Abstract
Background:
Dried Whitmania pigra is used for the treatment of cardiovascular and cerebrovascular diseases in traditional Chinese medicine and hot water and alcohol extracts also have anticogulant activity. However, a lower molecular weight and more stable anticogulant is needed.
Objective:
The objective of the following study is to purify and characterize of an anticoagulant oligopeptide from Hirudo (Whitmania pigra Whitman).
Materials and Methods:
Gel filtration on Sephadex G-50, ion exchange on diethylaminoethyl-cellulose, and semi-prepared high-performance liquid chromatography were used to purify Hirudo. Automated coagulation analyzer was used for evaluating anticoagulant activity. Molecular weight was measured by Matrix-assisted laser desorption ionization time of flight mass spectrometry. Amino acid sequence of the oligopeptide was measured by amino acid sequence analyzer.
Results:
A new anticoagulant, named whitide, isolated from Hirudo was purified, with a molecular weight 1997.1 Da. Amino acid sequence of the oligopeptide was identified as Gly-Pro-ALa-Gly-Hyp-Val-Gly-Ala-Hyp-Gly-Gly-Hyp-Gly-Val-Arg-Gly-Leu-Hyp-Gly-Asp-Arg-Gly. The results revealed that its amino acid sequence had strong homology to various types of collagen.
Conclusion:
Whitide might be an orally anticoagulant for its hot and trypsin stable.
Keywords: Amino acid sequence, anticoagulant, oligopeptide, Whitmania pigra Whitman
INTRODUCTION
Hirudo, including the Hirudo nipponica Whitman, Whitmania acranulata Whitman and Whitmania pigra Whitman in Chinese Pharmacopoeia 2010 edition,[1] have commonly been used as anticoagulants for thousands of years in traditional Chinese medicine. As potent anticoagulant medicine, they are also widely applied in the United States and Europe. Hirudin, which is the primary effective component in hirudo, is a specific and efficient inhibitor of thrombin, mainly used in prevention and treatment of thrombus in the clinic practice.[2]
It's reported that the dried Whitmania pigra is used for the treatment of cardiovascular and cerebrovascular diseases in traditional Chinese medicine and hot water and alcohol extracts also have anticogulant activity.[3] From Whitmania pigra some polypeptides were isolated, however their amino acid sequences and configurations were not reported or their molecular weight was high or anticoagulant activity was weak.[4,5] Therefore, a much lower molecular weight and biologically active peptide was needed. In present investigation, we reported a novel oligopeptide derived from dried Whitmania pigra Whitman, and its anticoagulant activity, structure and physicochemical properties were also studied.
MATERIALS AND METHODS
Materials
The whole animal of Whitmania pigra, which were purchased from Hubei province of China, were authenticated by author (Prof. Chen). Sephadex G-50 and diethylaminoethyl (DEAE) Cellulose DE-52 were purchased from Phamarcia Corporation, USA. Trypsin (from bovine pancrease, type II) was purchased from Sigma Chemical Corporation (St. Louis, USA). Thromboplastin, veronal barbital buffer and activated partial thromboplastin time reagents (APTT) were obtained from Dade Behring Marburg Corporation, Germany. Acetonitrile was purchased from Fisher. All other reagents used in this study were reagent grade chemicals.
Chromatographic analyses were performed on an Agilent 1100 LC system (Agilent, USA). Matrix-assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF-MS) was detected in Huazhong University of Science and Technology.
ISOLATION AND PURIFICATION OF OLIGOPEPTIDE
Preparation of crude extracts
The dried powder of Whitmania pigra (100 g) were degreased with petroleum ether (500 ml × 2), then heated to reflux for 2 h in 0.9% saline sodium chloride solution (1000 ml, 800 ml). The extracted solution were lyophilized for Sephadex and Ion-exchange chromatography.
