Abstract
Background:
Microwave-assisted reflux extraction of polysaccharides YPF-P from the famous Chinese traditional drug, Yupingfeng powder, optimization of extracting conditions and evaluation of their antioxidant activity were conducted in this study.
Results:
Single factor effect trends were achieved through yields and contends of YPF-P obtained from different extracting conditions. Then through a three-level, four-variable Box-Behnken design of response surface methodology adopting yield as response, the optimal conditions were determined as follows: Material/solvent ratio 1:23.37, microwave power 560 W, Extraction temperature 64°C, and extraction time 9.62 min. Under the optimal conditions, the YPF-P extraction yield was 3.23%, and its content was detected as 38.52%. In antioxidant assays, the YPF-P was tested to possess 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activities with an IC50 value of 0.262 mg/ml. In addition, YPF-P was also proved to have relatively low ferric reducing antioxidant power (FRAP), compared to Vc, through FRAP assay.
Conclusion:
In the microwave assisted reflux extraction research, good YPF-P yield was achieved from materials with relatively low YPF-P content. And for the first time, both DPPH and FRAP assays were conducted on YPF-P, which proved that the antioxidant activity of YPF-P contributed to the functions of this medicine.
Keywords: Antioxidant activity, microwave-assisted extraction, polysaccharides, response surface methodology, Yupingfeng powder
INTRODUCTION
Yupingfeng Powder is a famous Chinese traditional combination drug composed of Astragalus membranaceus (Fisch.) Bunge, Atractylodes macrocephala Koidz, and Saposhnikovia divaricata (Turcz.) Schischk by ratio of 3:1:1, first seen in “Danxi's experiential therapy” by Zhu Zhengheng (1281 A.D–1358 A.D) in Yuan Dynasty. From Chinese traditional medicine theory, the first two components focus on strengthening the body resistance while the third one is used to eliminate the pathogen. The combination of these three drugs well solved both the symptoms and root cause. Clinically, it is mainly adopted on the treatment and prevention of immunodeficiency associated inflammation like cold, respiratory infections, allergic rhinitis, chronic bronchitis, bronchial asthma, etc.[1]
Compiles of study indicate remarkable activities of polysaccharides extracted from medicinal plants, such as immunomodulation, activation of immune cells like T lymphocytes, B lymphocytes and macrophages, increasing plasma testosterone levels, protecting the kidney, antitumor, tumor transfer resistance, anti-oxidation, anti-aging, decreasing blood sugar and blood fat, anti-fibrosis, etc.,[2,3,4,5,6] which are similar to the pharmacological potency of Yupingfeng powder.
Researchers have adopted many different methods in polysaccharides extraction, including solvent extraction,[7] alkaline extraction,[8,9] enzymolysis approach,[10] ultrafiltration,[11] heat reflux extraction,[12] ultrasonic-assisted extraction,[13,14] microwave-assisted extraction,[15,16] etc., Compared with microwave-assisted extraction, conventional extraction methods are generally laborious and time-consuming.
Wang et al.[15] reported a comparison study of Potentilla anserina L. polysaccharides microwave-assisted extraction with conventional extraction method. In their research, P. anserina L. polysaccharides obtained from microwave-assisted extraction showed stronger antioxidant activities compared with polysaccharides obtained from hot water extraction.
Xie et al.[16] reported a study on microwave-assisted extraction of polysaccharides from Cyclocarya paliurus (Batal.) Iljinskaja, and also made a comparison with conventional methods. Their results showed that compared with heat reflux extraction and the ultrasonic-assisted extraction, C. paliurus (Batal.) Iljinskaja polysaccharides obtained from microwave-assisted extraction reached an optimal yield in much less time.
Studies on polysaccharides extracted from the three components of Yupingfeng powder could be easily seen in reports, and plenty of polysaccharides have been isolated, like acidic polysaccharide-I (APS-I), APS-II from Radix Astragali,[17] etc., However, as a classical Chinese traditional combination drug, Yupingfeng powder as well as YPF-P should rather be evaluated as a whole than separately. It is indicated that species, season and age could all contribute to diverse variation in polysaccharides contents.[18] In this research, active species were picked to make a good combination for extracting technology research as well as antioxidant activity evaluation.
