Significance
The prefrontal cortex (PFC) regulates multiple aspects of behavior. One hypothesis proposes that PFC segregates different functions into specific subregions: dorsal medial PFC [mPFC; prelimbic (PL)] regulates execution of behavior, or “going,” whereas ventral mPFC [infralimbic (IL)] regulates inhibition of behavior, or “stopping.” We demonstrate that neither PL nor IL neurons exhibited signals specifically tied to going or stopping, either during reward seeking or extinction sessions when rats stopped seeking reward. Instead, neurons better represented the outcome based context, firing more strongly for going during reward seeking and for stopping during extinction. The use of contextual information to guide responding is important for maximizing benefits vs. costs and may be a major role of the PFC in optimizing behavior.
Keywords: rat, extracellular, electrophysiology, instrumental, contingency
Abstract
The prefrontal cortex (PFC) guides execution and inhibition of behavior based on contextual demands. In rodents, the dorsal/prelimbic (PL) medial PFC (mPFC) is frequently considered essential for execution of goal-directed behavior (“go”) whereas ventral/infralimbic (IL) mPFC is thought to control behavioral suppression (“stop”). This dichotomy is commonly seen for fear-related behaviors, and for some behaviors related to cocaine seeking. Overall, however, data for reward-directed behaviors are ambiguous, and few recordings of PL/IL activity have been performed to demonstrate single-neuron correlates. We recorded neuronal activity in PL and IL during discriminative stimulus driven sucrose seeking followed by multiple days of extinction of the reward-predicting stimulus. Contrary to a generalized PL-go/IL-stop hypothesis, we found cue-evoked activity in PL and IL during reward seeking and extinction. Upon analyzing this activity based on resultant behavior (lever press or withhold), we found that neurons in both areas encoded contextually appropriate behavioral initiation (during reward seeking) and withholding (during extinction), where context was dictated by response–outcome contingencies. Our results demonstrate that PL and IL signal contextual information for regulation of behavior, irrespective of whether that involves initiation or suppression of behavioral responses, rather than topographically encoding go vs. stop behaviors. The use of context to optimize behavior likely plays an important role in maximizing utility-promoting exertion of activity when behaviors are rewarded and conservation of energy when not.
The prefrontal cortex (PFC) is involved in directing behavior and inhibiting inappropriate responses (1–4). Rodent medial PFC (mPFC) is functionally heterogeneous: prelimbic cortex (PL) is thought to be involved in behavioral execution, and infralimbic cortex (IL) in behavioral suppression, particularly during extinction (4). Fear conditioning studies support this dichotomy. PL stimulation elicits, and inactivation impairs, conditioned fear expression, and PL neurons fire during fear-related cues. Conversely, IL stimulation enhances, and inactivation blocks, extinction of fear conditioning, and IL neurons fire for extinguished cues that previously predicted an aversive stimulus (5, 6).
A similar dichotomy is proposed for appetitive behaviors, although support for this is not unequivocal (7). PL inactivation during discriminative stimulus (DS)-driven reward-seeking reduces cue-driven behaviors (8). However, PL inactivation also increases nonspecific (8) and premature lever responding (9, 10), and PL is important in inhibiting responses during a stop-signal reaction time task (11). IL inactivation during DS task performance increases responses generally, including those triggered by a nonrewarded stimulus (NS) (8), increases premature responding on five-choice serial reaction time tasks (12), and increases spontaneous recovery and reinstatement (13). Cocaine seeking is also associated with activation of PL, and extinction with activity in IL (4). However, IL is also implicated in driving seeking of cocaine, heroin, and other drugs of abuse, and PL activation inhibits compulsive cocaine seeking (7). Thus, even though the PL/IL go/stop dichotomy has been demonstrated in a number of studies, there are also a significant number of studies calling it into question (7). The goal of the present study was to test whether the PL/IL functional dichotomy seen in fear conditioning was also present in an appetitively motivated task and its extinction. This view predicts that PL neurons would fire during reward seeking, and IL neurons during suppression of seeking, particularly after extinction. Instead, we found that both regions displayed rapid, stimulus-evoked activity changes closely linked to contextually appropriate behavior, largely independent of whether the behavior involved response execution or inhibition.
Results
Task Performance.
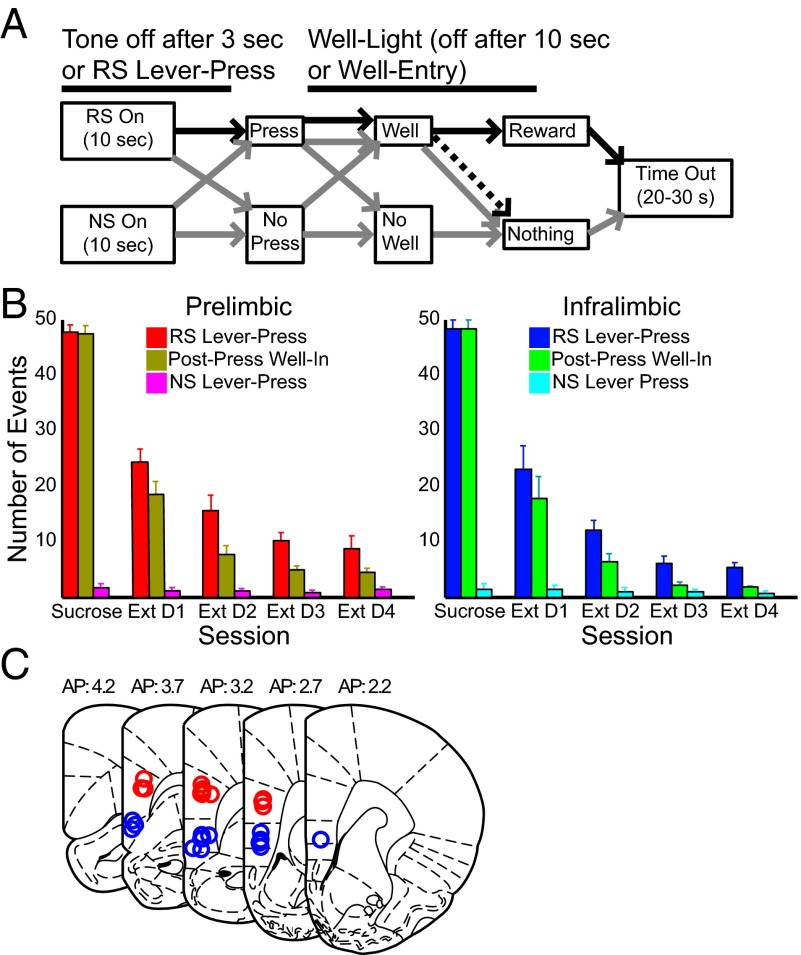
We recorded neuronal activity from PL and IL (n = 6 rats each) during performance of a DS-driven sucrose seeking task (DS-sucrose) and nonrewarded extinction sessions (Materials and Methods and Fig. 1A). In each trial, one of two lever/tone stimulus combinations was pseudorandomly presented: one rewarded [rewarded stimulus (RS)], and the other not (i.e., NS). RS lever presses followed by well entry were rewarded with sucrose. NS lever presses produced no effect. Rats accurately responded more to RSs than NSs (z = 4.78; P << 0.001, Wilcoxon test; Fig. 1B). RS lever presses (z = 1.15; P > 0.05, Mann–Whitney test) and reaction times (z = 0.36; P > 0.05) did not differ significantly between PL- and IL-implanted rats (median reaction time: PL, 2.41 s; IL, 2.33 s). During extinction, RS presses decreased significantly in PL-implanted [χ2(4) = 22.58; P << 0.001, Kruskal–Wallis test] and IL-implanted rats [χ2(4) = 22.07; P << 0.001] and equivalently (PL vs. IL: z = 1.15; P > 0.05, Mann–Whitney test).
Fig. 1.
(A) Task design. Rats were presented with RS or NS. NS responses (gray) produced no outcome. RS responses ended the RS tone, retracted the lever, and illuminated the reward well. Post-RS well entry resulted in sucrose reward (black). In extinction sessions, all events were the same except that well entry produced no reward (dashed line). (B) Lever press and well entry behavior for rats providing PL and IL neural recordings as indicated. RS lever presses and well entries decreased significantly across extinction days. For these and all following figures, error bars are mean ± SEM. Statistics are provided in Task Performance. (C) Placement of recording wire tips for PL-implanted (red) and IL-implanted (blue) animals. All wires tips were histologically localized to the respective prefrontal region.
PL and IL Neurons Signaled Rewarded and Nonrewarded Stimuli.
