Abstract
Background:
Parawixia bistriata is a semi-colonial spider found mainly in southeastern of Brazil. Parawixin 10 (Pwx 10) a compound isolated from this spider venom has been demonstrated to act as neuroprotective in models of injury regulating the glutamatergic neurotransmission through glutamate transporters.
Objectives:
The aim of this work was to evaluate the neuroprotective effect of Pwx 10 in a rat model of excitotoxic brain injury by N-methyl-D-aspartate (NMDA) injection.
Material and Methods:
Male Wistar rats have been used, submitted to stereotaxic surgery for saline or NMDA microinjection into dorsal hippocampus. Two groups of animals were treated with Pwx 10. These treated groups received a daily injection of the Pwx 10 (2.5 mg/μL) in the right lateral ventricle into rats pretreated with NMDA, always at the same time, each one starting the treatment 1 h or 24 h. Nissl staining was performed for evaluating the extension and efficacy of the NMDA injury and the neuroprotective effect of Pwx 10.
Results:
The treatment with Pwx 10 showed neuroprotective effect, being most pronounced when the compound was administrated from 1 h after NMDA in all hippocampal subfields analyzed (CA1, CA3 and hilus).
Conclusion:
These results indicated that Pwx 10 may be a good template to develop therapeutic drugs for treating neurodegenerative diseases, reinforcing the importance of continuing studies on its effects in the central nervous system.
Keywords: Excitotoxicity, glutamate, N-methyl-D aspartate, parawixia bistriata, spider venom
INTRODUCTION
Parawixia bistriata is a semi-colonial spider found in Central America and South America, mainly in South Eastern of Brazil.[1] This spider has habits of nocturnal hunting and diurnal quiescence, trooping in nests.
Some studies with the venom of P. bistriata using synaptosomes prepared from rat cerebral cortex (SRCC) demonstrated that the denatured venom causes a potent inhibition of the uptake of gama-aminobutyric acid (GABA) and greatly stimulates uptake of L-glutamate (L-Glu).[2]
In bioassays using termites (Sintermes sp), the crude venom of P. bistriata induced an irreversible paralysis caused dose-dependent manner when injected rectally.[3] Intracerebroventricular injection (icv) of the venom of this spider, as well as more pure fractions abolished tonic-clonic seizures induced by injection of picrotoxin, pentilenetetrazole (PTZ) and bicuculline in rats.[4]
After the separation process using high performance liquid chromatography (HPLC), a fraction of the venom of P. bistriata, now called FrPbAII, demonstrated to be a potent inhibitor of GABA uptake in SRCC as well as in synaptosomes prepared from rat retinas.[5] Other results have shown that icv injection of FrPbAII significantly inhibit seizures induced by bicuculline in rats.[4] This compound was neuroprotective in a rat model of experimental glaucoma and in ischemia/reperfusion injury in rat retinal model, reducing cell death and maintain normal morphology of all the layers of retinal cells.[6] Other studies have shown that this compound also has anticonvulsant and anxiolytic effects when injected into the rat hippocampus and tested on elevated plus maze.[7]
Beleboni et al.,[6] showed that FrPbAII isolated from the venom of P. bistriata, acts directly and primarily on GABA and glycine transporters, a feature that makes it an important tool in the study of neuroprotection.
Parawixin 1 also purified from the venom of P. bistriata by our group stimulates the uptake of L-Glu in SRCC in a dose-dependent manner, and appears to mediate this effect by increasing the Vmax for the uptake of L-Glu with any change in the Km.[2] This finding suggests that the active compound may act by increasing the conduction or alternatively increasing the number of functional carrier molecules at the cell surface. We also observed a neuroprotective effect of Parawixin 1 in both ischemia and ischemia/reperfusion retinas in experimental glaucoma rat model. When tested in immortalized cells derived from African monkey kidney (COS cells), which expressing subtypes of glutamate transporters, this compound stimulated an increase in uptake of L-Glu by subtype excitatory amino acid transporter type 2 (EAAT2).[2] Fontana et al.,[8] also reported that this compound promotes a direct increase of influx of L-Glu by subtype transporter EEAT2 through a mechanism that does not alter the apparent affinity for both co-substrates L-Glu and sodium.
