Abstract
Background:
The nuts of Juglans sinensis Dode, walnut tree, are rich in unsaturated fatty acids and bioactive compounds with antioxidant activity on liver damages. However, hepatoprotective activity of the leaves and twigs of J. sinensis have not intensively studied yet.
Objective:
Hepatoprotective activity of the refined ethanolic extract of J. sinensis (JSE3) was evaluated using carbon tetrachloride (CCl4)-intoxicated rats.
Materials and Methods:
Hepatotoxicity was induced in Sprague Dawley rats by intraperitoneal injection of CCl4 for 6 weeks in the presence or absence of JSE3 (100 and 200 mg/kg body weight). The hepatoprotective activity of JSE3 was assessed by biochemical parameters including plasma aspartate aminotransferase (AST), alanine aminotransferase (ALT), and antioxidant enzymes, such as superoxide dismutase (SOD), glutathione reductase, glutathione peroxide, reduced glutathione and oxidized glutathione, along with histopathological studies on hepatic tissue.
Results:
JSE3 significantly decreased the elevated levels of AST and ALT and restored the reduced levels of antioxidant enzymes. JSE3 also decreased the amounts of collagen content accumulated by CCl4 intoxication.
Conclusion:
These results suggested that the refined extract of J. sinensis may have a potential to be developed as a therapeutic agent to treat hepatic diseases, such as fatty liver and hepatic fibrosis.
Keywords: Carbon tetrachloride, flavonoid, hepatoprotective, Juglnas sinensis, oleanane, oxidative stress, ursane
INTRODUCTION
Damaged hepatocytes by oxidative stress play a central role in the sequential activation of hepatic fibrosis, the common pathophysiologic process of all chronic hepatic damage, such as chronic hepatitis C, alcoholic liver disease, nonalcoholic steatohepatitis, and Wilson's disease.[1,2] During hepatic fibrogenesis, quiescent hepatic stellate cells (HSCs) are activated and produce collagen and excessive extracellular matrix (ECM) proteins, which cause the distortion of hepatic architecture by forming the fibrous scar.[3] Thus, the attenuation of oxidative stress are suggested as a potential therapeutic target to suppress the activation of HSCs and manipulate hepatic damages.[4]
During the searching for anti-fibrotic agents from the natural resources using HSC-T6 cells, an immortalized rat HSC line, the refined ethanolic extract of Juglans sinensis Dode (Juglandaceae) was found to show a significant anti-proliferative activity. J. sinensis is a walnut tree native to Asian countries such as Korea and China.[5] As the anti-proliferative compounds of the refined ethanoic extract, oleanane-and ursane-type triterpene were isolated.[6,7,8] In addition to these triterpenes, phenolic compounds, such as quercetin and cathechin, which attenuate hepatic fibrosis by attenuating the oxidative stress were isolated from this plant.[9,10]
To the best of our knowledge, the study of the extract of J. sinensis on hepatoprotective activity in vivo models has not been reported. Based on the anti-fibrotic activity of the extract in vitro, it was attempted to evaluate the anti-fibrotic effect of the extract in vivo using the carbon tetrachloride (CCl4) intoxicated rat in the study.
MATERIALS AND METHODS
Chemicals
Carbon tetrachloride, chloramine T, and Ehrlich's reagent, malondialdehyde (MDA), superoxide dismutase (SOD), glutathione reductase (GR), glutathione peroxide (GSH-px), glutathione (reduced from, GSH), and glutathione (oxidized form, GSSG) were purchased from Sigma-Aldrich Chemical Co., (St. Louis, MO, USA). The assay kits for aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were purchased from Asan pharmaceutical Co. (Seoul, Korea).
Plant materials
Leaves and twigs of J. sinensis were collected at the Medicinal Plant Garden of Seoul National University located in Goyang Korea, in September 2008 and air-dried. J. sinensis was identified by Dr. Jong Hee Park, a professor of the College of Pharmacy, Pusan National University. A voucher specimen (SNUPH-0329) has been stored in the Herbarium of the Medicinal Plant Garden, Seoul National University.
