Abstract
Background:
Since antiquity, Zingiber officinale (ginger), pogostemonis herba, and radix aucklandiae have been used as traditional Chinese medicines to remit gastrointestinal discomfort. Recent evidences also show the efficacy of the three herbal medicines against nausea and vomiting.
Objective:
To optimize the CO2 supercritical fluid extraction (SFE-CO2) conditions for ginger and the ethanol reflux extraction conditions for radix aucklandiae, control the quality of pogostemonis herba essential oil, and evaluate anti-motion sickness activity of the compound recipes composed of the three herbal medicine extracts.
Materials and Methods:
Two orthogonal array designs L9 (3)4 were employed to optimize the SFE-CO2 conditions for enhancing yield of 6-gingerol from ginger and the ethanol reflux extraction conditions for enhancing yield of costunolide and dehydrocostus lactone from radix aucklandiae; a uniform design U5(53) was applied for evaluation of anti-motion sickness activity of the compound recipes.
Results:
Extraction pressure (P < 0.01), extraction temperature and extraction time (P < 0.05) have significant effects on the yield of 6-gingerol from ginger by SFE-CO2; ethanol concentration (P < 0.01) and times of repeating extraction (P < 0.05) have significant effects on the total yield of costunolide and dehydrocostus lactone from radix aucklandiae by ethanol reflux extraction; the anti-motion sickness effects of the optimized compound recipe composed of the three herbal medicine extracts were markedly better than those of dimenhydrinate.
Conclusion:
The compound recipe composed of ginger, pogostemonis herba, and radix aucklandiae could be developed as a promising anti-motion sickness medicine.
Keywords: Anti-motion sickness, ginger, pogostemonis herba, radix aucklandiae, supercritical fluid extraction
INTRODUCTION
Motion sickness is caused by exposure to unfamiliar motions such as repetitive angular and linear acceleration and deceleration. The typical symptom of motion sickness is gastrointestinal discomfort, including nausea and vomiting.[1] Zingiber officinale (ginger, fresh rhizome of Z. officinale Roscoe), pogostemonis herba (Pogostemon cablin [Blanco] Benth.), and radix aucklandiae (Aucklandia lappa Decne.) are recorded in the Chinese pharmacopoeia as traditional Chinese medicines that could be used to treat various diseases efficaciously, especially gastrointestinal discomfort. Ginger was dubbed “the holy medicine for anti-vomiting” in Bie Lu, an authoritative medicine book in antiquity. Pogostemonis herba was “a key medicine for regulation of the spleen and stomach in disorder” described in the Chinese ancient book Ben Cao Tu Jing. Radix aucklandiae was “an important medicine for treating vomiting and regurgitation” described in the Chinese ancient book Shen Nong Ben Cao Jing. The characteristic chemical constituents in ginger, pogostemonis herba, and radix aucklandiae are 6-gingerol, patchouli alcohol, costunolide, and dehydrocostus lactone, respectively.
In most studies, active compounds were extracted from ginger by aqueous-ethanol or some organic solvent such as methanol, ethanol, ethyl acetate, and hexane.[2,3,4] Squeezed and pressurized ethanol extraction of ginger also has been reported.[5] In addition, ginger oil extracted by hydro-distillation has been studied.[6] However, few studies on extracting active compounds from ginger by CO2 supercritical fluid extraction (SFE-CO2) have been reported except some,[7] in which active ingredients of ginger were extracted by SFE-CO2 under a random condition and without further investigation. In our previous studies, we compared the yields of extracts from ginger and the 6-gingerol contents in ginger extracts by three different extracting technologies (hydro-distillation, SFE-CO2, and aqueous-ethanol percolation). The results showed that the content percentages of 6-gingerol in SFE-CO2 extracts were much higher than those by the other two extracting technologies (SFE-CO2, 11.803–23.164%, ethanol percolation, 6.329–6.874%, hydro-distillation, 0.796%). However, the content percentages of 6-gingerol fluctuated widely under different conditions of ginger SFE-CO2. Thus, in this article, we optimized the SFE-CO2 condition for enhancing yield of 6-gingerol from ginger using orthogonal experimental design L9 (3)4. Meanwhile, the traditional ethanol reflux extraction has been optimized for enhancing yield of costunolide and dehydrocostus lactone from radix aucklandiae by another orthogonal experimental design L9 (3)4.