Furthermore, another compound has been isolated from the spider P. bistriata called Parawixin 10 (Pwx 10). It has anticonvulsant effect in rats submitted to acute chemical induction of seizures by kainic acid and N-methyl-D-aspartate (NMDA) (icv) and PTZ (ip). Furthermore, Pwx 10 caused a considerable increase in the uptake of L-Glu and glycine using SRCC and also stimulated the release of GABA.[9]
In recent decades, great efforts have been employed in an attempt to discover new substances or associations between existing substances that could be more effective to block processes of nerve tissue injury, such as chronic neurodegenerative diseases, trauma, status epilepticus (SE) and ischemic processes, which resulting from vascular changes in animal models or clinical trials.[10,11] However, so far no drug or politherapeutical scheme could block the death of neurons arising from these injuries. Accordingly, the use of animal toxins which acting on the central nervous system (CNS) can be a good template to improve the development of new compounds used as tools for the study of disease processes or as models for search for new therapeutic agents co-adjuvants.
Once demonstrated the action of spider toxins from P. bistriata in the transport of classical neurotransmitters in the CNS involved in brain changes induced by different injuries, it is important to check the activity of these toxins and their possible neuroprotective effects in different models of brain injury, such as excitotoxicity induced by NMDA microinjection into the dorsal hippocampus of rats.
The present study was designed to extend our earlier work about Pwx 10 and study the neuroprotective potential of Pwx 10 in a model of excitotoxic injury induced by microinjection of NMDA agonist in the dorsal hippocampus of rats.
Material and methods
Spider collection and venom fractionation
Spider collection and venom fractionation was performed according to Fachim et al.,[9] as well as the purification of the venom fractions. Briefly, specimens of P. bistriata were collected in the Ribeirão Preto region (São Paulo, Brazil), taken to the laboratory were frozen at −4°C, and kept at −20°C. Glands and venom reservoirs were homogenized in acetonitrile/milli-Q water (1:1; v/v), and centrifuged at 8000.g for 10 min at 4°C. Supernatants were collected and filtered in membranes with a 3000-Da cut-off (Millipore, Microcon, USA) under centrifugation at 8000.g and 4°C until complete filtration. Next, the extract was lyophilized, weighed, and submitted to fractionation.
Dry extract was dissolved in ultrapure water of milli-Q grade containing 0.1% trifluoroacetic acid (TFA). The solution was subjected to reverse-phase HPLC (Shimadzu, Japan) using an octadecylsilyl C18 column (15 μm, 20 mm × 250 mm; Phenomenex-Jupiter, USA) at the flow rate of 8.0 mL/min and monitored at 214 nm. Firstly, an isocratic gradient was run with 1% CH3 CN: H2O (v/v) containing 0.1% TFA for 10 min. Next, a linear gradient from 1% to 60% CH3 CN for 60 min was performed. Fractions were collected, lyophilized, weighed, and used in bioassays.[9]
Purity of Pwx 10 was verified by electrospray ionization mass spectra (ESI-MS). Dry fractions were dissolved in 50% CH3 CN: H2O (v/v) containing 0.1% formic acid (v/v). Molecular masses were determined by positive electrospray ionization (ESI+) on a high-resolution spectrometer. Fractions were injected with the aid of an infusion pump at a flow of 10 kL/min. ESI-MS spectra were acquired on an UltrOTOF apparatus (Bruker Daltonics, Billerica, USA) in the continuous acquisition mode, scanning from 50 m/z to 2000 m/z with a scan time of 5 s.[9]
Animals and housing
Male Wistar rats (220–250 g), from the animal house of the University of São Paulo were used. The animals were allocated in pairs in a temperature-controlled room (23°C ± 1°C), on a 12:12-h light: Dark cycle (lights on at 7:00 a.m.) with food and water ad libitum. Animal handling and all experiments were performed according to the Brazilian Society of Neuroscience and Behavior guidelines for care and use of laboratory animals, and all efforts were made to minimize animal suffering. This study was approved by the Ethics Committee for Care and Use of Laboratory Animals of the University of Sao Paulo, campus Ribeirão Preto (CEUA nr. 10.1.619.53.3).