Preparation of the refined extract of Juglans sinensis
Dried leaves and twigs (600 g) of J. sinensis were extracted with 80% ethanol in an ultrasonic apparatus. After removal of the solvent in vacuo, the concentrated extract was dissolved in distilled water, then subjected to a chromatography using macroporous adsorption HP-20 resin (Mitsubishi Chemical Co. Ltd, Tokyo, Japan) with successive elution of the mixtures of ethanol-H2O = 0:10, 2:8, 8:2, 10:0 to yield 4 sub-fractions ethanolic extract of J. sinensis (JSE1-4). The four sub-fractions were lyophilized, respectively, to yield brownish powders.
Condition for high-performance liquid chromatography-evaporative light scattering detector analysis
High-performance liquid chromatography (HPLC) system was consisted of a chromatographic pump (P680, Dionex, Germany), an automated sample injector (ASI-100, Dionex, Germany) and a thermostatted column compartment (TCC-100, Dionex, Germany) equipped with evaporative light scattering detector (ELSD) (PL-ELS2100, Polymer Laboratories, UK). Chromatographic separation was achieved on a Shiseido CapCell PAK C18 column (5 μm, 4.6 mm i.d. × 150 mm); the column temperature was set at 30°C. The mobile phase was consisted of (A) 0.1% formic acid and (B) acetonitrile at a flow rate of 1.0 mL/min, with gradient elution as follows: 0 min, 20% B; 18 min, 20% B; 25 min, 60% B; 45 min, 60% B; 45.1 min, 85% B; 60 min, 85% B; v/v). The temperature of evaporator for ELSD was set at 50tDowith a nitrogen gas flow rate of 1.5 L/min. The aliquot of JSE1-4 (10 μl/ml of 10 mg/ml) was injected into HPLC-ELSD system, respectively, to evaluate amounts of the active components.
Animals
Male Sprague-Dawley rats (180–200 g body weight) were purchased from Koatech Co. Ltd. (Yongin, Korea), housed in conventional cages (three per each cage) at room temperature under 12 h light-dark cycle and fed ad libitum with free access to water. Animal experiments were conducted under the guidelines of the Institutional Animal Use and Care Committee at Seoul National University. CCl4 was intraperitoneally injected to rats twice a week to induce hepatic failure (1 ml/kg, CCl4/olive oil = 1:1, [Figure 1a]) for 6 weeks. After 2 weeks of CCl4-intoxication of rats, JSE3 (100 and 200 mg/kg body weight) or silymarin (200 mg/kg body weight) were orally given to rats together with CCl4-intoxication for additional 4 weeks (three times a week; [Figure 1b]). The rats which were belong to normal control group were treated with JSE3 for 4 weeks (200 mg/kg body weight) without CCl4-intoxication (three times a week; [Figure 1c]). After the 6 week-experimental period, rats were anesthetized with zoletil 50 (10 mg/kg, i.p.) and blood samples were collected from inferior vena cava. Livers were quickly removed from the rats and weighed after washing with cold saline solution. The liver lobes were divided into three pieces. One piece was fixed with 10% formalin for histological analysis (Masson's trichrome staining, Sirius red staining). The second piece was quickly frozen at-80zenfor the determination of antioxidant enzymes activities and lipid peroxidation. The third piece was completely lyophilized for the determination of hydroxyproline content.
Figure 1.