Recently, evidence also showed that the efficacy of ginger against nausea and vomiting was as good as metoclopramide,[8] and patchouli alcohol could inhibit intestinal contraction by Ca2+ antagonism.[9] Remarkable inhibition activity of radix aucklandiae in gastric ulcer and possibility for the treatment of irritable bowel syndrome by radix aucklandiae also has been observed.[10,11,12] Our previous experiments on the screening of Chinese crude drugs for anti-motion sickness also proved that ginger, pogostemonis herba, and radix aucklandiae could abate the symptoms of motion sickness in mice. However, the anti-motion sickness effects of pogostemonis herba and radix aucklandiae through controlling gastrointestinal symptoms have not been studied. In this article, We first utilized the combination of the three herbal medicines to control gastrointestinal reaction during motion sickness. Screening the efficacious compound recipes for anti-motion sickness has been performed by the uniform design table U5 (53). Although mice had no emetic reaction, Yu XH et al. validated the motion sickness index as an evaluation criteria for motion sickness in mice.[13,14] The results that the anti-motion sickness effects of the optimized compound recipe of ginger, pogostemonis herba, and radix aucklandiae were markedly better than those of dimenhydrinate and other recipes, might provide the first evidence of the synergism of the three traditional Chinese medicines on anti-motion sickness.
MATERIALS AND METHODS
Apparatus
The high-performance liquid chromatography (HPLC) system used consisted of a low-pressure quaternary pump (model Agilent 1050), an autosampler (model Agilent 1050), and a ultraviolet (UV) visible detector (G79853A), and it employed an Agilent ZORBAX Eclipse DB C18 column (250 mm × 4.6 mm, 5 μm) (Agilent 1050 ChemStation, USA). The SFE-CO2 experiments were performed using a CL-10-J-3 SFE-CO2 device (He Si Corp., Beijing, China). Motion sickness was simulated using a DSL-1 minitype animal centrifuge unit (Peace Medical Equipment Factory, Beijing, China).
Chemicals and reagents
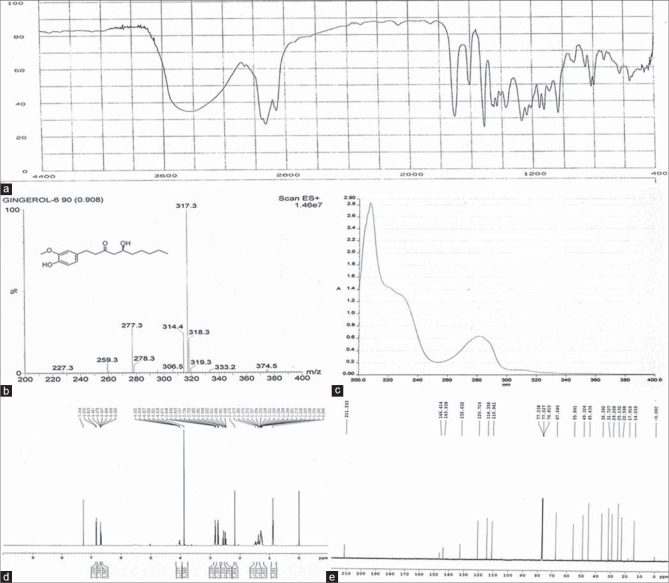
6-gingerol standard was obtained from Pharmacy Institution of Zhongxin (Tianjin, China. The 6-gingerol standard was qualitatively determined by 1hydrogen, 13carbon-nuclear magnetic resonance, infra-red, UV, and mass spectrum [Figure 1], and quantitatively determined by HPLC, chemical purity > 99%). Costunolide and dehydrocostus lactone were purchased from the National Institutes for Food and Drug Control (China). Ginger and radix aucklandiae were obtained from the Pharmacy of Beijing Military General Hospital (China) and authenticated by Prof. Zhang-mei (The director of Pharmacy of Beijing Military General Hospital, China). Pogostemonis herba essential oil was obtained from Shui-nan Pharmaceutical Corp. (Jiangxi, China). Dimenhydrinate was purchased from Yi-ming Pharmaceutical Corp. (Beijing, China). Ethanol was purchased from Beijing Chemical Corp. (China). HPLC-grade methanol was purchased from Sigma (USA), and water was double distilled for HPLC. All other chemicals used were of analytical grade.
Figure 1.