Surgery, N-methyl-D-aspartate intrahippocampal injection and treatment with Parawixin 10
To evaluate the neuroprotective potential of Pwx 10, the following animal groups were used: (1) Saline 1-week, (2) NMDA 1-week, (3) NMDA 1-week treated with Pwx 10 1 h after surgery and (4) NMDA 1 week treated with Pwx 10 24 h after surgery; (n = 06/each).
Each group was sacrificed at designated period of time after receiving the injection of saline or NMDA by via intrahippocampal. The Pwx 10 treated groups received a daily injection of the Pwx 10 (2.5 mg/μL) in the right lateral ventricle of 1-week, always at the same time, each one starting the treatment 1 h or or 24 h after injection of NMDA. The concentration of Pwx 10 was chosen according to the ED50 for protection of acute seizures induced in rats by NMDA.[9]
Histological procedures and quantification
After the behavioral test, all animals were anesthetized (urethane, 25%, Sigma) and killed by perfusion with intracardiac infusion of 0.1M phosphate buffered saline (PBS), pH 7.4, followed by 4% paraformaldehyde in PBS. The brains were rapidly removed and soaked in the same fixative solution for 2 h (4°C) and then cryoprotected by soaking in 30% sucrose in PBS for 48–72 h (4°C). Brains were frozen in isopentane cooled in dry ice (−40°C, Sigma) and stored at −70°C until sectioning. 24 h before sectioning the brains were transferred to a −20°C freezer. Selection of anatomical levels for sectioning was conducted with reference to illustrations from Paxinos and Watson (1986). Transverse sections (30 μm) containing hippocampus were obtained in a cryostat (−20°C, Leica) and processed for Nissl staining (3 section/animal from control and NMDA 2 and 4 weeks) or immunohistochemistry (3 sections/animal, all groups).
Histological sections were observed and the subfields of the hippocampus (CA1, CA3 and hilus dendate gyrus [HDG]) were captured in a light microscope with a digital camera (DFC300 FX, Leica) for further quantification using computerized image analysis software (QWin Plus, Leica).
Statistical analysis
The data from Nissl experiments were compared by analysis of variance (one-way ANOVA) followed by Newman–Keuls, both using Graph Prism software (version 4.0, GraphPad Software, USA). The significance level was set at P ≤ 0.05.
RESULTS
Histological evaluation of injury induced by N-methyl-D-aspartate in the hippocampus after treatment with Parawixin 10
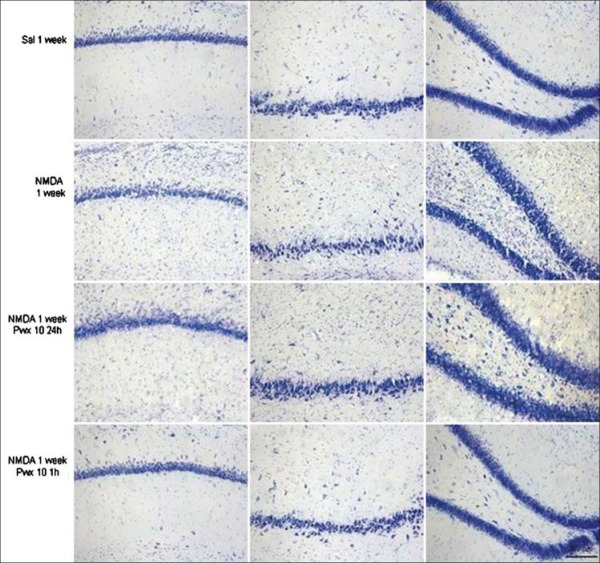
After treatment with Pwx 10, it was observed that both groups (24 h and 1 h after injection of NMDA), there were a decrease of glial proliferation in all hippocampal subfields studied, as well as the preservation of cell layers if compared to the NMDA treated group [Figure 1], and keeping the same characteristics as the control group. This effect was especially observed in the animals who received Pwx 10 starting 1 h after the microinjection of NMDA. This qualitative analysis can be confirmed by the quantitative data that are shown in Figure 2.