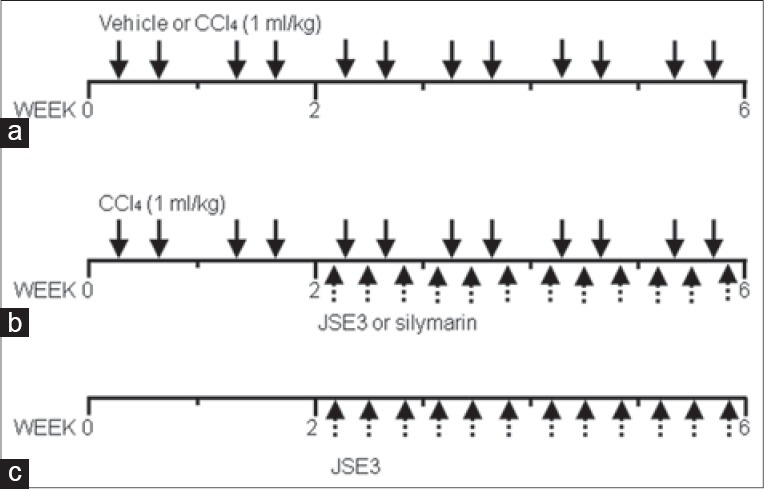
Treatment schedule. (a) Control rats or carbon tetrachloride (CCl4) – intoxicated rats were injected intraperitoneally with vehicle or CCl4 (1 ml/kg, CCl4/olive oil = 1:1, twice a week;) for 6 weeks; (b) ethanolic extract of Juglans sinensis (JSE3) were orally given to rats at a dose of 200 mg/kg, three times a week for 6 weeks to assess the toxicity of JSE3 (C) After 2 weeks of CCl4 intoxication, JSE3 (100 and 200 mg/kg) and silymarin (200 mg/kg) were orally given for additional 4 weeks; number o rats in each was 8 (n = 8)
Histopathological examination
For the assessment of hepatic fibrogenesis, the liver sections fixed in 10% formalin were stained with the Masson's trichrome and Sirius red dyes. The severity of hepatic fibrosis was evaluated by Knodell scoring system as follows: 0, no liver scarring; 1+, minimal liver scarring around liver blood vessels; 2+, scarring extended out from liver blood vessels; 3+ scarring that forms “bridges” between blood vessels; 4+, extensive scarring of cirrhosis.
Determination of serum aspartate aminotransferase and alanine aminotransferase activities, and hydroxyproline content
The activities of serum AST and ALT were determined by the Reitman-Frankel method with slight modifications.[11] Hydroxyproline content in liver hydrolysate was determined by the slightly modified method of Reddy and Enwemek.[12] Briefly, the lyophilized hepatic tissue (10 mg) was powdered and hydrolyzed with 6 N HCl for 60 min at 100°C. The supernatant of the hydrolyzed solution was oxidized by adding chloramine T solution and left for 10 min at room temperature. Finally, Ehrlich's solution was added and placed in dry oven at 60enefor 40 min. Absorbance of the final product was spectrophotometrically measured at 558 nm. The amount of hydroxyproline was determined from a standard curve obtained with 4-hydroxyproline.
Determination of the oxidative stress level in hepatic tissue
A piece of hepatic tissue was gently homogenized with homogenizing buffer (0.25 M sucrose, 10 mM Tris-HCl and 1 mM EDTA, pH 7.4) and centrifuged at 4000 rpm for 10 min at 4°C. The supernatant was taken for the determination of MDA level and antioxidant enzymes activities. The amounts of MDA were indirectly determined by the measurement of thiobarbituric acid-reactive substances level at 535 nm.[13] The activity of SOD was determined according to the method of McCord and Fridovich.[14] GR activity was measured according to the method based on the reduction of GSSG by GR in the presence of NADPH.[15] The activity of GSH-px was determined by quantifying the rate of oxidation of GSH to GSSG by cumene hydroperoxide.[16] Total GSH content in the supernatant was spectrophotometrically determined by the method of Tietze using the enzymatic cycling reaction.[17] The bicinchoninic acid kit with serum bovine albumin as a standard was used for the determination of the protein concentration.[18]
Statistical analysis
Statistical significance was evaluated by the One-way ANOVA test. A P ≤ 0.05 was considered to be statistically significant.
RESULTS
Preparation of ethanolic extract of Juglans sinensis
The 80% ethanolic extract of J. sinensis (JSE3) leaves and twigs was fractionated to give four sub-fractions (JSE1-4) using HP-20 resin. Among the four fractions, JSE3 was selected to the further study since JSE3 were found rich in oleanane-and ursane-type triterpenes, and flavonoids which were considered as anti-fibrotic components from the previous in vitro study [Figure 2].
Figure 2.