Qualitative identification of the 6-gingerol standard. (a) Infra-red spectrum of the 6-gingerol standard, (b) electrospray ionization-mass spectrometry of the 6-gingerol standard, (c) ultraviolet spectrum of the 6-gingerol standard, (d) 1hydrogen-nuclear magnetic resonance spectrum of the 6-gingerol standard, (e) 13carbon-nuclear magnetic resonance spectrum of the 6-gingerol standard. The 6-gingerol standard obtained from Zhongxin was validated to be 6-gingerol by serial qualitative analysis
Optimization of CO2 supercritical fluid extraction conditions for ginger with orthogonal experimental design
CO2 Supercritical fluid extraction of ginger under the conditions in the orthogonal design
An orthogonal experimental design L9(3)4 was employed in which extraction pressure, extraction temperature, separation pressure, and dynamic extraction time were considered the four major parameters that affect the extraction process.[15] The three different levels of each factor are as follows: Level 1, level 2, and level 3 of extraction pressure were 15, 20, and 25 MPa, respectively; extraction temperature, 35, 40, and 45°C; separation pressure, 4, 6, and 8 MPa; and extraction time, 2.0, 2.5, and 3.0 h. Nine extractions were carried out under the experimental conditions in L9 (3)4. In each extraction, 100.0 g of dried ginger (dried rhizome of Zingiber officinole, prepared from fresh ginger by atmospheric drying naturally until shrinking to 10% weight) was placed into the extraction vessel. Separation temperature was 2°C higher than extraction temperature. CO2 flow rate was 0.3–0.9 l/min. Finally, the extraction vessel was depressurized, the oil was collected from the separation vessel, and the extract was stored at −4°C.
High-performance liquid chromatography analysis of 6-gingerol in ginger CO2 supercritical fluid extraction extracts
High-performance liquid chromatography analytical conditions for 6-gingerol
The chromatographic conditions were as follows: Mobile phase, methanol: Water (62:38, v/v); column flow rate, 1.0 ml/min; column temperature, room temperature; chromatographic run time, 13.0 min; injection volumes, 10 μl; and wavelengths, 280 nm.
Method validation
The described method was validated according to the International Conference on Harmonization guidelines by determination of the linearity, detection and quantitation limits, precision, and recovery test.[16]
Assay of 6-gingerol in ginger CO2 supercritical fluid extraction extracts
Six hundred microgram per milliliter of 6-gingerol was prepared by dissolving a weighed amount in the mobile phase. Serial dilutions of the 600 μg/ml standard were made to produce the 300, 200, 100, 40, and 10 μg/ml working standards. These working solutions were used for calibration curves and method validation. 100–200 mg ginger SFE-CO2 extracts were weighed and added to 50 ml volumetric flasks containing 40 ml of the mobile phase. The samples were sonicated for 30 min in an ultrasound bath, and the 50 ml volume was completed with the mobile phase. After homogenization, samples were filtered through a 0.45 μm cellulose filter and analyzed by the previously developed HPLC method. The contents of 6-gingerol were calculated from the calibration curve of standards.
Optimization of ethanol reflux extraction for radix aucklandiae with orthogonal design
Ethanol reflux extraction of radix aucklandiae under the conditions in orthogonal design
Another orthogonal experimental design L9 (3)4 was employed to optimize the ethanol reflux extraction conditions of radix aucklandiae. Ethanol concentration, liquid-solid ratio, reflux time, and number of times of re-extraction were considered the four major factors that affect the extraction efficiency of radix aucklandiae. For each factor, three different levels were selected as follows: Level 1, level 2, and level 3 of ethanol concentration were 60%, 40%, and 80%, respectively; liquid-solid ratio, 8:1, 10:1, and 6:1; reflux time, 2, 1, and 3 h; and number of times of re-extraction, 2, 3, and 1 time. Nine extractions were carried out under the experimental conditions in L9 (3)4. In each extraction, active compounds were extracted from 18.0 g powder of radix aucklandiae by heating in a water bath at 85°C and reflux. The extract was concentrated, dried on a vacuum rotary evaporator, and stored at −4°C.
High-performance liquid chromatography analysis of costunolide and dehydrocostus lactone in radix aucklandiae ethanol extracts
High-performance liquid chromatography analytical conditions for costunolide and dehydrocostus lactone
The chromatographic conditions were as follows: Mobile phase, methanol: Water (68:32, v/v); column flow rate, 1.0 ml/min; column temperature, room temperature; chromatographic run time, 15.0 min, 17.0 min, respectively; injection volumes, 10 μl; and wavelengths, 225 nm.
Method validation
The described method was validated according to the International Conference on Harmonization guidelines.[16]
Assay of costunolide and dehydrocostus lactone in radix aucklandiae ethanol extracts
Three hundred and eighty microgram per millilitre of costunolide and 480 μg/ml of dehydrocostus lactone were prepared, respectively, by dissolving a weighed amount in the mobile phase. Serial dilutions were made to produce the 152, 76, 38, 15.2 and 7.6 μg/ml working standards of costunolide and 192, 96, 48, 19.2 and 9.6 μg/ml working standards of dehydrocostus lactone. One gram radix aucklandiae ethanol extracts were weighed for sample preparation, and the method of preparation was the same as that of the SFE-CO2 extract sample with the respective mobile phase. Samples were analyzed by the previously developed HPLC method. The contents of costunolide and dehydrocostus lactone were calculated from the calibration curve of standards.