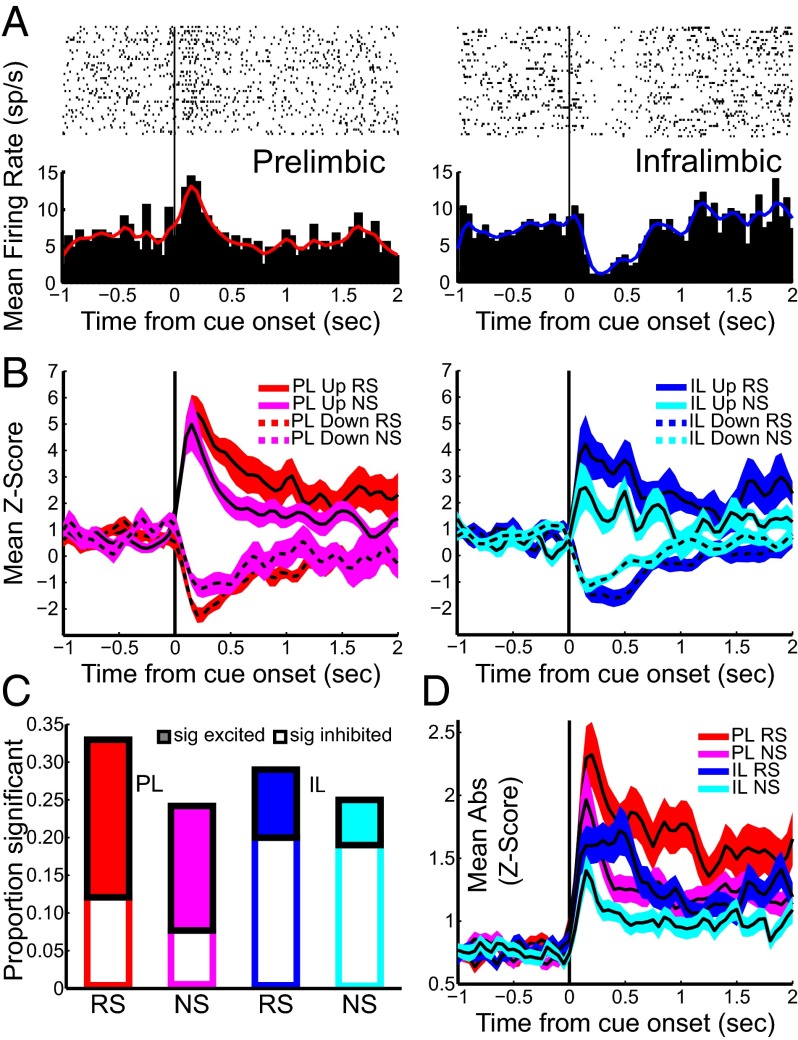
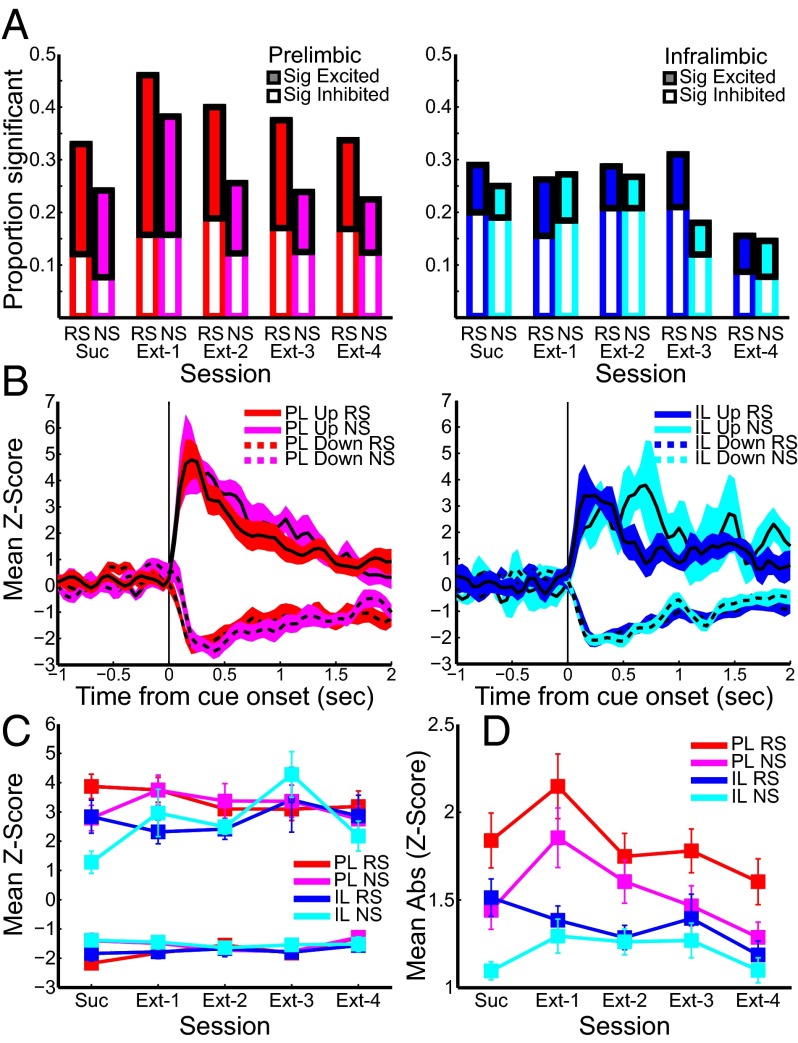
Neurons in PL (n = 91) and IL (n = 100; Fig. 1C) were recorded during DS-sucrose performance (representative neurons shown in Fig. 2A; sample waveforms and clusters shown in Fig. S1). Neurons included for analysis were likely primarily glutamatergic pyramidal neurons based on waveform and spontaneous firing rate (Fig. S1). Mean z-scored activity of significantly responding neurons is shown in Fig. 2B. Thirty-nine PL neurons (43%) significantly responded to the RS alone (n = 17), NS alone (n = 9), or both RS and NS (n = 13). Responses were biased toward excitation vs. inhibition, although this was not significant (RS and NS: both P > 0.05, binomial test; Fig. 2C). Similarly, 40 IL neurons (40%) were modulated by the RS alone (n = 15), NS alone (n = 11), or both (n = 14). More than twice as many IL neurons were more strongly inhibited than excited (RS, P = 0.06; NS, P < 0.01, binomial test; Fig. 2C). Significantly modulated neurons responded in the same direction (e.g., excited or inhibited) following RS and NS presentations (PL, 33 same vs. 6 different; P << 0.001; IL, 33 same vs. 7 different; P << 0.001, binomial test). Across the recorded population, neuronal responses to RS and NS were highly correlated (PL, r = 0.74; P << 0.001; IL, r = 0.66; P << 0.001). PL neurons significantly responsive to RS and NS appeared to be more strongly modulated than IL neurons (Fig. 2B), but this was not significant (excitation/inhibition, RS/NS; all P > 0.05, Mann–Whitney test). Neurons significantly modulated by RS presentation were less modulated by NS (PL, z = 4.37; P << 0.001; IL, z = 3.84; P << 0.001, Wilcoxon test on absolute value of z-scores). In contrast, PL neurons significantly modulated by NS presentation were equally modulated by RS (z = 1.06; P > 0.05), and IL neurons significantly modulated by NS presentations were more strongly modulated by RS (z = 2.65; P < 0.01). Peak/nadir neural responses to RS or NS presentation occurred rapidly (250–500 ms; Fig. 2 B and D) and far in advance of lever presses.
Fig. 2.
PL and IL neurons exhibited stimulus-evoked neural activity during DS-sucrose. (A) Single neuron examples of the prevalent response to RS presentation in each region. (B) Mean z-score activity for neurons that exhibited significant increases or decreases after RS or NS for PL (Left) or IL (Right) neurons. Solid/dashed lines represent activity of neurons with significantly increased/decreased activity, respectively. (C) Proportions of neurons that exhibited significant excitation (filled bars) or inhibition (empty bars) in response to RS or NS. (D) Mean absolute-value z-scores of all recorded neurons in response to RS or NS.
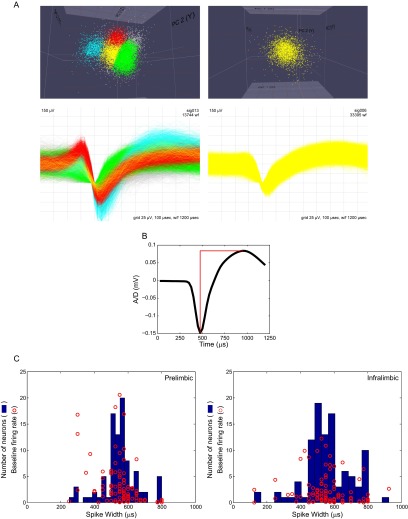
Fig. S1.
(A) Characterization of waveform clustering (Top) and the resultant waveforms (Bottom). (Right) Rare example of multiple neurons recorded on a single recording wire. (Left) more common (>50%) example of a single neuron recorded on a single recording wire. (B) Extracted mean waveform used for analysis of action potential width. Width measurements were taken as the time between spike valley and the following peak. (C) Distribution of spike widths and baseline firing rates for PL (Left) and IL (Right) neurons. Blue histograms show action potential widths, calculated as in B. Red circles indicate individual neurons with specific spike widths (x axis) and 5-min presession baseline firing rate (y axis). Note that the majority of the neurons are wide-spike and low-baseline firing, indicating that most neurons recorded are glutamatergic pyramidal neurons. A small number of neurons exhibited short-waveform action potentials, although baseline activity for these neurons were low in all cases but 2, arguing against their being GABAergic. Similarly, a small number of neurons exhibited relatively high baseline activity, but the combination of baseline activity and spike widths were not consistent with a GABAergic phenotype. We conclude that the vast majority of recorded neurons were glutamatergic, while noting that a small number may have been GABAergic interneurons.