Figure 1.
Nissl staining in subfields CA1, CA3 and hilus dendate gyrus control groups (saline), injected with N-methyl-D-aspartate (NMDA) and injected with NMDA and treated with Parawixin 10 in each study period (1 h and 24 h). 200 µm
Figure 2.
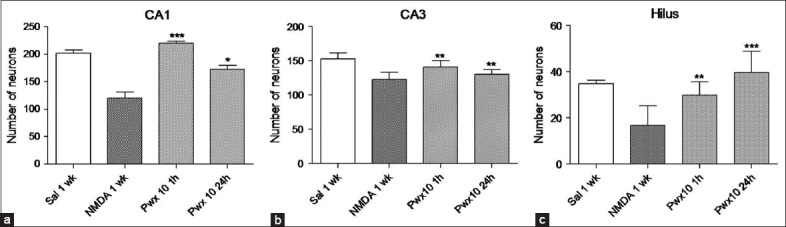
(a) Number of neurons in the CA1 subfield in animals in groups: Saline 1-week, N-methyl-D-aspartate (NMDA) 1-week, Parawixin 10 (Pwx 10) 1 h and Pwx 10 24 h. (b) Number of neurons in the CA3 subfield in animals in groups: Saline 1-week, NMDA 1-week, Pwx 10 1 h and Pwx 10 24 h. (c) Number of neurons in the hilus dendate gyrus subfield in animals in groups: Saline 1-week, NMDA 1-week, Pwx 10 1 h and Pwx 10 24 h. The values are represented as mean ± SEM of the number of cells quantified bilaterally into three sections/animal (n = 6). *P < 0.05, **P < 0.001; *** P < 0.0001 (one-way ANOVA followed by Newman–Keuls)
In the CA1 subfield [Figure 2a], as expected, was observed a decrease in the number of Nissl stained cells in the NMDA treated animals when compared with saline treated animals. However, when the both groups treated with Pwx 10 1 h or 24 h after NMDA were compared with saline-treated group we observed the neuroprotective effect of the spider toxin compound, since no significant differences were detected among them [Figure 2a]. When Pwx 10 treated animals were compared to NMDA treated animals, an increase in the number of Nissl stained cells in both Pwx 10 treated groups, these results confirm the preservation of the cells layer. This increase was 44% in the group that received Pwx 10 24 h after NMDA microinjection and 83% in the group that received Pwx 10 after 1 h (F3,24 =37.39; P < 0.001) [Figure 2a].
In the CA3 subfield [Figure 2b] we observed similar results to those observed in CA1 that is, the preservation of cell layer by Pwx 10 treatment. When the groups treated with Pwx 10 were compared to NMDA treated group, was observed an increase in the number of cells stained with Nissl, although this increase was less robust than as observed in CA1, it was 6% in Pwx 10 treated group after 24 h and 14% in the Pwx 10 treated group after 1 h (F3,24= 12.52, P < 0.0001) [Figure 2b].
In HDG subfield [Figure 2c] the results followed the same profile that was observed in CA1. The Pwx 10 treated groups showed no significant difference if compared to the saline treated group, which shows the neuroprotective effect of Pwx 10. This effect is confirmed by the significant increase in the number of Nissl stained neurons in Pwx 10 treated groups compared to the NMDA treated group. This increase was 81% in the Pwx 10 treated group 1 h after NMDA microinjection and 143% in the Pwx 10 treated group 24 h after NMDA microinjection (F3,24 = 13.18, P < 0.0001) [Figure 2c].