Optimization of the formula of the anti-motion sickness herbal medicine by uniform design
Experimental design
To enhance the effect of anti-motion sickness, ginger, pogostemonis herba, and radix aucklandiae were applied simultaneously to form a compound recipe of traditional Chinese medicine. Uniform design was applied to determine the optimum dosage and formula of the mixture.[17] According to the Chinese pharmacopoeia and related literature reported on the dosage and effectiveness of these herbal medicine,[8,9,18] the dosage range of ginger from 0 to 80 g, pogostemonis herba from 0 to 60 g, and radix aucklandiae from 0 to 10 g for human were selected for investigation by uniform design U5 (53). The levels and factors are listed in Table 1. Mice in the dimenhydrinate group were given 0.13 mg dimenhydrinate per 20 g weight, and mice in the control group were given the same volumes of 0.9% NaCl saline.
Table 1.
Factors and levels of uniform design U5 (53) and groups for anti-motion sickness evaluation
Evaluation of anti-motion sickness activity
Administration of drug in mice
Active ingredients were extracted from ginger and radix aucklandiae respectively, under the optimum conditions determined by previous experiments. A total of 70 male mice weighing 18–22 g were purchased from Sino-British SIPPR/BK Lab Animal Ltd (Certificate No.: SCXK (HU) 2007-0003, Shanghai, China). The mice were housed at a controlled temperature of 22°C ± 2°C, a relative humidity of 50–60%, lighting (8:00–20:00), and allowed free access to standard dry diet and tap water ad libitum. Mice were randomly divided into the seven groups in Table 1 (n = 10 for each group). The standard dry diet was removed, and the tap water remained on the night before the experiment. In the experiment, the drugs were given to mice at 0.5 h before rotary stimulus. The dosage given to mice conforms to this conversion: The dosage given to mice per 20 g weight was 0.0026 folders of dosage for humans shown in Table 1, and ginger SFE-CO2 extracts, radix aucklandiae ethanol extracts, and pogostemonis herba essential oil to which the dosage of crude drug for mice was converted, respectively, were administered to mice orally.
Motion stimulus execution
Each mouse was enclosed in an individual centrifuge cage. The device started rotating in the clockwise direction at a constant angular acceleration of 40°/s2. When the angular velocity reached 240°/s, it began slowing down at a constant angular deceleration of 40°/s2.[19] Without pause, the device rotated again in the counterclockwise direction in the same manner. Mice were stimulated by rotating for 40 min. Motion sickness symptoms were observed and recorded in 2 min after rotation.
Motion sickness index measurement
Evaluation criteria for motion sickness index included the following: [13] Each fecal granule scored 1, none scored 0; abundant urination scored 2, a little of urination scored 1, and none scored 0; piloerection scored 0.6, none scored 0; severe tremor scored 2.4, middle tremor scored 1.2, slight tremor scored 0.6, none scored 0. Motion sickness index was calculated by the sum of all the scores.
Statistical analysis
Analysis of variance (ANOVA) and t-test of group differences was performed by standard statistical software (Statistical Package for the Social Sciences 17.0). A value of P < 0.05 was considered as statistically significant.
RESULTS AND DISCUSSION
Validation of high-performance liquid chromatography methodology for 6-gingerol
The linear regression equation between the content of 6-gingerol and the peak area is y = 0.3267x − 5.0920 (x is the content of 6-gingerol (ng), and y is the peak area), R2 = 1. The limit of detection and limit of quantitation were in the range of 0.01–0.03 μg/ml. The relative standard deviation (RSD) values of intra-day and inter-day tests were found to be 1.80, 0.88, 0.72, and 2.29; 1.16, 0.95 (n = 5) for 100, 200, and 300 μg/ml of 6-gingerol standard solutions, respectively. The average recovery percentage of 6-gingerol ranged from 97.99 to 102.03%, and RSD values were 1.19% for 8, 10, and 12 mg added to 6-gingerol standard (n = 9). The results showed that the established method was reliable and accurate.