Responses across all recorded neurons, using the absolute value of firing to characterize signaling strength irrespective of response direction, exhibited the same general patterns (Fig. 2D). Evoked responses were stronger in PL than in IL neurons, but this difference was significant only for NS presentations (RS, z = 0.86; P > 0.05; NS, z = 2.20; P < 0.05, Mann–Whitney test). Population signaling was significantly greater for RS vs. NS presentations in PL (z = 2.85; P < 0.005) and IL (z = 3.90; P << 0.001). In sum, PL and IL neurons were strongly modulated during reward seeking. This modulation was excitation-biased for PL neurons and inhibition-biased for IL neurons, but both areas exhibited excitation and inhibition and both were more strongly engaged during RS than NS presentation (Fig. S2).
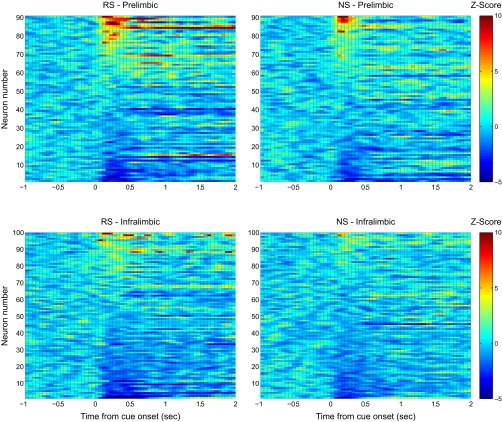
Fig. S2.
z-scored activity for all recorded PL (Top) and IL (Bottom) neurons during DS-sucrose illustrate the diverse stimulus-evoked responses in both regions. Activity is aligned on presentation of the RS (Left) or NS (Right). These data demonstrate that PL activity was approximately equally split between excitation and inhibition, whereas IL activity was primarily inhibited (although note some excitatory responses). In addition, the data show similar, although slightly weaker, evoked responses between RS and NS presentation.
Neuronal responses were stable across trials: only a small number of neurons exhibiting significant RS-evoked modulation showed significant changes over the course of a recording session (n = 6 PL and n = 2 IL; SI Materials and Methods and Fig. S3), indicating little to no influence of trial or sucrose consumption on mPFC activity in our task.
Fig. S3.
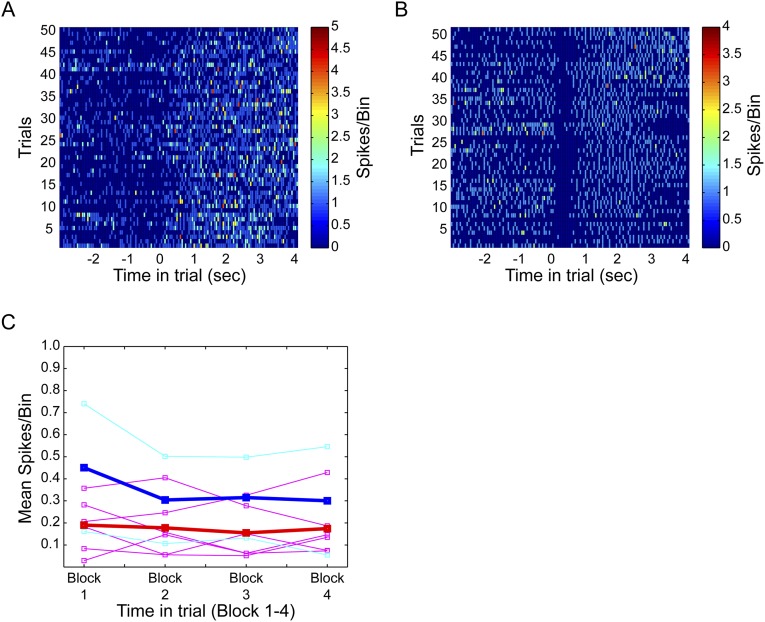
Activity of neurons in each trial of DS-sucrose recording sessions illustrate consistent evoked responses throughout the entire session. (A) An example of a RS-excited PL neuron. (B) An example of a RS-inhibited IL neuron. Trials are shown along the y axis and time in trial, aligned to RS presentation, is shown along the x axis. (C) Block-based responses for six PL (mauve; mean is red) and two IL (cyan; mean is blue) neurons that changed significantly across the recording session. Note the mild and inconsistent changes in most neurons across the session, even in this subset of significantly modulated neurons. These results indicate a minor influence of time and/or ingested sucrose on PL or IL activity during the DS-sucrose task.
PL/IL Response Patterns Persisted Throughout Extinction.
We next recorded activity from the same electrodes during 4 d of extinction (Materials and Methods and Fig. 1A). Surprisingly, stimulus-evoked activity in both PL and IL during extinction was similar to that seen during DS-sucrose (Fig. 3), despite greatly diminished lever pressing across extinction (Fig. 1B). The percentages of neurons significantly excited or inhibited by RS or NS presentation were largely consistent over the course of extinction in both regions (Fig. 3A), as were the proportions of neurons demonstrating significant evoked responses selectively for RS, NS, or both (Table S1). The strength of evoked responses also remained consistent across all sessions (Fig. 3 B–D) for both stimulus types in excited and inhibited neurons of PL and IL neurons (all χ2 < 8; all P > 0.05, Kruskal–Wallis test) except for two cases: (i) PL neurons significantly inhibited by RS presentation exhibited a small but significant diminution of inhibition [χ2(4,67) = 13.50; P < 0.01] and (ii) IL neurons significantly excited by NS presentation showed a significant enhancement of excitation [χ2(4,29) = 11.62; P < 0.05]. Similarly, activity in the entire population of recorded neurons did not change significantly across extinction when considering mean activity (all χ2 < 8; all P > 0.05, Kruskal–Wallis test). When considering absolute value activity, there were virtually no changes across extinction: PL activity related to the NS changed significantly [χ2(4,442) = 9.97.62; P < 0.05] but inconsistently, and there was a small and nonsignificant trend for diminished IL signaling in response to the RS [χ2(4,502) = 9.44; P = 0.051]. All other signals remained unchanged across extinction (Fig. 3D). Robust population activity on extinction day 2 is shown in Fig. 3B, and the stability of responses across all extinction days is shown in Fig. 3C (significantly modulated neurons) and Fig. 3D (absolute value of the whole population). Thus, despite some small fluctuations in firing over the transition from DS-sucrose to extinction, task-related signaling continued throughout extinction. These data indicate that PL and IL neurons signaled during reward seeking when rewarded (in DS-sucrose) and continued to signal stimuli throughout extinction despite absence of reward and substantial extinction-induced decreases in responding (Fig. 1B).
Fig. 3.
Stimulus-evoked neural activity during extinction was similar to that during DS-sucrose. Color/line conventions are as in Fig. 2. (A) Proportions of neurons significantly excited or inhibited by RS or NS presentation in DS-sucrose (as in Fig. 2) compared with all four extinction days [numbers recorded, n = 89, n = 90, n = 88, n = 89 (prelimbic; Left) and n = 103, n = 101, n = 100, n = 103 (infralimbic; Right)]. (B) Mean z-score activity for significantly excited or inhibited PL and IL neurons on extinction day 2 (PL, Left; IL, Right). (C) Mean z-score activity for significantly excited or inhibited PL and IL neurons poststimulus for DS-sucrose and all extinction days. (D) Mean absolute-value poststimulus z-scores for all recorded PL and IL neurons in response to RS and NS presentation on DS-sucrose and all extinction days.
Table S1.
Neurons exhibiting selective activation for stimuli at each stage
| Neuron | Stimulus | ||
| RS | NS | Both | |
| PL | |||
| DS-sucrose | 17 (18.7) | 9 (9.9) | 13 (14.3) |
| Extinction day 1 | 16 (18.0) | 9 (10.1) | 25 (28.1) |
| Extinction day 2 | 22 (24.4) | 9 (10.0) | 14 (15.6) |
| Extinction day 3 | 21 (23.9) | 8 (9.1) | 13 (14.8) |
| Extinction day 4 | 18 (20.2) | 8(9.0) | 12 (13.5) |
| IL | |||
| DS-sucrose | 15 (15.0) | 11 (11.0) | 14 (14.0) |
| Extinction day 1 | 11 (10.7) | 12 (11.7) | 16 (15.5) |
| Extinction day 2 | 13 (12.9) | 11 (10.9) | 16 (15.8) |
| Extinction day 3 | 20 (20.0) | 7 (7.0) | 11 (11.0) |
| Extinction day 4 | 9 (8.7) | 8 (7.8) | 7 (6.8) |
Significance was based on activity across trials 0–500 ms post stimulus presentation. Note that proportions remain largely consistent across sessions.
Baseline activity did not change across sessions. This was verified using two epochs: (i) 30 s preceding the first stimulus presentation in each recording session [F(1,4) = 0.18; P > 0.05, rank-ordered two-factor ANOVA], and (ii) 2–4 s preceding each stimulus presentation within a session, averaged across RS and NS [F(1,4) = 0.43; P > 0.05]. IL baseline activity, however, was significantly lower than PL [F(1,4) = 37.94 and 38.47; P << 0.001].