Considering the advantages of time-saving and high efficiency, microwave-assisted reflux extraction was adopted in our study. And Box-Behnken design of response surface methodology (RSM)[10,13,19,20] was applied in the determination of the optimal extracting conditions. In the antioxidant analysis, methods of 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay[21,22,23] and ferric reducing antioxidant power (FRAP) assay[24,25] were adopted.
MATERIALS AND METHODS
Materials
Dry R. Astragali, Gansu (processed with honey), dry Atractylodes (Fried), Zhejiang, dry Saposhnidoviae Radix, Hebei.
Chemicals
Petroleum ether, absolute ethanol, glucose standard substance, phenol, concentrated sulfuric acid, aluminium flake, NaHCO3, chloroform, n-butanol, HCl, DPPH, 2,4,6-tripyridyl-s-triazine (TPTZ), phosphate buffer, FeCl3.6H2O, etc., DPPH was purchased from Sigma chemical company (St. Louis, MO, USA). TPTZ was purchased from Solarbio Co., Ltd. (Beijing, China). All other chemicals were purchased from ChengDu Kelong Chemical Co., Ltd. and ChengDu Kelong Chemical Reagent Company (Chengdu, China) and were of analytical grade.
Preparation of Yupingfeng powder
About 1.2 kg R. Astragali, 400 g Atractylodes, and 400 g Saposhnidoviae Radix were placed in 50°C drying oven for 24 h, and the materials were smashed after drying, and screened through 20 mesh. The materials were precisely weighed in terms of 3 g R. Astragali, 1 g Atractylodes, 1 g Saposhnidoviae Radix, and then placed in a conical flask. 100 Petroleum ether was added into each flask, and degrease was conducted under ultrasound wave 345W for ½ h. After suction filtering and drying of the degreased materials, the Yupingfeng powder was prepared and transferred into 500 ml round-bottom flask.
Extraction
The prepared material was placed in corresponding material/solvent ratio of distilled water for ½ h. Then the microwave-assisted reflux extraction was conducted under corresponding extraction time. Absolute ethanol was added in the mixture to make a concentration of 30% after it cooled to room temperature, and the flask was put in 4°C refrigerator for 12 h. Then the precipitation was discarded after the material was centrifuged under 4000r/min for 15 min, and corresponding volume of absolute ethanol was added to make a concentration of 60%. Later put the mixture in −4°C refrigerator for15 h and the supernatant was discarded after centrifuged for 15 min under 4000 r/min. Crude polysaccharides (YPF-P) could be obtained through freeze drying the sediment.[10,13]
Yield of crude YPF−P (%W/W) = dried crude YPF−P weight (g)/power weight (5g) ×100
Preparation of the test solution
Test solution was prepared for ultraviolet (UV) spectrophotography to determine YPF-P contents obtained from different extracting conditions. The mixture was suction filtered right after the microwave-assisted reflux extraction of degreased Yupingfeng powder and the residue was washed for 3–4 times using hot distilled water. The filtrate was transferred into 250 ml volumetric flask after it cooled down and was diluted with distilled water to the scale. One milliliter of the solution was pipetted into 10 ml centrifuge tube (with plug) and 6 ml absolute ethanol was added. The mixture was shook up and centrifuged under 4000 r/min for 15 min. Then the supernatant was discarded and the precipitate was dissolved with deionized water to the scale of 50 ml after it was dried to no alcohol scent.
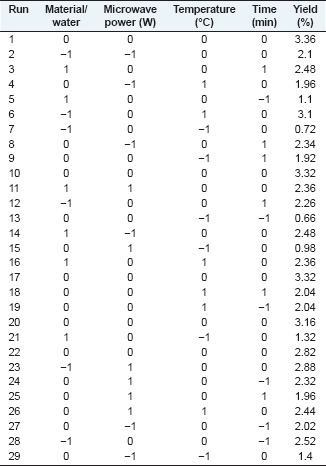
EXPERIMENTAL DESIGN
Single factor experiments
Three constants and one variable were set in one experiment, each containing three parallel experiments. And for each variable, four levels were chosen. Material/water ratio (1:15, 1:20, 1:25, 1:30), microwave power (400 W, 480 W, 560 W, 640 W), extraction temperature (50°C, 60°C, 70°C, 80°C), and extraction time (5 min, 10 min, 15 min, 20 min) were selected as the key variables, marked as X1, X2, X3, X4. Their impact on the yield and content of YPF-P was tested separately. Fixed conditions were determined as 1:25, 560 W, 80°C and 10 min.