Optimization of supercritical fluid extraction conditions for ginger
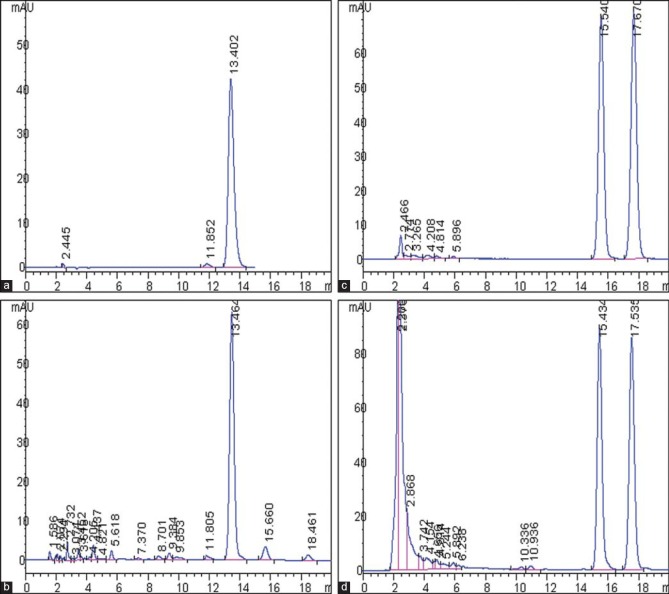
The assay of 6-gingerol in ginger SFE-CO2 extracts was performed by the HPLC method proposed in “Experimental.” The methodology showed a good separation of 6-gingerol in the samples of ginger SFE-CO2 extracts [Figure 2]. The yield of ginger SFE-CO2 extract, the content percentage of 6-gingerol in ginger SFE-CO2 extract, and the yield of 6-gingerol (the yield of ginger SFE-CO2 extract × the percentage content of 6-gingerol) in products obtained from L9 (3)4 test were quantitatively analyzed, and the experimental data and the results of the ANOVA are shown in Table 2. The yield of 6-gingerol extracted from 1 g dried ginger was considered the index for evaluation.
Figure 2.
High-performance liquid chromatography (HPLC) analysis of ginger supercritical fluid extraction (SFE-CO2) extracts and radix aucklandiae ethanol extracts. (a) HPLC chromatogram of 6-gingerol standard. (b) HPLC chromatogram of the ginger SFE-CO2 extract sample, the retention time of 6-gingerol was 13.464 min. (c) HPLC chromatogram of costunolide and dehydrocostus lactone standard. (d) HPLC chromatogram of the radix aucklandiae ethanol extract sample, the retention time of costunolide and dehydrocostus lactone were 15.434 and 17.535 min, respectively
Table 2.
Orthogonal array design matrix L9 (3)4 and experimental results for ginger SFE-CO2
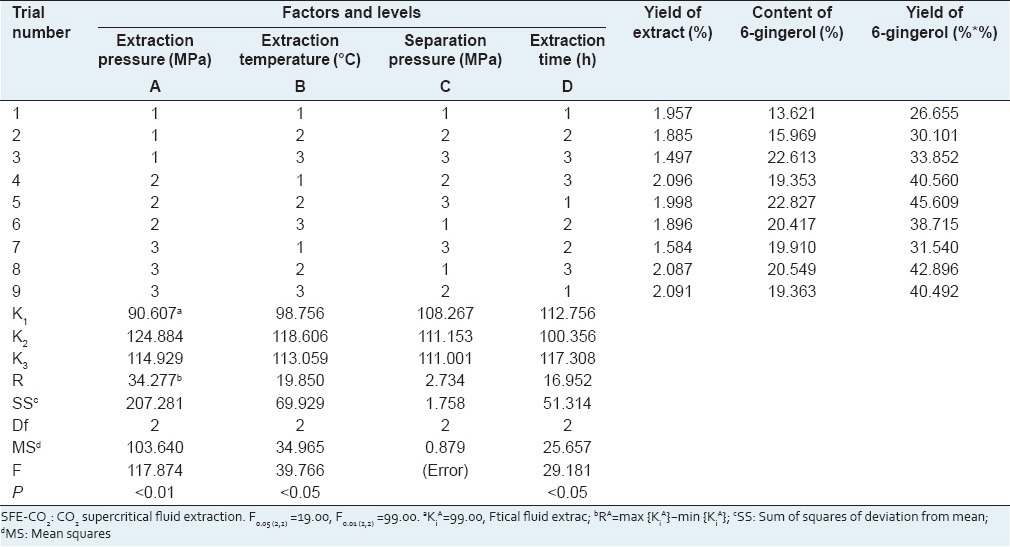
The experimental data in Table 2 reveals how the yield of 6-gingerol will change when the level of the factor is changed. The results of ANOVA in Table 2 indicate that with 95 or 99% confidence, extraction pressure factor has extremely significant effects on the yield of 6-gingerol extracted by SFE-CO2 (P < 0.01), and extraction temperature and extraction time have significant effects on the yield of 6-gingerol (P < 0.05), whereas the effects of separation pressure are small under the range from 4 to 8 MPa. The optimum values of factors for the extraction of 6-gingerol from dried ginger by SFE-CO2 were as follows: Extraction pressure 20 MPa, extraction temperature 40°C, extraction time 3 h, and separation pressure 6 MPa. Especially, extraction pressure was the most critical factor for SFE-CO2 of ginger.