Stimulus-Evoked PL/IL Activity Predicted Context-Appropriate Execution and Inhibition of Responding.
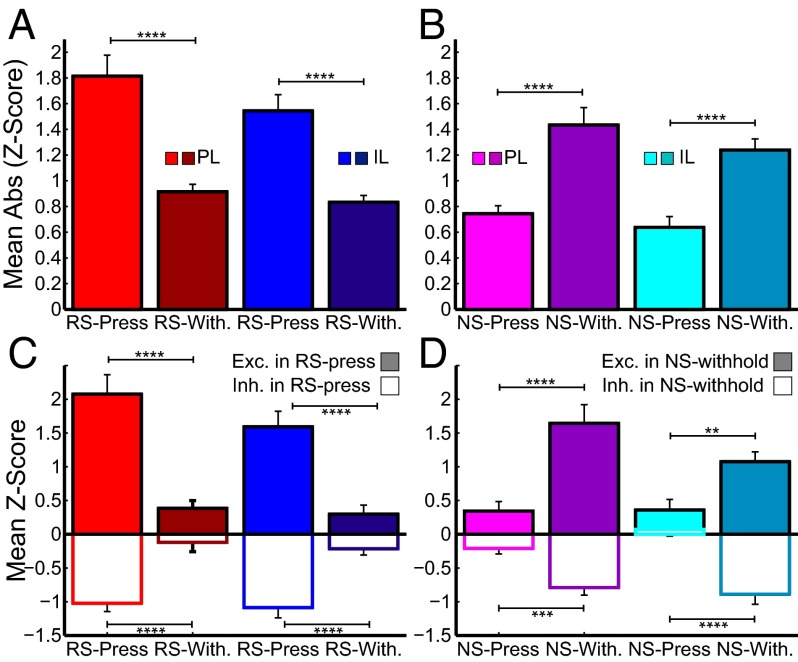
Because few differences in neural activity were observed during reward-seeking vs. extinction sessions, we investigated the relationship of stimulus-evoked neural responses to stimulus-evoked behaviors. Neural activity during DS-sucrose was characterized based on whether stimulus presentation resulted in a lever press or a withheld press. Instead of exclusively firing for one particular behavior or another (i.e., pressing or withholding, which would be predicted from a PL-go/IL-stop type hypothesis), neuronal activity reflected context-appropriate responding (i.e., press for RS, withhold for NS). We analyzed activity of neurons 0–500 ms post-RS/NS in recordings in which both types of responses were observed (RS-press vs. RS-withhold and NS-press vs. NS-withhold). The overall strength of signaling, measured by absolute value z-score in the same neuron across trials, was significantly greater following the RS in press compared with withhold trials (PL, z = 5.12; P << 0.001; IL, z = 5.44; P << 0.001; Fig. 4A). Conversely, in NS trials, the strength of signaling was significantly greater in withhold compared with press trials (PL, z = 4.49; P << 0.001; IL, z = 4.07; P << 0.001; Fig. 4B). This significant difference was seen in neurons excited and inhibited by RS-press and NS-withhold: both types of responses were significantly diminished in RS-withhold and NS-press in PL and IL neurons (all z > 3; all P < 0.01; Fig. 4 C and D). There were no significant differences between PL and IL neurons (all z < 1.6; all P > 0.05) except for a small difference between NS-press responses in neurons inhibited during NS-withhold (z = 2.24; P < 0.05; Fig. 4D, Right). Based on these results, we tentatively concluded that PL and IL neurons encode cue information that dictates a contextually appropriate response: press when reward is expected, withhold press when reward is not expected.
Fig. 4.
RS- and NS-evoked neural activity sorted by whether stimuli resulted in a lever press (lighter colors) or withhold (darker colors) during DS-sucrose. (A and B) Mean absolute-value z-scores of all recorded neurons in response to RS (A) and NS (B). Stimulus-evoked neural activity was strongest for RS-press (light red/blue) and NS-withhold (dark magenta/cyan) trials. (C and D) Neurons that were significantly excited (filled bars) or inhibited (empty bars) by RS (C) or NS (D) were analyzed with respect to how they responded to an executed press (RS/NS-press) or withheld press (RS/NS-withhold). Neurons significantly excited or inhibited by RS were more excited or inhibited when a press occurred after an RS (C). Neurons significantly excited or inhibited by NS were more excited or inhibited when no press occurred after an NS (D). Analysis described in Stimulus-Evoked PL/IL Activity Predicted Context-Appropriate Execution and Inhibition of Responding. ****P << 0.001, ***P < 0.001, and **P < 0.005.
Neurons in PL and IL Switched from Signaling Press During Sucrose-Seeking to Withhold During Extinction.
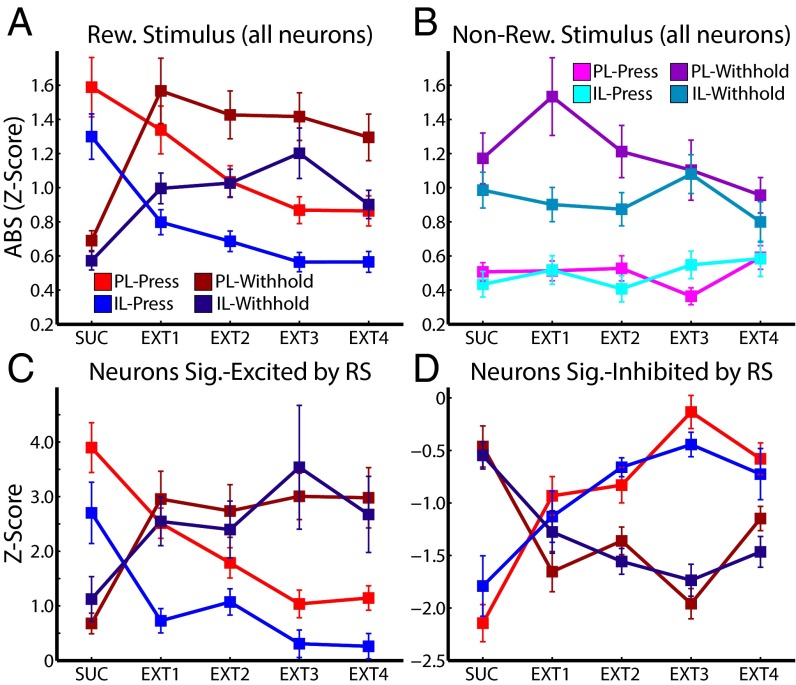
To verify that PL and IL neurons encode context-specific mappings between cues and behaviors, we analyzed press vs. withhold activity in PL and IL neurons during the 4 d of extinction of the RS. Over the course of extinction, rats switched from a behavioral strategy of pressing the RS lever to withholding responses (Fig. 1A). At the same time, PL and IL neurons significantly shifted from strong to weak signaling of RS-press [absolute-value z-score: PL, χ2(4,442) = 12.17; P < 0.05; IL, χ2(4,502) = 43.69; P << 0.001, Kruskal–Wallis test; Fig. 5A], and weak to strong signaling of RS-withhold [PL, χ2(4,442) = 34.28; P << 0.001; IL, χ2(4,502) = 27.09; P << 0.001], as predicted by the hypothesis that neurons encode contextual cue information for driving behavior. Thus, whereas evoked neuronal activity changes in DS-sucrose were stronger for executed vs. withheld responses, neuronal activity changes in extinction were stronger for withheld vs. executed responses. Analysis of mean activity across the population showed no significant changes (all χ2 < 5; all P > 0.05) except for IL neuronal responses during withheld presses [χ2(4,502) = 11.76; P < 0.05], consistent with the fact that averaging excitatory and inhibitory responses diminished observed effects and with the fact that IL signaling was more prominently inhibitory (and thus averaging across the entire population did not diminish significance). Signaling in response to the NS lever (NS-press or NS-withhold) did not change over the course of extinction (all χ2 < 9.2; all P > 0.05; Fig. 5B), indicating that changes in mPFC activity were specific to changes in RS outcome contingencies.
Fig. 5.
Stimulus-evoked neural activity switched from signaling press in DS-sucrose to withhold in extinction. (A) RS-evoked neural activity of all recorded neurons sorted by whether RS presentation resulted in a lever press (lighter colors) or withhold (darker colors) during DS-sucrose and extinction. (B) NS-evoked neural activity of all recorded neurons sorted as in A and C. Neurons in PL and IL neurons that were significantly excited by RS presentation were more excited for pressing than for withholding during DS-sucrose, and were more excited for withheld presses than elicited presses during extinction. (D) Neurons that were significantly inhibited by RSs were more inhibited for pressing than withholding presses during DS-sucrose, and also more inhibited for withholding than for pressing during extinction. Transitions from neural representation of press to withhold were highly significant. Statistics are provided in Neurons in PL and IL Switched from Signaling Press During Sucrose-Seeking to Withhold During Extinction.