Sephadex G-50 chromatography
The lyophilized powders (15 g) were dissolved in distilled water (40 ml) and centrifugated at 6000 rpm for 25 min (4°C), the supernatant were loaded on a Sephadex G-50 gel filtration column (100 cm × 1.6 cm) previously equilibrated with distilled water. The column was run at a flow rate of 1.5 ml/min of distilled water. Elution was monitored at 254 nm, and the resulting fractions were collected, divided into three fractions (S1, S2, S3). The fractions were screened for coagulation assay.
Ion exchange chromatography on diethylaminoethyl-cellulose
The lyophilized powders (2.0 g) of fraction S2 were dissolved in NH4HCO3 solution (0.01 M, pH 7.8) and centrifugated at 6000 rpm for 25 min (4°C), the supernatant were loaded on a DEAE-52 column (20 cm × 2.6 cm) previously equilibrated with NH4HCO3 solution (0.01 M, pH 7.8). The column was run at a flow rate of 1.5 ml/min. The unbound proteins were eluted by same buffer while the bound proteins were eluted with NH4HCO3 gradient from 0.01 to 0.2 M: Time 0–60 min, 0.01 M; time 60–120 min, 0.05 M; time 120–180 min, 0.1 M; time 180–280 min, 0.2 M. Elution was monitored at 254 nm, and the fractions were collected, divided into 4 fractions (D1, D2, D3, D4). The fractions were screened for coagulation assay.
Semi-preparative high performance liquid chromatography
The lyophilized powders (0.15 g) of fraction D1 were dissolved in ultrapure water (7.5 ml) and centrifugated at 6000 rpm for 5 min (4°C), the supernatant were injected onto a YMC-Pack ODS-A, S-5 μm, 12 nm, 50 × 20 mm id C-18 column, after simple filtration through 0.45 μm porosity membrane. The column was eluted with a linear gradient from 15% to 50% of acetonitrile at a flow rate of 1.0 ml/min. Elution was monitored at 214 nm, and the fractions were collected, divided into 6 fractions (P1, P2, P3, P4, P5, P6). The fractions were screened for coagulation assay.
High performance liquid chromatography purification
The lyophilized powders (2.0 mg) of fraction P6, which showed strong anticoagulant activity, were dissolved in ultrapure water (100 μl), and centrifugated at 6000 rpm for 5 min (4°C), the supernatant were injected onto Zorbax C18 column (4.6 mm × 250 mm, 5 μ). The column was eluted with a linear gradient from 20% to 40% of acetonitrile at a flow rate of 1.0 ml/min. Elution was monitored at 214 nm, and the fraction P6 was collected, named whitide.
Determination of molecular weight and amino acid sequence
Matrix-assisted laser desorption ionization -TOF-MS was performed to determine the accurate molecular weight of the high performance liquid chromatography (HPLC) purified whitide. The sample (10 μl) was injected into the column of the MALDI-TOF-MS. Applied voltage was upgraded to 20,000 V and the setting range of molecular weight was from 1000 to 5000 Da.
Amino acid sequence of whitide was determined using an Edman degradation protein sequencer (Applied Biosystems 477 A, Karolinska Institutet, Sweden) and coupled with amino acid sequence analyzer (120 A).
Database searching and sequence alignment
Whitide homologous sequences were identified by BlastpX[6] analyses at the NCBI database http://www.ncbi.nlm.nih.gov/. Search was performed in nonredundant protein sequence and SwissProt database. Multiple alignments were performed by the ClustalW program.[7]
Plasma-based coagulation assays
The platelet-poor plasma (PPP) used for coagulation assays was prepared by centrifuging citrated human blood at 3000 rpm for 15 min (4°C). Measurements of clotting time by the APTT and prothrombin time (PT) were determined on an automated coagulation analyzer (Thrombolyzer Compact, BE, Germany) using standard reagents according to the manufacturer's instructions.[8] The samples were solved in distilled water, and the concentration was 5 μg/μl. Normal citrated PPP, samples, and reagents were incubated for 5 min at 37°C. For activated APTT assay, 30 μl sample was loaded on test cup, then was automatic mixed with PPP, APTT reagent, and CaCl2 solution, and clotting time was recorded. In PT assay, the reaction was performed by adding PPP, thromboplastin, veronal barbital buffer and samples and determining the clotting time.