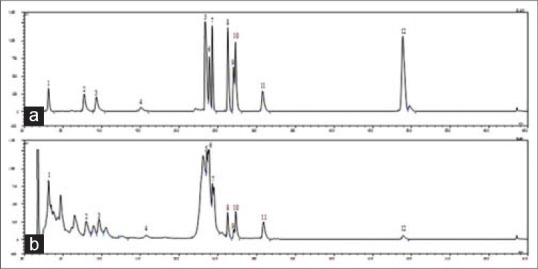
High-performance liquid chromatography-evaporative light scattering detector chromatograms of the mixture of standard compounds from Juglans sinensis (a) and Ethanolic extract of Juglans sinensis (b). The compounds were separated on on a Shiseido CapCell PAK C18 column (5 μm, 4.6 mm i.d. × 150 mm) using a periodic gradient solvent system with 20% B in 0–18 min, from 20% B to 60% B in 18–25 min, 60% B in 25–45 min and 85% B in 45–65 min. Solvent A: 0.1% formic acid, solven B: Acetonitrile. Flow rate: 1.0 mL/min. The peaks are (+)-catechin (1, tR 3.116 min), (+)-taxifolin 3-O-α-L-arabinofuranoside (2, tR 7.724 min), quercetin-3-O-α-L-arabinofuranoside (3, tR 9.288 min), kaempferol-3-O-α-L-arabinofuranoside (4, tR 15.079 min), quercetin (5, tR 23.424 min), 2α, 3β, 23-trihydroxy-olean-12-ene-28-oic acid 28-O-β-D-glucopyranoside (6, tR 23.875 min), 2α, 3α, 23-trihydroxy-urs-12-ene-28-oic acid 28-O-β-D-glucopyranoside (7, tR 24.294 min), arjunolic acid (8, tR 26.282 min), 1-oxo-3β, 23-dihydroxy-olean-12-en-28-oic acid (9, tR 27.049 min), 2α, 3α, 23-trihydroxy-urs-12-en-28-oic acid (10, tR 27.293 min), 23-hydroxyursolic acid (11, tR 30.826 min) and ursolic acid (12, tR 48.791 min)
The changes of body and liver weight of rats by ethanolic extract of Juglans sinensis
The body weights of rats intoxicated with CCl4 were significantly decreased as compared to those treated with vehicle (50% olive oil), and the liver weights were relatively increased [Table 1]. However, treatment of CCl4-intoxicated rats with JSE3 (100 and 200 mg/kg body weight) or silymarin (200 mg/kg body weight) significantly increased the decreased body weight and decreased the increased liver weight [Table 1].
Table 1.
Effect of JSE3 on body weight, relative liver weight and severity score of hepatic injury in CCl4-intoxicated rats
The morphological examination of hepatic tissue
The architecture of hepatic tissue of the rats treated with vehicle or treated with JSE3 alone (200 mg/kg body weight) showed normal structure in the Masson's trichrome and Sirius red staining results [Figure 3a and b, 1 and 2]. However, hepatic fibrosis characterized with fiber extension, collagen accumulation forming the partial compartments and pseudo lobe formation was phenotypically developed in rats intoxicated with CCl4 for 6 weeks. Furthermore, obvious fatty degeneration was observed in hepatocytes [Figure 3A and B, III and VII]. The treatment of CCl4-intoxicated rats with JSE3 at the dose of 100 mg/kg body weight significantly attenuated collagen accumulation even though the fatty degeneration seemed to be similar to rats intoxicated with CCl4 [Figure 3a and b, 4]. Both pathological pattern of fatty degeneration in hepatocytes and collagen accumulation were markedly ameliorated after the treatment CCl4-intoxicated rats with JSE3 at the dose of 200 mg/kg body weight. These results could be comparable to those treated with silymarin at the dose of 200 mg/kg body weight [Figure 3A and B, V, VI and VIII]. Consistently, the severity of the morphological changes of rats was scored and summarized in Table 1. The treatment of CCl4-intoxicated rats with JSE3 markedly lowered the average severity score which indicated the deterioration of hepatic fibrosis. In accord with the result, collagen content was significantly decreased in rats treated with JSE3 compared to those intoxicated with CCl4 [Table 1].
Figure 3.