Response surface methodology
Three levels of the four variables were selected [Table 1], based on single factor experiments, for Box-Behnken design of RSM adopting Design-Expert V8.0.6 (Stat-Ease, Inc) [Table 2].
Table 1.
Independent variables and levels used for Box-Behnken design

Table 2.
Box-Behnken design matrix and the YPF-P yield
Content determination
The content of YPF-P was determined by phenol/sulfuric acid method with D-glucose as standard at 490 nm. The result was expressed as YPF-P content (%) = C × N × V/W × 100%.
(C: Extract concentration; N: Dilution ratio; V: Volume; W: Powder weight)
Standard curve manufacture
0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6 ml glucose standard solution (100 μg/ml) was pipetted respectively into seven 10 ml test tubes (with plug), and deionized water was added to make each 2 ml, then 1 ml 6% distilled phenol solution was added. The mixture was shook up after a quick addition of 5 ml concentrated sulfuric acid. The solution was placed for 10 min, then 25°C water bathed for 20 min. Solvent-free was set as a reference and made zero. Scanning in the wavelength range of 200–800 nm by UV spectrophotography, and 490 nm was determined as the best detection wavelength. Keeping record of the absorbance values at 490 nm, and the glucose standard curve was obtained by Excel. Regression equation of the standard curve was obtained as y = 0.0155x − 0.0064; R2 = 0.9992.
Data analysis
Construction of binary quadratic equation
Variance analysis was conducted by performing ANOVA in Design-Expert v8.0.6, and the binary quadratic equation could be obtained as follows:
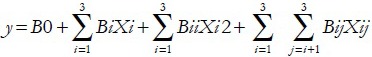
where Y is the predicted response, B0 is the intercept term, Bi are the linear coefficients, Bij are the quadratic coefficients and Xi and Xj are the levels of the independent variables.[13] In the present study, four variables were involved, so values of i and j were taking from 1 to 4. The actual design of experiments is given in Table 2. For a Box-Behnken design with four independent variables at three levels, 29 experimental runs were required. In this case, the binary quadratic equation could be converted to the following equation according to the coded values of the four variables:
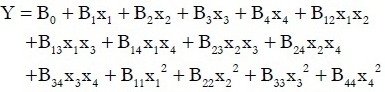
Mathematical modeling
Suitable math model was constructed according to the experimental data, and three-dimensional pictures were developed by Design-Expert v8.0.6. Optimum condition could be obtained by response surface analysis.
Antioxidant ability evaluation
Deproteinization of crude polysaccharides
The crude polysaccharides were redissolved with deionized water, and the crude polysaccharides were deproteinized adopting Sewage method[26] repeatedly with 0.3 times the polysaccharide solution volume of chloroform-n-butanol (5:1) mixed liquor. Refined polysaccharides could be achieved through rotary evaporation.
1,1-diphenyl-2-picrylhydrazyl assay
Preparation of 1,1-diphenyl-2-picrylhydrazyl
A volume of 0.00406 g DPPH was precisely weighed and dissolved with methanol to the scale of 100 ml to obtain 0.004% DPPH, and it was used right after it was ready.
1,1-diphenyl-2-picrylhydrazyl radical scavenging ability determination[21]
The solution of DPPH methanol was purple, and there was a linear relationship between its concentration and absorbance. Solution was added according to Table 3, and Ai was put in darkness for 1 h to make sure a complete reaction. Shallowing color and decreasing absorbance could indicate the DPPH radical scavenging ability of the sample solution.
Table 3.
DPPH adding method

1,1-diphenyl-2-picrylhydrazyl radical scavenging rate (SA%) =1− (Ai − Aj)/A0 × 100%
Ferric reducing antioxidant power assay[24,25]
Preparation of ferric reducing antioxidant power
Ferric reducing antioxidant power solution: 2.5 ml 10 mmol/L TPTZ working solution (dissolve by 40 mmol/L HCl), 2.5 ml 20 mmol/L FeCl3.6H2O and 25 ml 0.3 mol/L acetate buffer, pH 3.6.