We also characterized behavior-related signal changes in significantly-modulated neurons during each stage (sucrose and extinction days 1–4). For each session, we identified neurons in PL and IL that were significantly excited or inhibited by RS presentation (percentages shown in Fig. 3A). We then calculated the mean responses of these neurons in RS-press and RS-withhold conditions. As seen in the population as a whole, neurons significantly excited or inhibited by the RS shifted from signaling more robustly for RS-press in sucrose to RS-withhold in extinction (Fig. 5 C and D). In all cases except one, this shift was highly significant (all χ2 > 20; all P << 0.001). IL neurons excited by the RS strongly and significantly decreased activity in RS-press conditions. In RS-withhold conditions, these neurons increased activation on the whole (Fig. 5C), but the effect was not significant [χ2(4,38) = 5.49; P > 0.05], likely because of the small numbers of neurons in IL cortex significantly excited by the RS (Fig. 3A). Thus, across the entire population and in neurons selected for significant excitatory or inhibitory responses, neurons in both mPFC subregions switched selectivity from representing RS-press to RS-withhold. These data support the hypothesis that stimulus-evoked mPFC signaling reflected context-based stimulus behavior outcome mapping (press when rewarded, withhold when not rewarded).
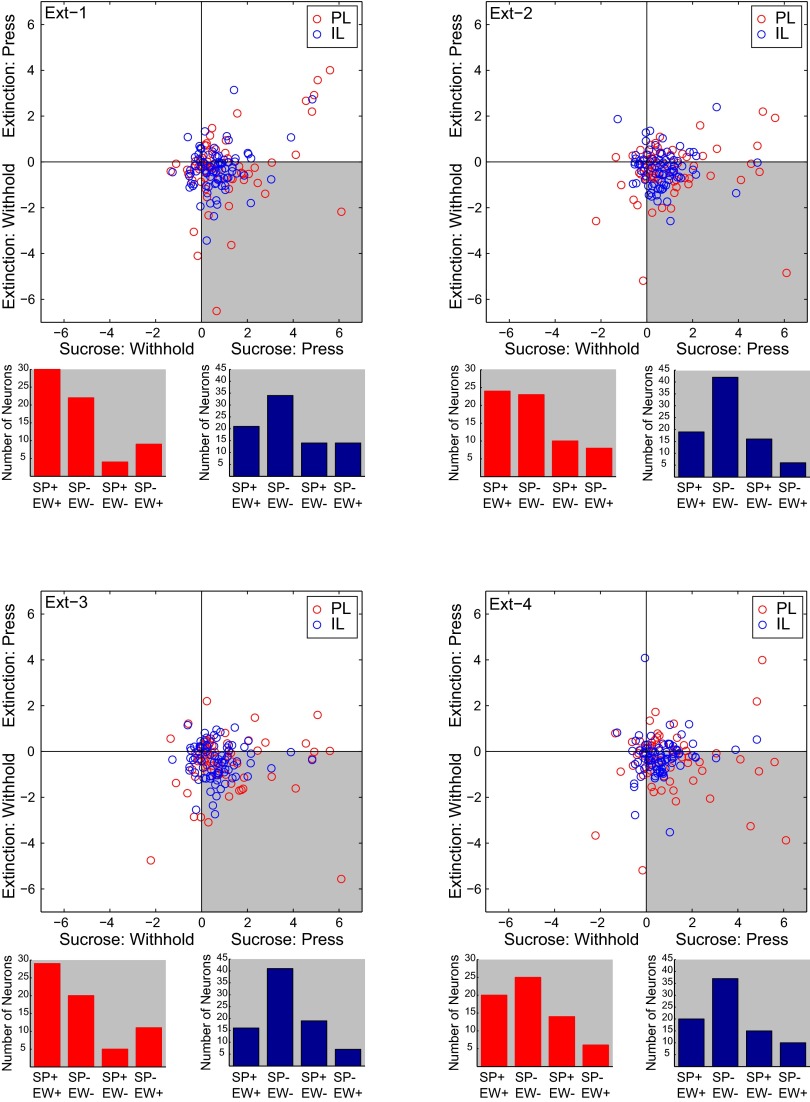
Individual neurons recorded across multiple sessions in PL and IL (SI Materials and Methods) most frequently shifted their activity from representing RS-press during DS-sucrose to RS-withhold during extinction (all P < 0.001, Fisher exact test; Fig. S4, shaded quadrants), and maintained the direction of modulation (excitation or inhibition) across sessions (Fig. S4, bar graphs). That individual neurons shifted representation across sessions further confirms the finding that PL and IL neuron activity was dynamically updated to reflect context-correct behaviors.
Fig. S4.
Neurons recorded in DS-sucrose and all four extinction sessions (PL, n = 65, IL, n = 72). Circles represent individual neurons (PL, red; IL, blue). For each neuron, we calculated an index of RS-evoked activity during press vs. withhold trials (SI Materials and Methods). Each figure represents a separate extinction session compared with DS-sucrose. Note that the majority of, although not all, neurons in PL and IL were strongly modulated for pressing during DS-sucrose and withholding during extinction. These points fall in the gray-shaded area. Data in scatter plots are based on absolute-value measurements of activity. Below each scatter plot is a measure of consistency of excitatory vs. inhibitory responses in each DS-sucrose pressing and extinction withholding. For neurons falling in the gray-shaded area, we measured whether neurons were excited or inhibited during DS-sucrose (when the animal pressed) and during extinction (when the animal withheld pressing). Note that, for both PL (red) and IL (blue), the majority of neurons were similarly modulated: excited or inhibited in both conditions (leftmost two bars). These data indicate that most individual neurons shifted their activity to represent pressing during DS-sucrose to withholding during extinction, and that these shifts in activity maintained the same valence of activity (excitation–excitation or inhibition–inhibition).
Inactivation of PL and IL Significantly Disrupted Sucrose Seeking and Extinction.
To assess potential causal roles for PL and IL neurons in reward seeking or extinction, we inactivated PL or IL by using baclofen/muscimol (bac/mus) during DS-sucrose and then on alternating extinction days in a separate group of animals (Materials and Methods). RS lever pressing was significantly decreased following bac/mus microinfusions into IL, but not PL, during DS-sucrose (Fig. S5A). Inactivation of either area dramatically influenced extinction, such that RS lever pressing decreased on inactivation days and rebounded on vehicle days (Fig. S5B). Bac/mus infusion also delayed extinction such that, on extinction day 6, when no treatment was given, the number of RS lever presses was equal to that on extinction day 1. These data indicate that strict relationships between PL and go vs. IL and stop are not maintained across all behaviors and that both PL and IL neurons play important roles in reward-seeking and extinction.
Fig. S5.
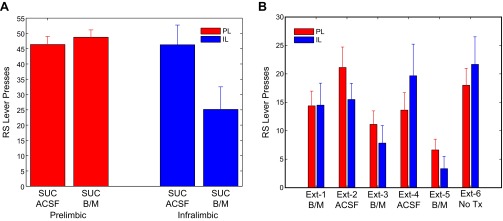
Pharmacological inactivation of PL (red) or IL (blue) using bac/mus (B/M) during DS-sucrose (A) or alternating days of extinction (B). ACSF was given on control days. Inactivation of IL, and not PL, decreased responding during DS-sucrose [PL, t(7) = 0.70; P > 0.05; IL, t(6) = 3.46; P < 0.05, paired t test]. During extinction, inactivation of PL and IL decreased lever presses, which rebounded on ACSF days [PL, χ2(4,28) = 9.53; P < 0.05; IL, χ2(4,20) = 14.67; P < 0.01, Friedman test]. Over the entire extinction session, inactivation of PL and IL neurons impaired extinction learning such that there were no significant differences between extinction day 1 and extinction day 6 (PL, signed-rank = 12.5; P > 0.05; IL, signed-rank = 7; P > 0.05, Wilcoxon test). These data indicate a broad influence of PL and IL manipulation on task performance not selectively limited to response execution or inhibition per se. Further discussion is provided in the main text.
RS Location Reversal Demonstrated a Stronger Influence of RS vs. NS than Spatial Location.
To investigate potential spatial influences on RS-evoked responses during DS-sucrose, we retrained four PL-implanted rats and four IL-implanted rats to perform the DS-sucrose task following extinction. After reacquisition of task performance, we reversed contingencies: RS became NS and vice versa (Materials and Methods). RS-evoked responses pre- and postreversal for RS-press trials were recorded from 59 PL and 80 IL neurons. There were no differences in RS-evoked responses pre- and postreversal in PL or IL neurons (PL, z = 0.41; P > 0.05; IL, z = 0.12; P > 0.05, Wilcoxon test), indicating that the location of the RS was less important in driving mPFC activity than the behavior and outcome.
Activity in PL and IL Was Selective for Rewarded vs. Nonrewarded Well Entries.
During DS-sucrose, PL and IL neurons were significantly modulated around well entry and during sucrose consumption (Fig. S6A). Neurons were minimally modulated by exploratory well entries not preceded by a lever press (Fig. S6B). Population absolute signaling around the well entry (−500 ms to 500 ms) was significantly stronger in PL and IL neurons for rewarded vs. nonrewarded well entries (PL, z = 3.96; P << 0.001; IL, z = 6.52; P << 0.001, Wilcoxon test). This was driven by significantly enhanced PL activity preceding well entry and a rapid, but transient decrease in IL activity immediately following well entry (Fig. S6A). During extinction, “rewarded” (i.e., following RS press) well entry modulation disappeared [PL, χ2(4,442) = 33.24; P << 0.001; IL, χ2(4,502) = 50.49; P << 0.001]. PL and IL neurons also showed a strong inverse relationship during sucrose receipt and consumption: IL activity was increased and PL activity was decreased. This divergence occurred ∼1 s after well entry and lasted ∼8–10 s, overlapping with consumption (Fig. S6A). This difference was significant (z = 3.08; P < 0.005, Mann–Whitney test), whereas the nonrewarded well entry difference was not (z = 0.18; P > 0.05; Fig. S6B).
