10.1093/schbul/sbr038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Braff DL. Connecting the “dots” of brain dysfunction in schizophrenia: What does the picture look like? Arch Gen Psychiatry. 1999;56(9):791–793. doi: 10.1001/archpsyc.56.9.791. [DOI] [PubMed] [Google Scholar]
- 52.Pettersson-Yeo W, Allen P, Benetti S, McGuire P, Mechelli A. Dysconnectivity in schizophrenia: Where are we now? Neurosci Biobehav Rev. 2011;35(5):1110–1124. doi: 10.1016/j.neubiorev.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 53.Keefe RSE, et al. CATIE Investigators Neurocognitive Working Group Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64(6):633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- 54.Lewis DA, et al. Subunit-selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. Am J Psychiatry. 2008;165(12):1585–1593. doi: 10.1176/appi.ajp.2008.08030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leucht S, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: A multiple-treatments meta-analysis. Lancet. 2013;382(9896):951–962. doi: 10.1016/S0140-6736(13)60733-3. [DOI] [PubMed] [Google Scholar]
- 56.Elkis H. Treatment-resistant schizophrenia. Psychiatr Clin North Am. 2007;30(3):511–533. doi: 10.1016/j.psc.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 57.Brunoni AR, et al. Lithium as a treatment of clozapine-induced neutropenia: A case report. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(8):2006–2007. doi: 10.1016/j.pnpbp.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 58.Brunoni AR, et al. Understanding tDCS effects in schizophrenia: A systematic review of clinical data and an integrated computation modeling analysis. Expert Rev Med Devices. 2014;11(4):383–394. doi: 10.1586/17434440.2014.911082. [DOI] [PubMed] [Google Scholar]
- 59.Coyle JT. NMDA receptor and schizophrenia: A brief history. Schizophr Bull. 2012;38(5):920–926. doi: 10.1093/schbul/sbs076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nitsche MA, et al. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. 2003;553(Pt 1):293–301. doi: 10.1113/jphysiol.2003.049916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nitsche MA, et al. Consolidation of human motor cortical neuroplasticity by D-cycloserine. Neuropsychopharmacology. 2004;29(8):1573–1578. doi: 10.1038/sj.npp.1300517. [DOI] [PubMed] [Google Scholar]
- 62.Favalli G, Li J, Belmonte-de-Abreu P, Wong AH, Daskalakis ZJ. The role of BDNF in the pathophysiology and treatment of schizophrenia. J Psychiatr Res. 2012;46(1):1–11. doi: 10.1016/j.jpsychires.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 63.Fritsch B, et al. Direct current stimulation promotes BDNF-dependent synaptic plasticity: Potential implications for motor learning. Neuron. 2010;66(2):198–204. doi: 10.1016/j.neuron.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu EQ, et al. The economic burden of schizophrenia in the United States in 2002. J Clin Psychiatry. 2005;66(9):1122–1129. doi: 10.4088/jcp.v66n0906. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.