ENZYME STABILITY
The resistance of fractions to trypsin was detected at 37°C for 12 h on the shaking table with the speed of 180 rpm. The reaction products were injected onto ODS-C18 column (4.6 mm × 150 mm, 5 μ) by semi-preparative HPLC. The column was eluted with a linear gradient from 15% to 50% of acetonitrile at a flow rate of 1.0 ml/min. Elution was monitored at 214 nm.
Statistics
Results are presented as mean ± standard deviation of the mean (n = 3). Student's t-test was used to determine the level of significance.
RESULTS
Isolation and purification of oligopeptide
The fractions collected by Sephadex G-50 Chromatography were divided into 3 fractions: S1, S2, S3 [Figure 1a]. The fractions S1 and S3 were turbid, and fraction S2 was used for Ion-exchange chromatography.
Figure 1.
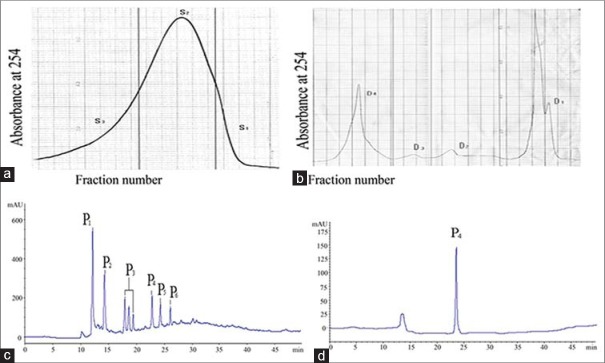
Purification of an anticoagulant oligopeptide from Whitmania pigra Whitman. (a) Elution profile of sephadex G-50 chromatography. (b) Elution profile of ion-exchange chromatography. (c) Elution profile of semi-preparative HPLC chromatography. (d) Elution profile of HPLC-purified whitide
The fractions collected by Ion-exchange chromatography were divided into four fractions (D1, D2, D3, D4): Time 0–60 min, 0.01 M NH4HCO3 (D1); time 60–120 min 0.05 M (D2); time 120–180 min, 0.1 M (D3); time 180–280 min, 0.2 M (D4) [Figure 1b]. The fraction D1, showed relatively higher anticoagulant activity and a higher peak, was used for semi-preparative HPLC chromatography.
The fractions collected by semi-preparative HPLC chromatography were divided into 6 fractions: P1, P2, P3, P4, P5, P6 [Figure 1c]. Fraction P3 combined three peaks together for its not isolated absolutely. Then all fractions were lyophilized. The fraction P6 was then purified by of HPLC as showed in Figure 1d, and HPLC-purified P6 named whitide. The lyophilized powder of whitide was green, used for detecting molecular weight and amino acid sequence.
Molecular weight and amino acid sequence of whitide
As shown in Figure 2, the accurate molecular weight of HPLC-purified whitide analyzed by MALDI-TOF-MS was 1997.1 Da.
Figure 2.
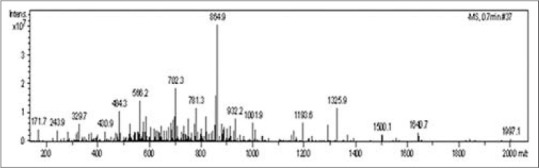
Molecular weight determination of high performance liquid chromatography (HPLC)-purified Whitide. Matrix-assisted laser desorption ionization time of flight mass spectrometry was performed to identify the native molecular mass
Amino acid sequence of whitide was identified using a degradation protein sequencer, coupled with amino acid sequence analyzer. The amino acid sequence was shown to be Gly-Pro-ALa-Gly-Hyp-Val-Gly-Ala-Hyp-Gly-Gly-Hyp-Gly-Val-Arg-Gly-Leu-Hyp-Gly-Asp-Arg-Gly. The molecular weight was 2001.25 Da, which was almost consistent with MALDI-TOF-MS analysis.