Fig. S6.
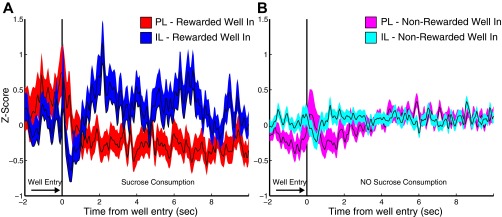
PL and IL neurons exhibited changes in activity related to reward well entry and consumption. (A) Mean z-scores for all recorded neurons aligned on well entry following RS lever press during DS-sucrose. (B) Mean z-scores for the same neurons aligned on all other (nonrewarded) well entries. Neuronal activity in both areas is more strongly modulated for rewarded well entries than for nonspecific well entries. In addition, IL activity is strongly elevated and PL activity is mildly inhibited during sucrose consumption.
SI Materials and Methods
Animals.
Male Sprague–Dawley rats [∼300–400 g upon arrival; n = 12 (behavioral electrophysiology); n = 15 (pharmacological inactivation); Charles River] were used in the experiments. Rats were single-housed under temperature- and humidity-controlled conditions on a reversed light cycle (6:00 AM off to 6:00 PM on) and allowed free access to commercial chow and tap water. Studies were conducted during the active (dark) cycle. Six rats were implanted with electrode arrays in PL, and six different rats were implanted with electrode arrays in IL. Eight different rats were implanted with cannulae in PL, and seven different rats were implanted with cannulae in IL.
Surgery.
Anesthesia was induced with isoflurane in a closed container, and maintained with 1.5–2.5% (vol/vol) isoflurane in air delivered through a nosecone. Animals were placed in a stereotaxic frame, and body temperature was maintained at ∼37 °C by using a thermistor-controlled electric heating pad. All incision points were infiltrated with a long-lasting anesthetic agent [2% (wt/vol) lidocaine]. The skull was exposed, and unilateral holes were drilled above the mPFC (∼3.0 mm rostral and 0.4–0.8 mm lateral to bregma). Three or four additional holes were drilled, and screws were implanted to secure array implants. Arrays of 16 preinsulated stainless steel microwires (50-μm diameter) arranged in a 4 × 4 pattern (∼200 μm spacing between microwires) were lowered to PL (dorsal/ventral = 3.7 mm; n = 6) or IL (dorsal/ventral = 5.2 mm; n = 6). For pharmacological inactivation studies, guide cannulae (Plastics One) were implanted at the same coordinates but 1 mm dorsal (dorsal/ventral = 2.7 mm and 4.2 mm). Animals were given antibiotic (0.1 mL cefazolin, 330 mg/mL, i.m.) and analgesic (meloxicam, 1 mg/kg, s.c.) and allowed to recover at least 1 wk following surgery, during which time weight, activity, and other measures of general health were monitored.
Behavioral Training and Task.
Following recovery, rats were trained to self-administer sucrose by using an FR1 paradigm in sound-attenuated operant chambers (Med-Associates). Two levers were presented on each trial, one active and the other inactive. The positions of active and inactive levers varied among animals but remained constant within an animal. Active lever presses resulted in retraction of both levers and illumination of a light in the reward well. The well light (signaling reward availability) remained illuminated 10 s. Upon well entry, the well light turned off and 0.1 mL of 15% (wt/vol) sucrose was delivered. Inactive lever presses were recorded but produced no outcome. A 20–30-s time out occurred following well entry or lever retraction (if the active lever was pressed but the well was not entered).
Following successful acquisition of the FR1 sucrose task, animals were trained on the DS-sucrose task (illustrated in Fig. 1A). Active and inactive levers were presented individually in a pseudorandom fashion. Lever presentations were paired with unique tones (active, 10 KHz; inactive, 5 KHz), forming an RS and an NS complex. Tones were played for a maximum of 3 s, and levers remained extended for a maximum of 10 s. RS lever presses resulted in tone offset, lever retraction, and illumination of the light in the reward well. The well light (signaling reward availability) remained illuminated for 10 s or until well entry, at which point 0.1 mL of a 15% sucrose solution was delivered. NS lever presses were recorded but produced no outcome. Animals received a 20–30-s time out following well entry or lever retraction (after the NS lever was retracted or if the RS lever was pressed but the well was not entered). Rats ran for 1 h per day on the DS-sucrose task. Recording commenced upon stable performance (>75% responses to RS presentation along with <25% responses to NS presentation for >2 d).
Neurons were recorded for one or two sessions per rat during performance of the DS-sucrose task. Following the final DS-sucrose session, neuronal activity was recorded during extinction sessions for four consecutive days. During this time, stimuli were presented as during the DS-sucrose task. RS lever presses resulted in lever retraction and illumination of the well light. Well entry, however, resulted only in offset of the well light and no delivery of sucrose. NS lever presses produced no results. Extinction was considered complete when RS lever presses decreased to fewer than 10 per session. This almost always occurred on extinction day 3 or 4. Recordings from the same population of neurons were made every day of extinction.
Electrophysiological Recording.
During recording sessions, arrays were connected to a head stage (20× gain; Plexon). Signals were passed through a cable (Omnetics Connector) to an electrical commutator (Keyo Electric) to allow free movement. Commutator output was delivered to a Plexon recording system (MAP/16; Plexon), in which signals were amplified (50×) and filtered (100 Hz to 8 kHz) and sampled at 40 kHz. Action potentials were recorded by using RASPUTIN software (Plexon), in which gain and thresholds were set to isolate single-neuron activity. Recorded spikes were further sorted offline using Offline Sorter (Plexon) by using a combination of template-matching and manual sorting using principal-components analysis. Well-isolated single units that fired consistently throughout the recording session were included for analysis. Thresholds were set at 2× noise band during recording, and offline sorting included only action potentials crossing a 2.5× threshold (neurons typically crossed a 3× threshold). More than 50% of active recording wires yielded one isolated neuron. For those wires on which more than one neuron was recorded, we verified our manual sorting with statistical criteria in Offline Sorter (e.g., multivariate ANOVA; P < 0.05). In the infrequent instances in which multiple neurons were clearly sorted into different clusters but not significant, we accepted the manual sort, having noted on previous occasions that statistical cluster sorts can be overly conservative. Examples of sorted neurons are shown in Fig. S1. Time stamps for behavioral events were sent from the Med-Associates behavioral control system to the Plexon recording system for use in aligning neural activity to behavior.
Reversal.
Following conclusion of the extinction study a subset of animals (n = 4 PL and n = 4 IL) were retrained on DS-sucrose (rewarded). After reacquisition of the task, animals were then trained in a reversal version of the task in which, after 30 min of initial task contingencies, the RS and NS were switched. Animals were trained on the reversal version of the task to reach reliable task performance before recording. We analyzed recordings with a focus on RS presentation followed by a lever press to test for spatiomotor properties in neural signaling.
Pharmacological Inactivation.
Following consistently accurate performance on the DS-sucrose task (>10 contiguous days of >75% responses to RS presentation), animals were given bilateral microinjections of 0.3 μL of artificial cerebrospinal fluid (ACSF; vehicle) or a 0.3 nmol/0.03 nmol mixture of bac/mus dissolved in ACSF. Injections were given over 1 min, injection cannulae were left in place for 2 min to allow diffusion, and rats were tested immediately afterward. Animals received ACSF or bac/mus in a counterbalanced order during DS-sucrose performance. The following week, animals began extinction. Animals received alternating days of bac/mus and ACSF over the course of 5 d of extinction (bac/mus: extinction days 1, 3, and 5). All animals received the same treatment so that comparisons could be made across brain regions. All extinction days after day 5 were untreated, and extinction day 6 was included for comparison with treatment days.
Histology.
Following experimental testing and recording, rats were anesthetized with 1.5–2.5% (vol/vol) isoflurane, and constant current (25 μA) was delivered to each recording wire for 15 s to produce lesions and iron deposits to mark the tips of recording electrodes. Rats were killed 1 d later to allow development of lesion-induced gliosis. Animals were perfused with ∼50 mL of 0.9% NaCl solution followed by 400–500 mL of 4% (wt/vol) paraformaldehyde followed by 50–100 mL of a 5% (wt/vol) potassium ferricyanide/5% (vol/vol) HCl solution to stain iron deposits. Brains were postfixed overnight in 4% paraformaldehyde and transferred to a 20% (wt/vol) solution of sucrose/0.1% sodium azide in phosphate buffer at 4 °C for at least 3 d. Coronal 40-μm-thick sections of brains were cut on a cryostat. Prefrontal sections were transferred to slides, counterstained with neutral red (Fisher), dehydrated with graded alcohol solutions, cleared with xylene, and coverslipped with Permount (Fisher). Lesion sites were used to confirm accurate electrode placement (Fig. 1C). The same histological procedures were applied to the brains of animals tested with pharmacological inactivation of PL/IL, and cannula implant sites were verified to be accurately targeted.