Antioxidant determination
Ferric reducing/antioxidant power assay follows the principle that antioxidants could reduce Fe3 + to Fe2+, while Fe2 + could form blue complex with TPTZ, and have optimum absorbance at 593 nm.
A volume of 0.3 ml sample solution was pipetted, and 2.7 ml FRAP working solution (preheat to 37°C) was added. The mixture was placed for 1 h after shook up, and its absorbance was detected at 593 nm. Absolute ethanol was used to displace sample as blank control and made zero. FeSO4 concentration (μmol/L) could be obtained from the standard curve according to the absorbance, and defined as FRAP value. Sample with higher FRAP has higher antioxidant ability.
Manufacture of FeSO4 standard curve
A volume of 6.08 mg FeSO4 was dissolved with moderate volume of distilled water 0.25 ml, 18 mol/L H2SO4 was added, and the mixture was diluted with distilled water to the scale of 50 ml, then an iron nail was put into it. Five milliliter of the solution was pipetted into 50 ml volumetric flask, and was diluted with distilled water to the scale to make 800 μmo1/L FeSO4 standard solution. The 200, 400, 600 μmol/L standard solutions were prepared sequentially. Then TPTZ was added as stated above and the standard curve was manufactured according to the absorbance at 593 nm. Regression equation of the standard curve was obtained as y = 0.0002x − 0.0012; R2 = 0.9991.
RESULTS AND DISCUSSION
Single factor results
Effect of material/Water ratio
Effects of material/water ratio on YPF-P yields and contents were determined under 80°C, 560 W, 10 min, and material/water ratio of 1:15, 1:20, 1:25, 1:30, respectively. And the trends were obtained as Scheme 1 showed.
Scheme 1.
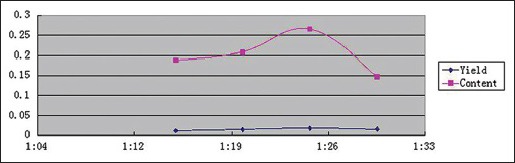
Effect of material/water ratio on Yupingfeng powder yields and contents
Effect of microwave power
Effects of microwave power on YPF-P yields and contents were determined under 80°C, 1:25, 10 min, and microwave power of 400 W, 480 W, 560 W, 640 W, respectively. And the trends were obtained as Scheme 2 showed.
Scheme 2.
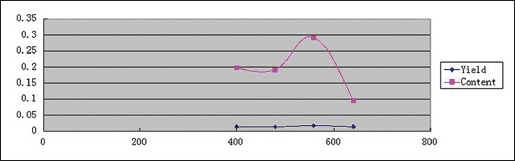
Effect of microwave power on Yupingfeng powder yields and contents
Effect of extraction temperature
Effects of extraction temperature on YPF-P yields and contents were determined under 560W, 1:25, 10 min, and extraction temperature of 50°C, 60°C, 70°C, 80°C, respectively. And the trends were obtained as Scheme 3 showed.
Scheme 3.
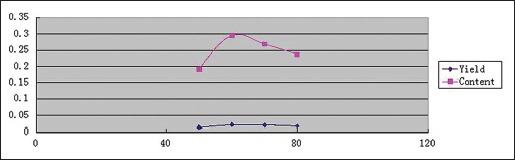
Effect of extraction temperature on Yupingfeng powder yields and contents
Effect of extraction time
Effects of extraction time on YPF-P yields and contents were determined under 560 W, 1:25, 80°C and extraction time of 5 min, 10 min, 15 min, 20 min, respectively. And the trends were obtained as Scheme 4 showed.
Scheme 4.