Quality control of pogostemonis herba essential oil
Pogostemonis herba essential oil is marron liquid that has special aromatic odor. The relative density was 0.965. The specific rotation was − 45°. The refractive index was 1.508. The yield of the oil was 1%. The content of patchouli alcohol in the oil was 26.12% by GC. All the results meet the criterion in Chinese pharmacopoeia.[18]
Validation of high-performance liquid chromatography methodology for costunolide and dehydrocostus lactone
The linear regression equation between the content of costunolide and the peak area is y = 1.1308x − 1.7289 and the equation is y = 0.9849x − 1.9114 for dehydrocostus lactone (x is the content of standard [ng], and y is the peak area), R2 = 1. The RSD values of intra-day and inter-day tests were found to be 1.69, 1.15, 0.86, and 2.31; 1.42, 1.07 (n = 5) for 38, 76, and 152 μg/ml of costunolide standard solutions, respectively; and 1.93, 1.17, 1.04 and 2.47, 1.85, and 1.26 (n = 5) for 48, 96, and 192 μg/ml of dehydrocostus lactone standard solutions, respectively. The average recovery percentage of costunolide and dehydrocostus lactone ranged from 96.86 to 103.11%, 96.96 to 102.81%, respectively, and RSD values were 2.26% and 2.03% for 4, 5, and 6 mg, respectively, added to costunolide and dehydrocostus lactone standards (n = 9). The results showed that the established method was reliable and accurate.
Optimization of ethanol reflux extraction conditions for radix aucklandiae
The contents of costunolide and dehydrocostus lactone in each product obtained from L9 (3)4 test were quantitatively analyzed by the HPLC method proposed in “Experimental.” The methodology showed a good separation of costunolide and dehydrocostus lactone in the samples of radix aucklandiae ethanol extracts [Figure 2]. The total yield of costunolide and dehydrocostus lactone extracted from 1 g radix aucklandiae was considered the index for evaluation, and the experimental data and the results of ANOVA are shown in Table 3.
Table 3.
Orthogonal array design matrix L9 (3)4 and experimental results for ethanol reflux extraction of radix aucklandiae
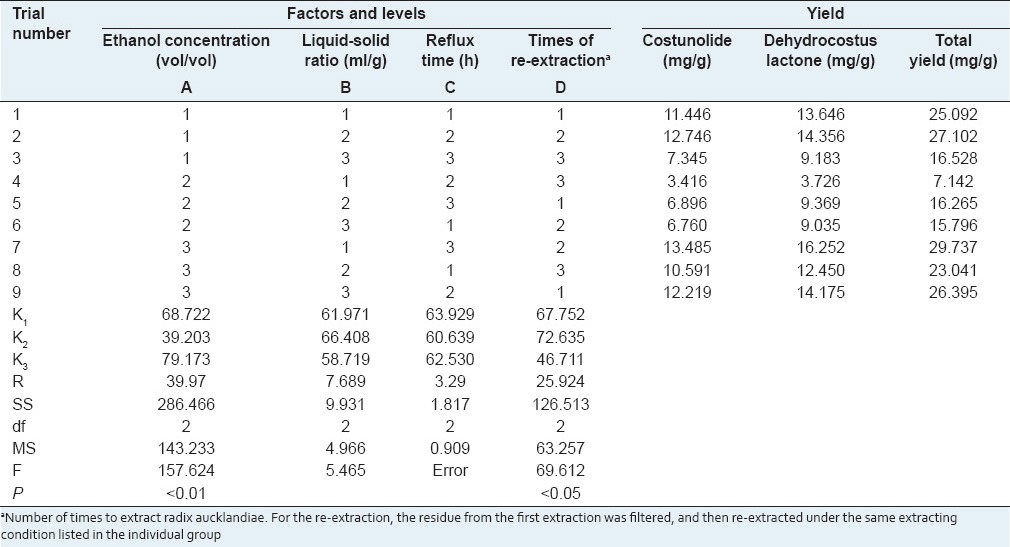
The results of ANOVA in Table 3 indicate that with 95 or 99% confidence, ethanol concentration for extraction has extremely significant effects on the total yield of costunolide and dehydrocostus lactone (P < 0.01), and the number of times of repeating extraction has significant effects (P < 0.05), whereas the effects of liquid-solid ratio and reflux time are small under the range from 6:1 to 10:1 and 1 to 3 h, respectively. The optimum values of factors for the extraction of costunolide and dehydrocostus lactone from radix aucklandiae by ethanol reflux extraction were as follows: Ethanol concentration 80%, number of times of repeating extraction three times, liquid-solid ratio 10:1, and reflux time 2 h. Especially, ethanol concentration and times of repeating extraction were the two most critical factors for ethanol extraction of radix aucklandiae.