Data Analysis.
Neural activity and behavior were analyzed with custom programs written in Matlab (MathWorks). All well-isolated neurons were included in analyses, although subsets were selected for additional analysis based on response criteria, as described. Neuronal activity aligned on task events was grouped in 50-ms bins, and spike density functions were generated by Gaussian smoothing the resulting event-related histogram. Activity was z-score normalized against mean and SD of a 2,000-ms epoch preceding RS/NS presentations for comparisons across neurons. Neurons were characterized as significantly modulated based on paired Wilcoxon signed-rank tests (Wilcoxon) comparing firing rate 0–500 ms pre stimulus to 0–500 ms post stimulus across trials. Absolute-value z-scored firing rate calculations were performed to characterize signaling strength across the entire population of recorded neurons and to avoid potential cancelation of averaged excitatory and inhibitory responses. Population analysis of significantly excited or inhibited neurons were also performed. To measure potential changes across trials within recording sessions, we grouped trials into four even blocks (typically of 12–13 trials each). We computed mean activity 0–500 ms post-RS presentation for each trial and used a repeated-measures ANOVA to calculate changes in RS-evoked response across blocks of trials. Similar results were observed if analyses were performed on different numbers of blocks. For single-neuron analysis of RS/NS encoding across extinction, we calculated, for each neuron, (press − withhold) indices for DS-sucrose and extinction, by using absolute value of z-scored firing rate (Fig. S4, scatter plot), followed by analysis of excitation of individual neurons in each condition (Fig. S4, bar graph). Analyses were focused on electrodes that maintained the same number of isolated neurons with the same waveform shape across all sessions. We analyzed RS trial results, as behavior/neuronal activity in NS trials were similar in DS-sucrose and extinction sessions. Additional parametric or nonparametric analyses, as appropriate, were applied to firing rate data as described. Main effects were calculated by using ANOVA or Kruskal–Wallis or Friedman tests depending on whether the data were parametric and whether data were paired. Within-neuron comparisons were made using paired t tests/Wilcoxon signed-rank tests when trials were equivalent. Nonequivalent or cross-neuron analyses were performed using unpaired t tests or Mann–Whitney U tests. The same analysis scheme was used for behavioral data calculation (counts of lever press and well entry, reaction time). Significance thresholds were defined as P < 0.05.
Discussion
Neuronal Activity in PL and IL Did Not Differentially Encode Go vs. Stop.
We tested the hypothesis that PL activity signals behavioral execution during reward seeking and IL activity signals response inhibition during extinction (4, 14). This view posits that PL neurons would fire for reward-predicting stimuli during DS-sucrose, and IL neurons would fire for the same stimuli during extinction. Instead, PL and IL neurons responded specifically to stimuli that triggered context-appropriate behavioral responses (lever press or withheld press) during reward seeking or extinction. During DS-sucrose, neurons in both areas preferentially responded to RSs that produced a rewarded lever press and for NSs followed by (nonrewarded) withheld press. Throughout extinction, individual neuronal responses in both areas shifted to become selective for RS presentation when pressing was (appropriately) suppressed. These results support the hypothesis that, in addition to or instead of encoding opposing behaviors (e.g., “going” vs. “stopping”), PL and IL neuronal signals reflect the representation of context, here defined by an outcome-based contingency, irrespective of the motor output involved.
The relationship of PL and IL to expression and extinction of fear learning and cocaine seeking has been very well characterized (4, 7). Our results in no way call these findings into question. However, they are aligned with previous studies demonstrating that these relationships are not absolute across all types of behavioral execution/suppression. For example, activity is increased in IL neurons following cue- and context-induced cocaine and heroin seeking, and IL inactivation diminishes reinstatement of extinguished cocaine, methamphetamine, and heroin seeking (7). Furthermore, PL inactivation increases spontaneous and premature behaviors (8–10) and impairs response inhibition (11). Together, these results imply that neuronal networks within PL and IL can contribute to both response execution and inhibition. Our data support this conclusion and indicate that one way in which both regions may contribute to adaptive behavior is by signaling the contingency-defined context to be used in appropriately executing or inhibiting behavior.
PL and IL Signaling Was Not Homogeneous.
PL responses were equally divided between excitation and inhibition, whereas most IL responses were inhibitory. PL and IL neurons also fired differentially around the reward: PL neurons were excited at well entry and inhibited during consumption, whereas IL neurons were inhibited at well entry and excited during consumption, in line with a recent report (15). The time course of extinction-related changes in RS signaling also differed across PL and IL neurons: IL neurons shifted to signaling withholding more rapidly than PL neurons (Fig. 5). This IL lead is the opposite of observations that PL neurons signaled learned strategy shifts ahead of IL neurons (16), raising the possibility that PL and IL neurons may differentially signal learning-related changes for separate behaviors via temporal differences in onset of representation. Another intriguing possibility is that IL inhibition may serve as a permissive, or gating, role for excitatory responses observed in PL neurons (17). Thus, although our data do not support the idea that PL and IL exclusively control execution and inhibition of behavior respectively, there are clear differences across the regions, in line with previous findings, that warrant further investigation.
Previous studies found mixed results with respect to the activity of mPFC neurons during reward-related extinction. mPFC neurons were found to exhibit lever press responses during seeking, extinction, and reinstatement for natural rewards (18), but other studies have found an overall decrease in signaling by PL neurons during a within-session extinction session (19). Neither of those studies compared prefrontal subregions during reward seeking and extinction, ultimately making direct comparisons vs. our results difficult.
The change in PL and IL signaling to reflect changing context has not been observed in all frontal regions. In lateral orbitofrontal cortex, we found strong RS-evoked responses that consistently diminished over extinction, even when animals withheld responses (20). These data are consistent with differential roles of mPFC and orbitofrontal cortex in guiding reward-related and cognitive behaviors. Similarly, nucleus accumbens neurons decrease their activity during extinction of a DS task (14). In contrast, a sizeable subset of basolateral amygdala neurons showed enhanced responding during extinction (21), indicating that neurons in basolateral amygdala and mPFC may interact in signaling context-related information to shape behavior.
Prefrontal Networks Participate in Many Cognitive Functions.
Although our recordings do not provide causal evidence, our data are consistent with previous work indicating that a major aspect of prefrontal signaling is integrating contextual information to produce goal-directed responses (2, 3, 22). This concept aligns with studies indicating that an important role of the PFC is to generate context-appropriate behavior (23). Neurons recorded from PL exhibit context-specific responses (24), activity representing the rule or set being followed (25, 26), activity signaling cues driving correct vs. incorrect behavior during an attention task (27), and delay-related firing rate changes influenced by the results (correct or error) in the incipient or previous trial (10). In the present study, context was defined as the specific response–outcome contingencies the animal experienced (i.e., RS press produces reward vs. RS press produces no reward), which corresponded with adaptive (high utility) behaviors (i.e., press to obtain reward vs. withhold pressing to decrease effort). An important question for future research is the degree to which mPFC neurons encode different types of contextual information [e.g., environmental (22) vs. rule- or outcome-based (24, 25) context].
Inactivation of PL vs. IL Revealed Differential Roles in Task Performance.
Although PL and IL neurons were strongly modulated during DS-sucrose, inactivation of IL, but not PL, significantly decreased responding. One possible explanation for this divergence may be that animals were overtrained (>2 wk of DS-sucrose, preceded by fixed-ratio training), so that responding may have become habitual. IL plays a prominent role in habit-based behaviors (28–30), whereas PL has been associated with goal-directed behaviors (23, 28, 30, 31). We propose that PL responses during DS-sucrose reflected task or contingency representation that was maintained after the initial stages of learning. These data indicate a previously unidentified dimension of PL activity, whereby task representations are maintained even when not necessary to drive behavior. PL activity may be involved not only in goal-directed task acquisition, but also in later behavioral refinement after a task is well-learned to, e.g., detect changes in or update rule/context representations. During extinction, PL and IL neurons were strongly modulated, and inactivation produced pronounced deficits in acute behavior and extinction learning. Thus, PL and IL neurons play essential roles in extinction and other types of learning (16, 30, 31), but IL (not PL) may be important for expression of well-learned behavior (29, 30). An important future investigation will be the relationship between PFC representations of context and goal-directed or habitual behaviors.
The mPFC as a whole is involved in a wide range of behavioral processes and holds a massively integrative position, both anatomically and physiologically (32). One possibility is that PFC neurons or networks adapt to the present demands. Thus, in tasks in which spatial orientation is essential, for example, neurons show strong spatial signaling (33). In studies using rule-related tasks, activity represents specific rules (25). In our task, neurons represent contingency-based contexts to drive execution or extinction of behavior. PL and IL neurons may show dichotomous function in some cases, such as during learning and extinction related to fear and drug use (4), but not in other cases. Ultimately, these results, including ours, are all aligned with the perspective that a major role of mPFC is to maximize behavioral utility (34). In the present study, PL and IL signaling was strongest under conditions of high utility: when animals exerted effort to receive reward or when animals conserved effort in the absence of reward. In situations of low utility—exerting effort for no reward or withholding rewarded responses—mPFC activity was diminished. Although our findings were not specifically designed to test economic utility, they indicate this subject as an important future direction. Another important future line of research is a focus on how different mPFC neuronal ensembles encode these diverse cognitive elements to support optimal behavior (7). Critical issues include understanding how these networks are defined, how flexibly they encode behavioral components, and how they interact with each other. A truly comprehensive understanding of PFC function, and its relationship to other brain areas, will involve specifically addressing the complexity and heterogeneity of the region.