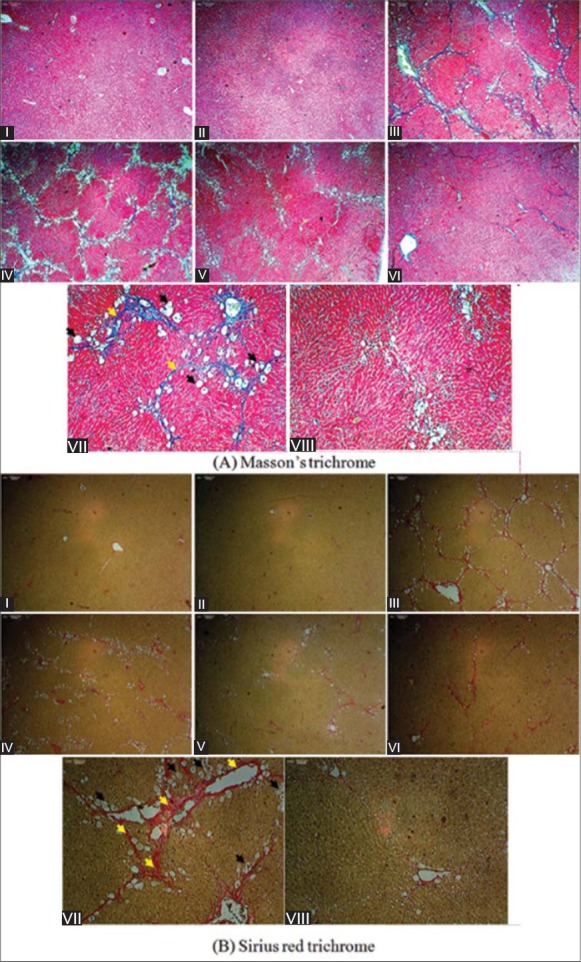
The effect of ethanolic extract of Juglans sinensis (JSE3) on hepatic fibrosis developed in carbon tetrachloride (CCl4)-intoxicated rats. (a) Masson's trichrome staining, (b) Sirius red staining, (1) control group; (2) JSE3-treated group (200 mg/kg); (3) and (7) CCl4 intoxicated group (1 mg/kg, CCl4/olive oil = 1:1); (4) CCl4 + 100 mg/kg of JSE3-treated group; (5) and (8) CCl4 + 200 mg/kg of JSE3-treated group; (6) CCl4 + 200 mg/kg of silymarin-treated group. (a) Masson's trichrome and (b) Sirius red staining; magnification: (1–6); 40×, (7–8); 100×. Black arrows indicate fatty degeneration and yellow collagen accumulation
Figure 4.
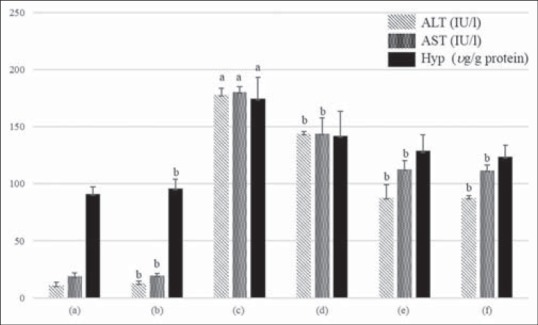
Effect of ethanolic extract of Juglans sinensis (JSE3) on serum alanine aminotransferase and aspartate aminotransferase activities, and the hydroxyproline content in carbon tetrachloride (CCl4)-intoxicated rats. (a) Control group; (2) JSE3-treated group (200 mg/kg); (c) CCl4-intoxicated group (1 mg/kg, CCl4/olive oil = 1:1); (d) CCl4 + 100 mg/kg of JSE3-treated group; (e) CCl4 + 200 mg/kg of JSE3-treated group; (f) CCl4 + 200 mg/kg of silymarin-treated group. Values are mean of ± standard deviation (n = 8). aSignificantly different from the control rats, P < 0.05. bSignificantly different from rats intoxicated treated with CCl4 alone, P < 0.05
The determination of serum levels of alanine aminotransferase and aspartate aminotransferase and the collagen content
The levels of serum ALT and AST of CCl4-intoxicated rats were significantly increased when compared to those of rats treated with vehicle [Figure 4]. However, the administration of JSE3 (100 and 200 mg/kg body weight) to CCl4-intoxicated rats significantly reduced the elevated serum transaminases activities. The treatment of CCl4-intoxicated rats with silymarin at the dose of 200 mg/kg body weight exerted almost similar protection effect of JSE3 at the same dose of 200 mg/kg body weight. After the treatment off CCl4-intoxicated rats with JSE3 (100 and 200 mg/kg body weight), the content of hydroxyproline as an index of collagen formation was also decreased to the similar level of silymarin-treated rats [Figure 4].