DISCUSSION
Many neurotoxins exhibit high affinity and selectivity for receptors, transporters and neuronal or glial ion channels[12] among them are the venoms of spiders that are considered important sources of neuroactive molecules, that acting specifically on different types of ion channels.[13] These molecules have a great potential for the study of neuroscience, which act as pre- and post-synaptically, and can modulate the neurotransmission.[13]
A detailed analysis of the structure and activity of neuroactive molecules allows a better understanding of the organization and functioning of the CNS. Also enables the development of new drugs for the treatment of CNS disorders.[12]
In view of the neuroprotective potential of Pwx 10 isolated and characterized in our laboratory[9] and its effect on the glutamatergic system, in this study this molecule was used in order to treat and/or ameliorate the injury caused by NMDA in the rat hippocampus. In an attempt to visualize its neuroprotective effect, it was administered a daily dose of Pwx 10, in a 1-week period in rats given intrahippocampal injection of NMDA.
According to our results, we observed that the treatment with Pwx 10 was effective in this type of injury, since the number of cells in the evaluated regions after NMDA injection and treated with Pwx 10 to increase significantly when compared with the animals injected with NMDA and untreated. These data add further evidence to the therapeutic potential of Pwx 10, since in our previous findings was demonstrated that the Pwx 10 has anticonvulsant potential to block the occurrence of acute seizures induced by NMDA, PTZ, and kainic acid (KA) in rats.[9] Therefore, besides the anticonvulsant effect in the induction of acute seizures model also has neuroprotective potential in the excitotoxic model used in this work.
This effect can be attributed to the action of Pwx 10 in glutamate transport, since at low concentrations neurotoxin that increases the uptake of this neurotransmitter in SRCC,[9] making this excess of neurotransmitter provoked by the NMDA injection to be attenuated by acting in the transport mechanism of this excitatory amino acid. Similar activity, with activities on the glutamatergic transport, it was found in a compound of the P. bistriata the Parawixina 1, which promoted an increased influx through glutamate transporter EAAT2 in liposomes and COS-7 cells directly and selectively, through a mechanism that does not alter the affinity of its co-substrates, sodium and LGlu not thereby increasing the reverse transport.[8]
Furthermore, the neuroprotection found after treatment with Pwx 10 may be due to the period in which treatment was initiated, since with 1 h and 24 h after NMDA injection the mechanisms of neuronal death triggered by excitotoxicity may have been blocked or minimized by Pwx 10. Evidence-based literature have shown that neuronal death after an insult varies according to brain region studied and the cell type examined.[14,15]
The evidence showed that peak neuronal death (cells positive for Fluoro-Jade) in HDG and dendate gyrus occurred between 3 h and 12 h after SE in rats, while the most damage in pyramidal neurons in subfields CA1 and CA3 were visualized after 1-week. Moreover, recent results performed in our laboratory concerning the gliosis resulting from the NMDA-induced injury showed a significant increase of glial fibrillary acidic protein-positive cells in the CA1 and CA3 layer after 1-week (paper in elaboration).
The mechanism by which Pwx 10 acts on glutamate transport remains unknown and more studies are required to clarify it. As the Pwx 10, further spider neurotoxins have affinity for receptors and ion channels in the CNS, among them we have the argiotoxin 636, a noncompetitive NMDA antagonist,[16] JSTX-3 (an analogue of Jorotoxin) that blocks epileptiform activity in both rat hippocampal sections as human.[17] Some toxins from the spider Phoneutria nigriventer (PhTx-3, TX 3-3, TX 3-4) were neuroprotective in the induction of a model of retinal ischemia[18] and PhTx-56, an analogue of philantotoxina-433 that acts as potent and selective antagonist of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors[19] and transport of glutamate by inhibiting its reuptake dose-dependent manner.