Materials and Methods
All protocols and procedures followed National Institutes of Health guidelines for the care and use of laboratory animals, and were approved by the Medical University of South Carolina Institutional Animal Care and Use Committee, where studies were performed. Rats were trained on DS-sucrose (Fig. 1A and SI Materials and Methods) in an operant environment (Med-Associates) and as described in our previous studies (20). All studies were approved by the Medical University of South Carolina Institutional Animal Care and Use Committee. Recording commenced upon stable performance. Animals were implanted with arrays of 16 stainless steel microwires (50 μm) in PL (dorsal/ventral = 3.7 mm, n = 6) or IL (dorsal/ventral = 5.2 mm, n = 6). Neurons were recorded using a Plexon MAP/16 system (Plexon). Following DS-sucrose recording, neurons were recorded during four extinction days during which all conditions were the same except that no reward was delivered. After extinction recording, neurons were recorded from a subset of animals during a DS-sucrose reversal task in which the RS and NS lever switched position midway through the session. After final recordings, lesions were made at electrode tips, animals were perfused, brains were extracted and sectioned, and Nissl-staining procedures were used to verify electrode placement. A second group of animals received bac/mus inactivation of PL or IL immediately preceding DS-sucrose task performance and on alternate extinction days. All data were analyzed by using custom routines in Matlab (MathWorks) and consisted of parametric/nonparametric variants of ANOVA and t tests. Additional analyses are described in Results. Significance was set at P < 0.05. Detailed methods are provided in SI Materials and Methods.
Acknowledgments
The authors thank Cody Weidenthaler for excellent technical assistance. This work was supported by National Institutes of Health (NIH) Grants R21-DA032005, P50-DA015369, R37/R01-DA06214, R01-MH092868, P50-AA010761, and UL1-RR029882.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1507611112/-/DCSupplemental.
References
- 1.Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: Neural and neurochemical substrates. NeurosciBiobehav Rev. 2004;28(7):771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Euston DR, Gruber AJ, McNaughton BL. The role of medial prefrontal cortex in memory and decision making. Neuron. 2012;76(6):1057–1070. doi: 10.1016/j.neuron.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 4.Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16(5):279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5(11):844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- 6.Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci. 1995;109(4):681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- 7.Moorman DE, James MH, McGlinchey EM, Aston-Jones G. Differential roles of medial prefrontal subregions in the regulation of drug seeking. Brain Res. 2014:S0006-8993(14)01708-9. doi: 10.1016/j.brainres.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishikawa A, Ambroggi F, Nicola SM, Fields HL. Contributions of the amygdala and medial prefrontal cortex to incentive cue responding. Neuroscience. 2008;155(3):573–584. doi: 10.1016/j.neuroscience.2008.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jonkman S, Mar AC, Dickinson A, Robbins TW, Everitt BJ. The rat prelimbic cortex mediates inhibitory response control but not the consolidation of instrumental learning. Behav Neurosci. 2009;123(4):875–885. doi: 10.1037/a0016330. [DOI] [PubMed] [Google Scholar]
- 10.Narayanan NS, Laubach M. Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron. 2006;52(5):921–931. doi: 10.1016/j.neuron.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bari A, et al. Prefrontal and monoaminergic contributions to stop-signal task performance in rats. J Neurosci. 2011;31(25):9254–9263. doi: 10.1523/JNEUROSCI.1543-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy ER, Dalley JW, Robbins TW. Local glutamate receptor antagonism in the rat prefrontal cortex disrupts response inhibition in a visuospatial attentional task. Psychopharmacology (Berl) 2005;179(1):99–107. doi: 10.1007/s00213-004-2068-3. [DOI] [PubMed] [Google Scholar]
- 13.Rhodes SE, Killcross AS. Lesions of rat infralimbic cortex enhance renewal of extinguished appetitive Pavlovian responding. Eur J Neurosci. 2007;25(8):2498–2503. doi: 10.1111/j.1460-9568.2007.05486.x. [DOI] [PubMed] [Google Scholar]
- 14.Ghazizadeh A, Ambroggi F, Odean N, Fields HL. Prefrontal cortex mediates extinction of responding by two distinct neural mechanisms in accumbens shell. J Neurosci. 2012;32(2):726–737. doi: 10.1523/JNEUROSCI.3891-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burgos-Robles A, Bravo-Rivera H, Quirk GJ. Prelimbic and infralimbic neurons signal distinct aspects of appetitive instrumental behavior. PLoS One. 2013;8(2):e57575. doi: 10.1371/journal.pone.0057575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rich EL, Shapiro M. Rat prefrontal cortical neurons selectively code strategy switches. J Neurosci. 2009;29(22):7208–7219. doi: 10.1523/JNEUROSCI.6068-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji G, Neugebauer V. Modulation of medial prefrontal cortical activity using in vivo recordings and optogenetics. Mol Brain. 2012;5:36. doi: 10.1186/1756-6606-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters YM, O’Donnell P, Carelli RM. Prefrontal cortical cell firing during maintenance, extinction, and reinstatement of goal-directed behavior for natural reward. Synapse. 2005;56(2):74–83. doi: 10.1002/syn.20129. [DOI] [PubMed] [Google Scholar]
- 19.Bouret S, Sara SJ. Reward expectation, orientation of attention and locus coeruleus-medial frontal cortex interplay during learning. Eur J Neurosci. 2004;20(3):791–802. doi: 10.1111/j.1460-9568.2004.03526.x. [DOI] [PubMed] [Google Scholar]
- 20.Moorman DE, Aston-Jones G. Orbitofrontal cortical neurons encode expectation-driven initiation of reward-seeking. J Neurosci. 2014;34(31):10234–10246. doi: 10.1523/JNEUROSCI.3216-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tye KM, Cone JJ, Schairer WW, Janak PH. Amygdala neural encoding of the absence of reward during extinction. J Neurosci. 2010;30(1):116–125. doi: 10.1523/JNEUROSCI.4240-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hyman JM, Ma L, Balaguer-Ballester E, Durstewitz D, Seamans JK. Contextual encoding by ensembles of medial prefrontal cortex neurons. Proc Natl Acad Sci USA. 2012;109(13):5086–5091. doi: 10.1073/pnas.1114415109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Killcross S, Coutureau E. Coordination of actions and habits in the medial prefrontal cortex of rats. Cereb Cortex. 2003;13(4):400–408. doi: 10.1093/cercor/13.4.400. [DOI] [PubMed] [Google Scholar]
- 24.Mulder AB, Nordquist RE, Orgüt O, Pennartz CM. Learning-related changes in response patterns of prefrontal neurons during instrumental conditioning. Behav Brain Res. 2003;146(1-2):77–88. doi: 10.1016/j.bbr.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 25.Durstewitz D, Vittoz NM, Floresco SB, Seamans JK. Abrupt transitions between prefrontal neural ensemble states accompany behavioral transitions during rule learning. Neuron. 2010;66(3):438–448. doi: 10.1016/j.neuron.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 26.Sul JH, Kim H, Huh N, Lee D, Jung MW. Distinct roles of rodent orbitofrontal and medial prefrontal cortex in decision making. Neuron. 2010;66(3):449–460. doi: 10.1016/j.neuron.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Totah NK, Kim YB, Homayoun H, Moghaddam B. Anterior cingulate neurons represent errors and preparatory attention within the same behavioral sequence. J Neurosci. 2009;29(20):6418–6426. doi: 10.1523/JNEUROSCI.1142-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balleine BW, O’Doherty JP. Human and rodent homologies in action control: Corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35(1):48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coutureau E, Killcross S. Inactivation of the infralimbic prefrontal cortex reinstates goal-directed responding in overtrained rats. Behav Brain Res. 2003;146(1-2):167–174. doi: 10.1016/j.bbr.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 30.Smith KS, Graybiel AM. A dual operator view of habitual behavior reflecting cortical and striatal dynamics. Neuron. 2013;79(2):361–374. doi: 10.1016/j.neuron.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tran-Tu-Yen DA, Marchand AR, Pape JR, Di Scala G, Coutureau E. Transient role of the rat prelimbic cortex in goal-directed behaviour. Eur J Neurosci. 2009;30(3):464–471. doi: 10.1111/j.1460-9568.2009.06834.x. [DOI] [PubMed] [Google Scholar]
- 32.Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51(1):32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- 33.Hok V, Save E, Lenck-Santini PP, Poucet B. Coding for spatial goals in the prelimbic/infralimbic area of the rat frontal cortex. Proc Natl Acad Sci USA. 2005;102(12):4602–4607. doi: 10.1073/pnas.0407332102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rushworth MF, Behrens TE. Choice, uncertainty and value in prefrontal and cingulate cortex. Nat Neurosci. 2008;11(4):389–397. doi: 10.1038/nn2066. [DOI] [PubMed] [Google Scholar]