Evaluation of anti-motion sickness effect and determination of the optimum formulation
The uniform design method was used for preliminary evaluation of anti-motion sickness effect of the compound recipes composed of ginger, pogostemonis herba, and radix aucklandiae, as well as more efficient optimization of the combination of the three extracts. During rotation, mice showed a general reduction in spontaneous activity. The motion sickness indexes of 10 mice in each group were recorded and shown in Table 4.
Table 4.
Comparison of motion sickness indexes among seven groups
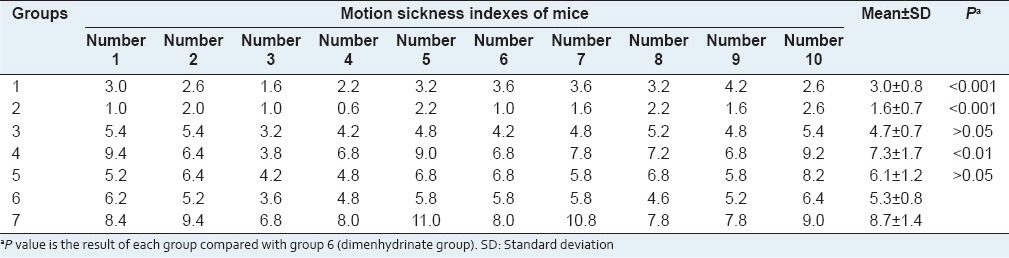
The lower the motion sickness index is, the better the anti-motion sickness effect is. The results in Table 4 showed that the motion sickness indexes in group 6 (dimenhydrinate group) were significantly lower than those in group 7 (0.9% NaCl saline group). It means that dimenhydrinate has significant effects of anti-motion sickness. The motion sickness indexes in group 1 and group 2 were markedly lower than those in group 6 (P < 0.001). It indicated that the effects of anti-motion sickness of groups 1 and 2 were significantly greater than those of dimenhydrinate. The motion sickness indexes in group 3 and group 5 were not significantly different from those in the dimenhydrinate group (P > 0.05), whereas the motion sickness indexes in group 4 were higher than those in the dimenhydrinate group. Thus, the optimum formulation determined by uniform design U5 (53) for anti-motion sickness was found to be group 2: ginger 60 g, pogostemonis herba 45 g, and radix aucklandiae 5 g.
CONCLUSIONS
Gingerols present in ginger would be oxidized easily under an O2 surrounding, and high temperature will accelerate the oxidization. The SFE-CO2 conditions of ginger applied in this article were low temperature and CO2 surrounding, which were advantageous for keeping the natural chemical structures and biological activities of gingerols, and the extracts were pure with no residual solvent. Moreover, in our previous research, the content of 6-gingerol in SFE-CO2 extracts was found to be two folders above that in ethanol oleoresin extracts (about 10%). Therefore, SFE-CO2 is a suggestive and preferential method for ginger extractions after optimization. For radix aucklandiae extraction, the total yields of costunolide and dehydrocostus lactone from radix aucklandiae by ethanol reflux extraction were significantly higher than 1.8%, which was the total content of costunolide and dehydrocostus lactone in radix aucklandiae guided by Chinese pharmacopoeia. It means that costunolide and dehydrocostus lactone have been extracted almost completely after the optimization of extraction in our research. In conclusion, all the investigations provide a scientific basis for the development and utilization of the compound recipe of ginger, pogostemonis herba, and radix aucklandiae as a promising anti-motion sickness medicine.
ACKNOWLEDGMENTS
This study was supported in part by the National Nature Science Foundation of China (81121004, 81230041, 81171798) and the National Basic Science and Development Programme (973 Programme, 2012CB518105).
Footnotes
Source of Support: This study was supported in part by the National Nature Science Foundation of China (81121004, 81230041, 81171798) and the National Basic Science and Development Programme (973 Programme, 2012CB518105)
Conflict of Interest: None declared.