Given the association of cell death attributed to glutamate excitotoxicity with stroke, head trauma, ischemia, among other insults,[20] several alternatives have been studied in an attempt to attenuate neuronal damage, by reducing the function is glutamate receptor, or modulating its transport. In animal models of ischemia, it has been speculated that ischemic insult triggers a decrease in the GluR2 subunit causing an increase in the permeability of Ca2+ through AMPA receptors, eventually leading to neuronal cell death. Interestingly, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs)-permeable are highly expressed in CA1 pyramidal neurons, and this area of the hippocampus is more vulnerable to cell death after an ischemic insult or seizures than other regions of the hippocampus for review see Chang et al., 2012.[21] Some antagonists of AMPARs appear to be more effective in preventing neuronal than antagonists of NMDA receptors death, highlighting the importance of modulation of these receptors as therapeutic targets. In this context polyamines, are important modulators of AMPARs, since when the GluR2 subunit is absent from its composition, this receptor becomes sensitive to blockage by polyamines.[22] In addition, NMDA receptors have in its structure a site for polyamines located in the extracellular region of the NR2B subunit. The binding of polyamines that site has regulatory action, which may contribute to or protect cells from excitotoxic damage.[23]
Among the isolated toxins of spiders that act on glutamatergic transmission, are the polyamines or amides acilpolyamines.[24] The investigations on the pharmacological effects of polyamines present in toxins have shown that these compounds interact with several important targets in insects, in both the periphery and CNS as well as in the CNS of mammals.[24] Since glutamate is the first chemical messenger present at neuromuscular junctions of insects, it would be no surprise that iGluRs insects are the main targets of polyamines present in toxins.[25] These compounds are present in the venom of Nephila clavata and Argiope lobata, among others[26] and are noncompetitive antagonists of glutamatergic NMDA, AMPA and KA receptors.[25]
Given that GluRs are involved in the pathophysiology of epilepsy as well as in seizures,[27] blockade of these receptors by polyamines theoretically produce an anticonvulsant and/or neuroprotective effects. In this context, there are reports that polyamines have anticonvulsant effects in models of induced seizures. Jackson and Parks[28] observed that a poliaminic fraction (called AG2) isolated from the spider Agelenopsis aperta alleviated or suppressed seizures induced by injection of kainic acid, bicuculline and picrotoxin. The 1-naftilacetil spermine, which is a synthetic derivative of polyamine AG2, showed anticonvulsive effect on kindling model (“kindling”) in rats when injected into the lateral ventricle.[29]
Until now the structure of Pwx 10 not characterized, however there is evidence based on previous studies, considering its fractionation and mass spectrometry, suggesting that it may be a polyamine. This explains in part its neuroprotective activity, since at low concentrations the polyamines can exert anticonvulsant and neuroprotective activities.[30] However, high doses of polyamines can cause deficits in memory and learning functions. Shimada et al.,[31] observed that rats treated with spermidine and were submitted to a memory test on the elevated plus maze had presented deficits in cognitive functions. Furthermore, it is worth mentioning that the blocking of polyamines found in venoms of spiders and wasps by type AMPA receptors may differ according to the constitution of this receptor, which exhibits properties of different permeability due to lack of GluR2 subunit. In this particular model, we note that after 24 h and 1-week of injection of NMDA (at what stage of the treatment was started with the Pwx 10) there is a sharp decrease of GluR2 subunits, making the environment more sensitive to blockade by polyamines (paper in elaboration). Thus, the Pwx 10 could act in blocking Ca2+-permeable AMPARs, thereby decreasing glutamatergic excitotoxicity, justifying its neuroprotective effect in this model.
In addition to our results, a parallel study is being conducted in our laboratory using the Pwx 10 as well with interesting results. When administered chronically in a rat model of pilocarpine-induced seizures, the Pwx 10 has demonstrated anticonvulsant and neuroprotective effect, increasing the latency of recurrent seizures and diminishing the duration and severity average of those. In addition, the treatment with Pwx 10 showed no behavioral deficits in treated rats when exposed to Morris water maze (unpublished data).