BlastX analysis showed strong homology to collagens from various organisms [Figure 3], and all showed above 65% identity, for example, homo sapiens collagen alpha-1 type II (Sequence ID: Emb CAA32030.1, 68% identity).
Figure 3.
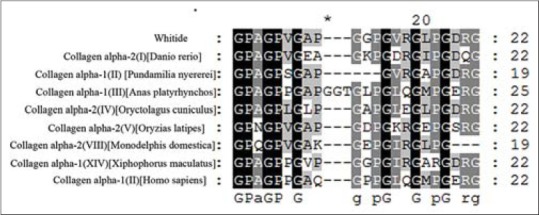
Multiple alignments (ClustalW) of the Whitide with various types of collagen from different organisms
Determination of anticoagulant activity
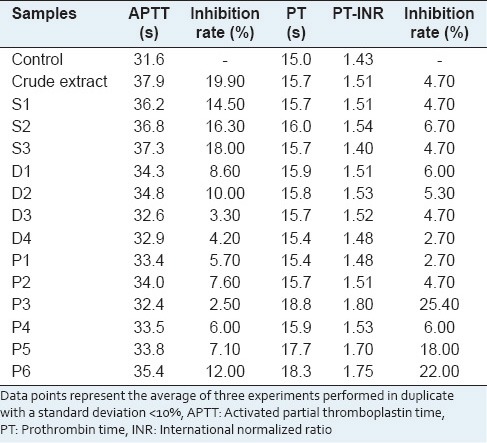
The clotting times in the control group were within the normal range for human [Table 1]. Crude extract was the most potent in prolonging APTT but inactive in PT. Furthermore, the crude extract had significant inhibition on formation of fibrin from fibrinogen.[3] Prolongation of PT was observed for fraction P3 and P6.
Table 1.
Effects of samples in the clotting assays
ENZYME STABILITY
The enzyme stability of fraction D1 (20 mg/ml) was performed in 0.25% trypsin. After an incubation on a bath shaker at 180 rpm for 12 h (37°C), the results showed that all samples were stable except fraction P2 [Figure 4].
Figure 4.
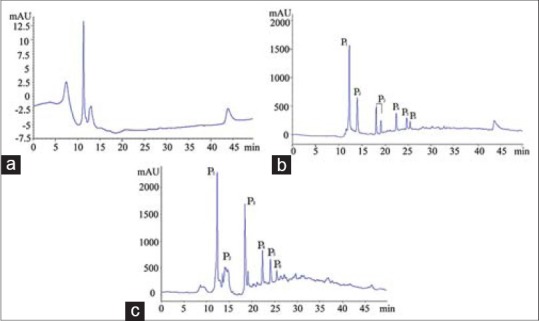
Enzyme stability of fraction D1. (a) High performance liquid chromatography (HPLC) chromatography of 0.25% trypsin after incubation. (b) HPLC chromatography of 20 mg/ml fraction D1 after incubation. (c) HPLC chromatography of 20 mg/ml fraction D1 and 0.25% trypsin after incubation
DISCUSSION AND CONCLUSIONS
In this study, a novel oligopeptide-whitide with anticoagulant activity was isolated from Hirudo (Whitmania pigra Whitman). This might be a clue to discover new resources from Hirudo and make full use of the existing resources.
The original cascade models described two distinct pathways for initiating coagulation, triggered by extrinsic or intrinsic factors, which converge on a common pathway leading to thrombin generation and fibrin formation. The anticoagulant activity of fractions was initially screened in vitro in human plasma by APTT and PT. The APTT is an assay for the activation of coagulation through the intrinsic pathway, the clot formation in response to a nonphysiological stimulus; the PT assay measures the activity of coagulation factors of the extrinsic pathway.[9] In this study, we found that crude extract selectively caused an increase in the APTT value without affecting the PT values, indicating that it targets the intrinsic rather than extrinsic pathway at a level, and also it could inhibit the formation of fibrin from fibrinogen. However, whitide prolonged PT value more than APTT value, indicating that it could activate the extrinsic coagulation cascade.