REFERENCES
- 1.Shupak A, Gordon CR. Motion sickness: Advances in pathogenesis, prediction, prevention, and treatment. Aviat Space Environ Med. 2006;77:1213–23. [PubMed] [Google Scholar]
- 2.Bak MJ, Ok S, Jun M, Jeong WS. 6-shogaol-rich extract from ginger up-regulates the antioxidant defense systems in cells and mice. Molecules. 2012;17:8037–55. doi: 10.3390/molecules17078037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gunathilake KD, Rupasinghe HP. Inhibition of human low-density lipoprotein oxidation in vitro by ginger extracts. J Med Food. 2014;17:424–31. doi: 10.1089/jmf.2013.0035. [DOI] [PubMed] [Google Scholar]
- 4.Gull I, Saeed M, Shaukat H, Aslam SM, Samra ZQ, Athar AM. Inhibitory effect of Allium sativum and Zingiber officinale extracts on clinically important drug resistant pathogenic bacteria. Ann Clin Microbiol Antimicrob. 2012;11:8. doi: 10.1186/1476-0711-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu J, Guo Z, Glasius M, Kristensen K, Xiao L, Xu X. Pressurized liquid extraction of ginger (Zingiber officinale Roscoe) with bioethanol: An efficient and sustainable approach. J Chromatogr A. 2011;1218:5765–73. doi: 10.1016/j.chroma.2011.06.088. [DOI] [PubMed] [Google Scholar]
- 6.Mangprayool T, Kupittayanant S, Chudapongse N. Participation of citral in the bronchodilatory effect of ginger oil and possible mechanism of action. Fitoterapia. 2013;89:68–73. doi: 10.1016/j.fitote.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Meng Q, Feng YF, Guo XL, Chen GF, Cai JC. Study on quality standard of extracts from Rhizoma Zingiberis by supercritical fluid extraction. Zhongguo Zhong Yao Za Zhi. 2005;30:750–2. [PubMed] [Google Scholar]
- 8.Ernst E, Pittler MH. Efficacy of ginger for nausea and vomiting: A systematic review of randomized clinical trials. Br J Anaesth. 2000;84:367–71. doi: 10.1093/oxfordjournals.bja.a013442. [DOI] [PubMed] [Google Scholar]
- 9.Ichikawa K, Kinoshita T, Sankawa U. The screening of Chinese crude drugs for Ca2+antagonist activity: Identification of active principles from the aerial part of Pogostemon cablin and the fruits of Prunus mume. Chem Pharm Bull (Tokyo) 1989;37:345–8. doi: 10.1248/cpb.37.345. [DOI] [PubMed] [Google Scholar]
- 10.Han J, Lin H, Zhong Z, Rong X. Study of the effect of exceed critical extracts from Radix Aucklandiae on experimental gastric ulcer model. Zhong Yao Cai. 2005;28:1017–9. [PubMed] [Google Scholar]
- 11.Zhang BH, Gao R, Li ZH, Li BS, Wang FY, Tang XD. Treatment of irritable bowel syndrome by Chinese medicine and pharmacy: An analysis of data mining on experiences of experts. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2013;33:757–60. [PubMed] [Google Scholar]
- 12.He HH, Shen H, Zheng K. Observation of the curative effect of qingchang huashi recipe for treating active ulcerative colitis of inner-accumulation of damp-heat syndrome. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2012;32:1598–601. [PubMed] [Google Scholar]
- 13.Yu XH, Cai GJ, Liu AJ, Chu ZX, Su DF. A novel animal model for motion sickness and its first application in rodents. Physiol Behav. 2007;92:702–7. doi: 10.1016/j.physbeh.2007.05.067. [DOI] [PubMed] [Google Scholar]
- 14.Wei X, Wang ZB, Zhang LC, Liu WY, Su DF, Li L. Verification of motion sickness index in mice. CNS Neurosci Ther. 2011;17:790–2. doi: 10.1111/j.1755-5949.2011.00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Y, Wei L, Wang J, Bi J, Liu Z, Wang Y, et al. Optimization of supercritical fluid extraction of saikosaponins from Bupleurum falcatum with orthogonal array design. J Sep Sci. 2010;33:1161–6. doi: 10.1002/jssc.200900529. [DOI] [PubMed] [Google Scholar]
- 16.Q2 (R1). Validation of Analytical Procedures: Text and Methodology. International Conference on Harmonization (ICH) 2005 [Google Scholar]
- 17.Li DJ, Song JF, Xu AQ, Liu CQ. Optimization of the ultrasound-assisted synthesis of lutein disuccinate using uniform design. Ultrason Sonochem. 2014;21:98–103. doi: 10.1016/j.ultsonch.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Beijing: Chemical Industry Press; 2005. Chinese Pharmacopoeia Committee. Chinese Pharmacopoeia: The First Part; pp. 30–1. 45, 66. [Google Scholar]
- 19.Wang ZB, Han P, Tu Y, Liu WY, Tao BL, Zhang LC, et al. Oxidative stress is not involved in motion sickness in mice. CNS Neurosci Ther. 2013;19:611–6. doi: 10.1111/cns.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]