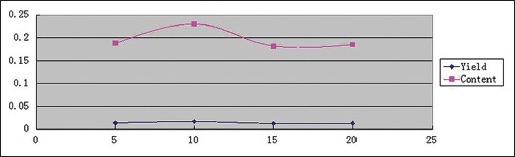
Effect of extraction time on Yupingfeng powder yields and contents
Response analysis and interpretation
Yupingfeng powder yield was obtained as:

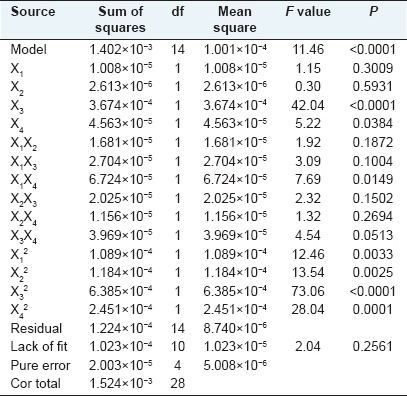
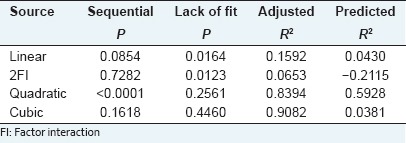
Analysis of variance results, shown in Table 4, suggested that the independent variables (X3, and X4), quadratic terms (X1 × 1, X2 × 2, X3 × 3, X4 × 4) and cross product coefficient (X1 × 4) significantly affected the polysaccharides yield (P < 0.05). The results of the study showed that extraction temperature was the most significant single parameter that influenced YPF-P yield, followed by extraction time. The Model F-value of 11.46 implied the model was significant, and the “lack of fit F-value” of 2.04 implied the lack of fit was not significant relative to the pure error.
Table 4.
Surface quadratic model analysis of variance table (partial sum of squares-type III)
Fit summary results, shown in Table 5, indicated that this model was not aliased. The adjusted R2 close to 1 indicated a high degree of correlation between the observed and predicted values.
Table 5.
Fit summary
In Figures 1-6, A, B, C, D represented for coded factors of x1, x2, x3, x4, respectively.
Figure 1.
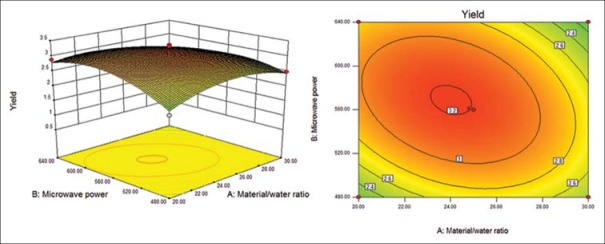
Response surface (left) and contour (right) plots for effects of material/water ratio and microwave power on the polysaccharide yield
Figure 6.
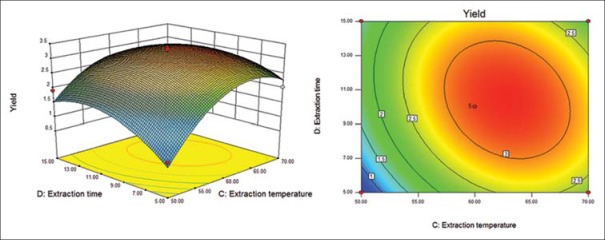
Response surface (left) and contour (right) plots for effects of extraction temperature and extraction time on the polysaccharide yield.
Figure 2.
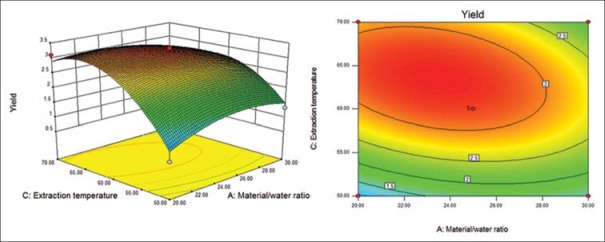
Response surface (left) and contour (right) plots for effects of material/water ratio and extraction temperature on the polysaccharide yield
Figure 3.
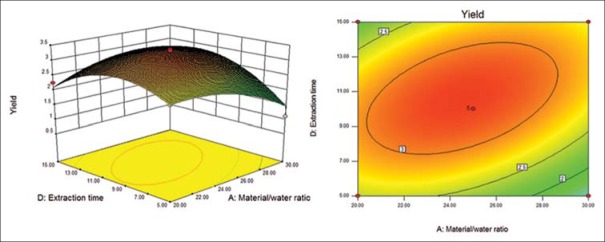
Response surface (left) and contour (right) plots for effects of material/water ratio and extraction time on the polysaccharide yield
Figure 4.