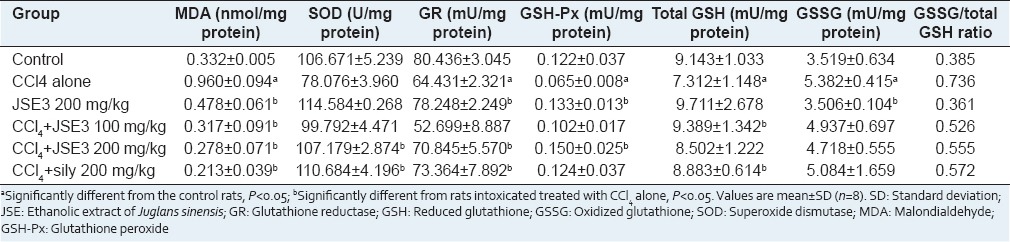
Malondialdehyde and reduced glutathione contents, and activities of superoxide dismutase, glutathione peroxide and glutathione reductase
Continuous exposure of CCl4 to rats deteriorated the defense system against oxidative stress in liver. The level of MDA formed during lipid peroxidation of poly-unsaturated fatty acids in liver was significantly increased in the CCl4-intoxicated rats, whereas the activities of antioxidant enzymes, such as SOD, GSH-px and GR, were decreased [Table 2]. However, the treatment of CCl4-intoxicated rats with JSE3 (100 and 200 mg/kg body weight) effectively decreased MDA level and increased antioxidant enzymes activities in CCl4-intoxicated rats. The intoxication of rats with CCl4 also increased the ratio of GSH and GSSG in liver, used as an index of a balance of reduction and oxidation during oxidative stress. The ratio of GSH and GSSG was reversed by the treatment of CCl4-intoxicated rats with JSE3 (100 and 200 mg/kg body weight) compared with CCl4-intoxicated rats.
Table 2.
Effect of JSE3 on the levels of MDA and GSH, and the activities of antioxidant enzymes in CCl4-intoxicated rats
DISCUSSION
Liver fibrosis, an early stage of cirrhosis, was thought to be a passive and irreversible process due to the collapse of the balance between fibrogenesis and fibrolysis. Currently, it is generally considered that hepatic fibrosis is reversible and the wound-healing response to chronic liver injury.[19] If treated optimally at this stage, hepatic injury can be reversed, thus not be progressed to the end-stage, irreversible chronic damage that ultimately leads to the high mortality and morbidity.
Fibrogenesis is generally characterized by increased ECM deposition and reduced matrix degradation.[19] Numerous studies uncovered HSCs are the main collagen-and ECM-producing cells in the process of hepatic fibrosis.[20,21] Also HSCs are activated by external insults such as virus, drug and toxicants that produce ROS and lead to the injury of parenchymal hepatocytes. The apoptotic removal of activated HSCs has been suggested the potential target for therapeutic agents.[22]
Juglans sinensis leaves and twigs contained oleanane-and ursane-type triterpenes and flavonoids which have protective or therapeutic effect on hepatic fibrosis in vitro and in vivo models.[23,24,25,26] Recently, it was reported that ursolic acid, a major components of J. sinensis, induced selective apoptotic cell death in activated primary cultured HSCs, but not in primary hepatocytes and quiescent HSCs, which was confirmed as an in vivo model that the compound inhibited the progression of liver fibrosis.[6] Flavonoids of J. sinensis, mainly flavonols (quercetin derivatives) and dihydroflavonol (taxifolin derivatives), suppress ROS generation by the inhibition of enzymatic activity involved in free radical production.[27]
Based on these studies, we expected that the extract of J. sinensis having these triterpenes and flavonoids may have the therapeutic potential for ameliorating the progression of hepatic fibrosis. The 80% JSE3 was fractionated using adsorption resin (HP-20) to obtain the triterpenes and flavonoids-concentrated fraction. The HPLC-ELSD analysis revealed that JSE3 had a variety of chromatographic peaks in consistent with those of ursane-, oleanane-type triterpenes and flavonoids used as the external standards.