The amino acid sequence of whitide was obtained. BlastX analysis showed strong homology to various types of collagens. Collagens represent up to 40% of the total protein of the vessel wall, it plays a crucial role in regulating thrombin formation. Collagen can directly interact with either glycoprotein receptors on the platelet surface or indirectly via binding of von Willebrand factor to the platelet surface.[10] Aside from its well-established role in primary hemostasis, collagen was shown to initiate blood coagulation through the intrinsic pathway.[11] However, whitide was found to inhibit extrinsic coagulation, the mechanisms need to further study.
Whitide was derived from dried Whitmania pigra Whitman, it would also have anticoagulant activity if extracted by hot water. This proved that it is a hot stable oligopeptide. Although whitide with trypsin was well-tolerated, if it could be used as oral anticoagulants may be worth further study.
CONCLUSION
Whitide is a novel oligopeptide from Whitmania pigra Whitman with anticoagulant activity. Our results indicated that its amino acid sequence had strong homology to various types of collagens, and its anticoagulant action was mainly mediated by targeting the extrinsic pathway. Most significantly, whitide might be an orally active compound for its hot and trypsin stable.
ACKNOWLEDGMENTS
This work was supported by Project of China Academy of Traditional Chinese Medicine (ZZ20090402) and Project of Hubei University of Traditional Chinese Medicine (XJ2014KJ008).
Footnotes
Source of Support: This work was supported by Project of China Academy of Traditional Chinese Medicine (ZZ20090402)
Conflict of Interest: None declared.
REFERENCES
- 1.I. Beijing: Chemical Industry Press; 2010. Chinese Pharmacopoeia Editorial Committee. The Pharmacopoeia of the People's Republic of China; p. 77. [Google Scholar]
- 2.Markwardt F. Hirudin as alternative anticoagulant – A historical review. Semin. Thromb Hemost. 2002;28:405–14. doi: 10.1055/s-2002-35292. [DOI] [PubMed] [Google Scholar]
- 3.Wan M, Wang M, Liu YM, Yu S, Chen KL. Studies on extraction methods of the materials with anticoagulant activity from Whitmania pigra. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2012;32:414–7. [Google Scholar]
- 4.Zhong S, Yang DP, Cui Z. Studies on an anticoagulant constituents in dried Whitmania pigra. Zhongguo Zhong Yao Za Zhi. 2008;33:2781–4. [PubMed] [Google Scholar]
- 5.Salzet M, Chopin V, Baert J, Matias I, Malecha J. Theromin, a novel leech thrombin inhibitor. J Biol Chem. 2000;275:30774–80. doi: 10.1074/jbc.M000787200. [DOI] [PubMed] [Google Scholar]
- 6.Barhoom S, Sharon A. Bcl-2 proteins linkprogrammed cell death with growth and morphogenetic adaptations in the fungal plant pathogen Colletotrichum gloeosporioides. Fung Genet Biol. 2007;44:32–43. doi: 10.1016/j.fgb.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuk DY, Ryu CK, Hong JT, Chung KH, Kang WS, Kim Y, et al. Antithrombotic and antiplatelet activities of 2-chloro-3-[4-(ethylcarboxy)-phenyl]-amino-1,4-naphthoquinone (NQ12), a newly synthesized 1,4-naphthoquinone derivative. Biochem Pharmacol. 2000;60:1001–8. doi: 10.1016/s0006-2952(00)00411-1. [DOI] [PubMed] [Google Scholar]
- 9.Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:1687–93. doi: 10.1161/ATVBAHA.107.141911. [DOI] [PubMed] [Google Scholar]
- 10.Andrews RK, Berndt MC. Platelet physiology and thrombosis. Thromb Res. 2004;114:447–53. doi: 10.1016/j.thromres.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 11.Versteeg HH, Heemskerk JW, Levi M, Reitsma PH. New fundamentals in hemostasis. Physiol Rev. 2013;93:327–58. doi: 10.1152/physrev.00016.2011. [DOI] [PubMed] [Google Scholar]