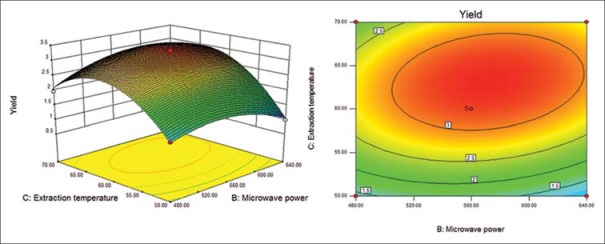
Response surface (left) and contour (right) plots for effects of microwave power and extraction temperature on the polysaccharide yield
Figure 5.
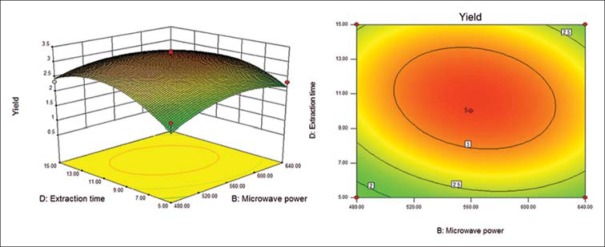
Response surface (left) and contour (right) plots for effects of microwave power and extraction time on the polysaccharide yield
Optimization of the procedure
Six response surface and contour pictures are shown in Figures 1-6. Effects of two variables on yields within the experimental range were depicted in three-dimensional surface plots while the two other variables were kept constant at 0 level. And of the contour plots, circular or elliptical shapes indicated whether the mutual interactions between the two variables were significant or not.[10] In the case of YPF-P extraction, the yield first increased with the increase of the variables, then decreased with the increase of the variables after the threshold level.
It could be concluded from the contour lines that the optimum range for C was 57–70°C while, for other variables, the optimum range changed largely in different figs. And it could also be seen that, the YPF-P yield highly increased from 50°C to 60°C.
Through numerical analysis by balancing domain decomposition, the optimal conditions for the extraction of YPF-P were obtained as 1:23.37, 579.46 W, 63.61°C, 9.62 min.
Validation of the models
The adequacy of the model equation for predicting optimum response value was validated under the following conditions: 1:23.37, 560W, 64°C, 9.62 min. Under the optimal conditions, the model predicted a maximum response of 3.30906%, while a mean value of 3.23% ± 0.07% (n = 3) was obtained from experiments. The good correlation between the predicted value and experimental value confirmed a satisfactory model for reflecting the expected optimization.
Antioxidant ability evaluation results
1,1-diphenyl-2-picrylhydrazyl radical scavenging result
Comparison of DPPH radical scavenging rate of refined YPF-P and Vc was made as Figure 7 shows. It could be seen that, refined YPF-P had relatively low DPPH radical scavenging ability compared to Vc.
Figure 7.
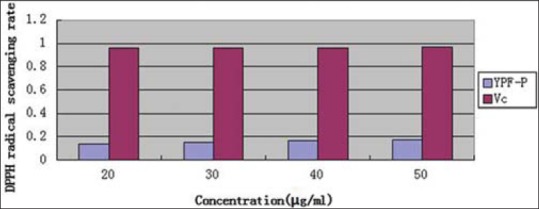
Comparison of 1,1-diphenyl-2-picrylhydrazyl radical scavenging rate of refined Yupingfeng powder and Vc
Regression equation of YPF-P concentration and DPPH radical scavenging rate was obtained as y = 0.0015x + 0.1069, R2 = 0.9783 [Figure 8]. So when scavenging rate reached 50%, IC50 = 0.262 mg/ml.
Figure 8.
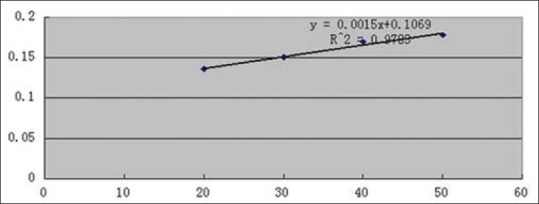
Regression equation of Yupingfeng powder concentration and 1,1-diphenyl-2-picrylhydrazyl radical scavenging rate
Ferric reducing antioxidant power assay result
Comparison of FRAP value of refined YPF-P and Vc was made as Figure 9 shows. In this assay, it could be concluded that compared to Vc, refined YPF-P had a much lower antioxidant ability.
Figure 9.