Carbon tetrachloride initiates lipid peroxidation resulting in necrosis of hepatocytes and eventually induce hepatic fibrosis.[28] The model of CCl4-induced hepatic fibrosis has been widely used for assessing the efficacy of anti-fibrotic agents especially in the context of the redox modulation in liver.[29] The treatment of CCl4 intoxicated rats with JSE3 significantly restored liver weights and the activities of ALT and AST compared to those of CCl4 intoxicated rats alone. The treatment of JSE3 attenuated CCl4-induced oxidative stress by up-regulating the activities of antioxidant enzymes. Fortunately, the rats given JSE3 alone did not show any risk of toxicity and the significant alteration of antioxidant markers compared to the normal rats. Interestingly, the body weight of rats treated with JSE3 was not significantly restored in comparison with those with silymarin. This result demonstrated that JSE3 have the hepatoprotective effect without regaining the body weight. By the morphological evidences of collagen staining and fibrotic scoring system, it could be postulated that JSE3 is beneficial for the treatment of oxidative stress-induced hepatic fibrosis.
CONCLUSION
Ethanolic extract of J. sinensis significantly reversed the pathological parameters and normalized biochemical indices of liver injury. Although the action mechanisms were not completely elucidated, it is expected that JSE3 prevents CCl4-induced hepatic oxidative stress due to the strong antioxidative effect. In the study, J. sinensis attenuated a part of hepatic fibrosis and might be useful to develop as a therapeutic agent against liver fibrosis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Liu T, Wang X, Karsdal MA, Leeming DJ, Genovese F. Molecular serum markers of liver fibrosis. Biomark Insights. 2012;7:105–17. doi: 10.4137/BMI.S10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singal AK, Jampana SC, Weinman SA. Antioxidants as therapeutic agents for liver disease. Liver Int. 2011;31:1432–48. doi: 10.1111/j.1478-3231.2011.02604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Popov Y, Schuppan D. Targeting liver fibrosis: Strategies for development and validation of antifibrotic therapies. Hepatology. 2009;50:1294–306. doi: 10.1002/hep.23123. [DOI] [PubMed] [Google Scholar]
- 4.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–50. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 5.Park JH, Sung SH. Seoul: Sin-II Books; 2007. Haeksim-Yakyongsikmul; pp. 442–3. [Google Scholar]
- 6.Wang X, Ikejima K, Kon K, Arai K, Aoyama T, Okumura K, et al. Ursolic acid ameliorates hepatic fibrosis in the rat by specific induction of apoptosis in hepatic stellate cells. J Hepatol. 2011;55:379–87. doi: 10.1016/j.jhep.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 7.Wu LM, Wu XX, Sun Y, Kong XW, Zhang YH, Xu Q. A novel synthetic oleanolic acid derivative (CPU-II2) attenuates liver fibrosis in mice through regulating the function of hepatic stellate cells. J Biomed Sci. 2008;15:251–9. doi: 10.1007/s11373-007-9216-9. [DOI] [PubMed] [Google Scholar]
- 8.Yang H, Jeong EJ, Kim J, Sung SH, Kim YC. Antiproliferative triterpenes from the leaves and twigs of Juglans sinensis on HSC-T6 cells. J Nat Prod. 2011;74:751–6. doi: 10.1021/np1008202. [DOI] [PubMed] [Google Scholar]
- 9.Pavanato A, Tuñón MJ, Sánchez-Campos S, Marroni CA, Llesuy S, González-Gallego J, et al. Effects of quercetin on liver damage in rats with carbon tetrachloride-induced cirrhosis. Dig Dis Sci. 2003;48:824–9. doi: 10.1023/a:1022869716643. [DOI] [PubMed] [Google Scholar]
- 10.Abe K, Suzuki T, Ijiri M, Koyama Y, Isemura M, Kinae N. The anti-fibrotic effect of green tea with a high catechin content in the galactosamine-injured rat liver. Biomed Res. 2007;28:43–8. doi: 10.2220/biomedres.28.43. [DOI] [PubMed] [Google Scholar]