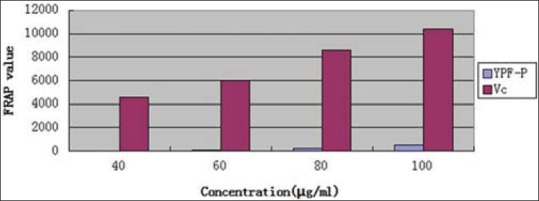
Comparison of ferric reducing antioxidant power value of refined Yupingfeng powder and Vc
CONCLUSION
In this study, the optimization of microwave-assisted reflux extraction of polysaccharides from Yupingfeng Powder and evaluation of antioxidant activity were conducted. The crude YPF-P was obtained by 30–60% ethanol precipitation. Single factor effect trends were achieved through yields and contends of YPF-P obtained from different extracting conditions. Then through RSM of yield, the optimal conditions were determined as follows: Material/solvent ratio 1:23.37, microwave power 560 W, extraction temperature 64°C, and extraction time 9.62 min. Under the optimal conditions, the YPF-P yield was 3.23%, and its content was 38.52%. Refined YPF-P was proved to contain antioxidant ability through both DPPH and FRAP assays.
ACKNOWLEDGMENTS
This research was funded by NSFC (No. 81001383) and Applied Basic Research Programs of Science and Technology Department of Sichuan Province (No. 2012JY0035).
Footnotes
Source of Support: This research was funded by NSFC (No.81001383) and Applied Basic Research Programs of Science and Technology Department of Sichuan Province (No. 2012JY0035)
Conflict of Interest: None declared.
REFERENCES
- 1.Gao J, Li J, Shao X, Jin Y, Lü XW, Ge JF, et al. Antiinflammatory and immunoregulatory effects of total glucosides of Yupingfeng powder. Chin Med J (Engl) 2009;122:1636–41. [PubMed] [Google Scholar]
- 2.Zhao C, Li M, Luo Y, Wu W. Isolation and structural characterization of an immunostimulating polysaccharide from fuzi, Aconitum carmichaeli. Carbohydr Res. 2006;341:485–91. doi: 10.1016/j.carres.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 3.Yin JY, Nie SP, Zhou C, Wan Y, Xie MY. Chemical characteristics and antioxidant activities of polysaccharide purified from the seeds of Plantago asiatica L. J Sci Food Agric. 2010;90:210–7. doi: 10.1002/jsfa.3793. [DOI] [PubMed] [Google Scholar]
- 4.Yan JK, Wang WQ, Wu JY. Recent advances in Cordyceps sinensis polysaccharides: Mycelial fermentation, isolation, structure, and bioactivities: A review. J Funct Foods. 2014;6:33. doi: 10.1016/j.jff.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Wang M, Ling Y, Fan W, Wang Y, Yin H. Structural determination and antioxidant activity of a polysaccharide from the fruiting bodies of cultured Cordyceps sinensis. Am J Chin Med. 2009;37:977–89. doi: 10.1142/S0192415X09007387. [DOI] [PubMed] [Google Scholar]
- 6.Jing Y, Huang L, Lv W, Tong H, Song L, Hu X, et al. Structural characterization of a novel polysaccharide from pulp tissues of Litchi chinensis and its immunomodulatory activity. J Agric Food Chem. 2014;62:902–11. doi: 10.1021/jf404752c. [DOI] [PubMed] [Google Scholar]
- 7.Rioux LE, Turgeon SL, Beaulieu M. Characterization of polysaccharides extracted from brown seaweeds. Carbohydr Polym. 2007;69:530–7. [Google Scholar]
- 8.Yu RM, Yin Y, Yang W, Ma WL, Yang L, Chen XJ, et al. Structural elucidation and biological activity of a novel polysaccharide by alkaline extraction from cultured Cordyceps militaris. Carbohydr Polym. 2009;75:166–71. [Google Scholar]
- 9.Júnior DL, Ayoub A, Venditti RA, Jameel H, Colodette JL, Chang HM. Ethanol precipitation of hetero-polysaccharide material from hardwood by alkaline extraction prior to the Kraft cooking process. Bioresources. 2013;8:5319–32. [Google Scholar]
- 10.Deng Q, Zhou X, Chen H. Optimization of enzyme assisted extraction of Fructus mori polysaccharides and its activities on antioxidant and alcohol dehydrogenase. Carbohydr Polym. 2014;111:775–82. doi: 10.1016/j.carbpol.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 11.Majdoub H, Ben Mansour M, Chaubet F, Roudesli MS, Maaroufi RM. Anticoagulant activity of a sulfated polysaccharide from the green alga Arthrospira platensis. Biochim Biophys Acta. 2009;1790:1377–81. doi: 10.1016/j.bbagen.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Wang ZJ, Luo DH. Antioxidant activities of different fractions of polysaccharide purified from Gynostemma pentaphyllum Makino. Carbohydr Polym. 2007;68:54–8. [Google Scholar]
- 13.Wang Y, Liu Y, Hu Y. Optimization of polysaccharides extraction from Trametes robiniophila and its antioxidant activities. Carbohydr Polym. 2014;111:324–32. doi: 10.1016/j.carbpol.2014.03.083. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Gu X, Huang SQ, Li J, Wang X, Tang J. Optimization of ultrasonic/microwave assisted extraction (UMAE) of polysaccharides from Inonotus obliquus and evaluation of its anti-tumor activities. Int J Biol Macromol. 2010;46:429–35. doi: 10.1016/j.ijbiomac.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Wang JL, Zhang J, Zhao BT, Wang XF, Wu YQ, Yao J. A comparison study on microwave-assisted extraction of Potentilla anserina L. polysaccharides with conventional method: Molecule weight and antioxidant activities evaluation. Carbohydr Polym. 2010;80:84–93. [Google Scholar]
- 16.Xie JH, Xie MY, Shen MY, Nie SP, Li C, Wang YX. Optimisation of microwave-assisted extraction of polysaccharides from Cyclocarya paliurus (Batal.) Iljinskaja using response surface methodology. J Sci Food Agric. 2010;90:1353–60. doi: 10.1002/jsfa.3935. [DOI] [PubMed] [Google Scholar]
- 17.Xu DJ, Xia Q, Wang JJ, Wang PP. Molecular weight and monosaccharide composition of Astragalus polysaccharides. Molecules. 2008;13:2408–15. doi: 10.3390/molecules13102408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma XQ, Shi Q, Duan JA, Dong TT, Tsim KW. Chemical analysis of Radix Astragali (Huangqi) in China: A comparison with its adulterants and seasonal variations. J Agric Food Chem. 2002;50:4861–6. doi: 10.1021/jf0202279. [DOI] [PubMed] [Google Scholar]
- 19.Ferreira SL, Bruns RE, Ferreira HS, Matos GD, David JM, Brandão GC, et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal Chim Acta. 2007;597:179–86. doi: 10.1016/j.aca.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Thirugnanasambandham K, Sivakumar V, Maran JP. microwave-assisted extraction of polysaccharides from mulberry leaves. Int J Biol Macromol. 2015;72:1–5. doi: 10.1016/j.ijbiomac.2014.07.031. [DOI] [PubMed] [Google Scholar]
- 21.Yang B, Zhao MM, Shi J, Yang N, Jiang YM. Effect of ultrasonic treatment on the recovery and DPPH radical scavenging activity of polysaccharides from longan fruit pericarp. Food Chemistry. 2008;106:685–90. [Google Scholar]
- 22.Kriengsak T, Unaroj B, Kevin C, Luis CZ, David HB. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Compost Anal. 2006;19:669–75. [Google Scholar]
- 23.Ge Q, Huang J, Mao JW, Gong JY, Zhou YF, Huang JX. Optimization of total polysaccharide extraction from Herba Lophatheri using RSM and antioxidant activities. Int J Biol Macromol. 2014;67:37–42. doi: 10.1016/j.ijbiomac.2014.02.055. [DOI] [PubMed] [Google Scholar]
- 24.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 25.Wang ZB, Pei JJ, Ma HL, Cai PF, Yan JK. Effect of extraction media on preliminary characterizations and antioxidant activities of Phellinus linteus polysaccharides. Carbohydr Polym. 2014;109:49–55. doi: 10.1016/j.carbpol.2014.03.057. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Huang Y, Hou T, Wang Y. Hypoglycaemic effect of Artemisia sphaerocephala Krasch seed polysaccharide in alloxan-induced diabetic rats. Swiss Med Wkly. 2006;136:529–32. doi: 10.4414/smw.2006.11348. [DOI] [PubMed] [Google Scholar]