- 11.Crowley LV. The Reitman-Frankel colorimetric transaminase procedure in suspected myocardial infarction. Clin Chem. 1967;13:482–7. [PubMed] [Google Scholar]
- 12.Lin YK, Kuan CY. Development of 4-hydroxyproline analysis kit and its application to collagen quantification. Food Chem. 2010;119:1271–7. [Google Scholar]
- 13.Stocks J, Gutteridge JM, Sharp RJ, Dormandy TL. Assay using brain homogenate for measuring the antioxidant activity of biological fluids. Clin Sci Mol Med. 1974;47:215–22. doi: 10.1042/cs0470215. [DOI] [PubMed] [Google Scholar]
- 14.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–55. [PubMed] [Google Scholar]
- 15.Carlberg I, Mannervik B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J Biol Chem. 1975;250:5475–80. [PubMed] [Google Scholar]
- 16.Flohé L, Günzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–21. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 17.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–22. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 18.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 19.Albanis E, Friedman SL. Hepatic fibrosis. Pathogenesis and principles of therapy. Clin Liver Dis. 2001;5:315–34. doi: 10.1016/s1089-3261(05)70168-9. v. [DOI] [PubMed] [Google Scholar]
- 20.Forbes SJ, Russo FP, Rey V, Burra P, Rugge M, Wright NA, et al. A significant proportion of myofibroblasts are of bone marrow origin in human liver fibrosis. Gastroenterology. 2004;126:955–63. doi: 10.1053/j.gastro.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 21.Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J Cell Mol Med. 2006;10:76–99. doi: 10.1111/j.1582-4934.2006.tb00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elsharkawy AM, Oakley F, Mann DA. The role and regulation of hepatic stellate cell apoptosis in reversal of liver fibrosis. Apoptosis. 2005;10:927–39. doi: 10.1007/s10495-005-1055-4. [DOI] [PubMed] [Google Scholar]
- 23.Bai X, Qiu A, Guan J, Shi Z. Antioxidant and protective effect of an oleanolic acid-enriched extract of A. deliciosa root on carbon tetrachloride induced rat liver injury. Asia Pac J Clin Nutr. 2007;16(Suppl 1):169–73. [PubMed] [Google Scholar]
- 24.Lee MK, Ha NR, Yang H, Sung SH, Kim GH, Kim YC. Antiproliferative activity of triterpenoids from Eclipta prostrata on hepatic stellate cells. Phytomedicine. 2008;15:775–80. doi: 10.1016/j.phymed.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Mandal AK, Das S, Basu MK, Chakrabarti RN, Das N. Hepatoprotective activity of liposomal flavonoid against arsenite-induced liver fibrosis. J Pharmacol Exp Ther. 2007;320:994–1001. doi: 10.1124/jpet.106.114215. [DOI] [PubMed] [Google Scholar]
- 26.Shyu MH, Kao TC, Yen GC. Hsian-tsao (Mesona procumbens Heml.) prevents against rat liver fibrosis induced by CCl (4) via inhibition of hepatic stellate cells activation. Food Chem Toxicol. 2008;46:3707–13. doi: 10.1016/j.fct.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 27.Cazarolli LH, Zanatta L, Alberton EH, Figueiredo MS, Folador P, Damazio RG, et al. Flavonoids: Prospective drug candidates. Mini Rev Med Chem. 2008;8:1429–40. doi: 10.2174/138955708786369564. [DOI] [PubMed] [Google Scholar]
- 28.Basu S. Carbon tetrachloride-induced lipid peroxidation: Eicosanoid formation and their regulation by antioxidant nutrients. Toxicology. 2003;189:113–27. doi: 10.1016/s0300-483x(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 29.Wu J, Norton PA. Animal models of liver fibrosis. Scand J Gastroenterol. 1996;31:1137–43. doi: 10.3109/00365529609036901. [DOI] [PubMed] [Google Scholar]