These results are additional evidence that Pwx 10 has a neuroprotective potential, reinforcing the importance of continuing studies on its effects in the CNS. These studies may provide new tools for the investigation of pathological processes that contribute to the clarification of excitotoxic mechanisms, as well as new alternatives for the exploration of drugs that could act in the treatment of neurodegenerative diseases of the CNS.
CONCLUSION
These results indicated that Pwx 10 has a great therapeutic and neuroprotective potential, reinforcing the importance of continuing studies on its effects in the CNS.
ACKNOWLEDGMENT
Financial support of Fapesp, CNPq and CAPES from Brazil.
Footnotes
Source of Support: Financial support of Fapesp, CNPq and CAPES from Brazil
Conflict of Interest: None declared.
REFERENCES
- 1.Levi HW. The orbweavers of the genera Molinaranea and Nicolepeira, a new species of Parawixia, and comments on orb weavers of temperate South America (Araneae: Araneidae) [Last accessed on 2014 Dec];Bull Mus Comp Zool. 2001 155:445–75. Available from: http://www.biodiversitylibrary.org/page/4272540#page/493/mode/1up . [Google Scholar]
- 2.Fontana AC, Guizzo R, de Oliveira Beleboni R, Meirelles E Silva AR, Coimbra NC, Amara SG, et al. Purification of a neuroprotective component of Parawixia bistriata spider venom that enhances glutamate uptake. Br J Pharmacol. 2003;139:1297–309. doi: 10.1038/sj.bjp.0705352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pizzo AB, Fontana AC, Coutinho-Netto J, dos Santos WF. Effects of the crude venom of the social wasp Agelaia vicina on gamma-aminobutyric acid and glutamate uptake in synaptosomes from rat cerebral cortex. J Biochem Mol Toxicol. 2000;14:88–94. doi: 10.1002/(sici)1099-0461(2000)14:2<88::aid-jbt4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 4.Cairrão MA, Ribeiro AM, Pizzo AB, Fontana AC, Beleboni RO, Coutinho-Netto J, et al. Anticonvulsant and GABA uptake inhibition properties of venom fractions from the spiders parawixia bistriata and scaptocosa raptoria. [Last cited on 2013 Jan 24];Pharm Biol. 2002 40:472–7. Available from http://www.informahealthcare.com/doi/abs/10.1076/phbi.40.6.472.8436 . [Google Scholar]
- 5.Beleboni RO, Carolino RO, Pizzo AB, Castellan-Baldan L, Coutinho-Netto J, dos Santos WF, et al. Pharmacological and biochemical aspects of GABAergic neurotransmission: Pathological and neuropsychobiological relationships. Cell Mol Neurobiol. 2004;24:707–28. doi: 10.1007/s10571-004-6913-z. [DOI] [PubMed] [Google Scholar]
- 6.Beleboni RO, Guizzo R, Fontana AC, Pizzo AB, Carolino RO, Gobbo-Neto L, et al. Neurochemical characterization of a neuroprotective compound from Parawixia bistriata spider venom that inhibits synaptosomal uptake of GABA and glycine. Mol Pharmacol. 2006;69:1998–2006. doi: 10.1124/mol.105.017319. [DOI] [PubMed] [Google Scholar]
- 7.Liberato JL, Cunha AO, Mortari MR, Gelfuso EA, Beleboni Rde O, Coutinho-Netto J, et al. Anticonvulsant and anxiolytic activity of FrPbAII, a novel GABA uptake inhibitor isolated from the venom of the social spider Parawixia bistriata (Araneidae: Araneae) Brain Res. 2006;1124:19–27. doi: 10.1016/j.brainres.2006.09.052. [DOI] [PubMed] [Google Scholar]
- 8.Fontana AC, de Oliveira Beleboni R, Wojewodzic MW, Ferreira Dos Santos W, Coutinho-Netto J, Grutle NJ, et al. Enhancing glutamate transport: Mechanism of action of Parawixin1, a neuroprotective compound from Parawixia bistriata spider venom. Mol Pharmacol. 2007;72:1228–37. doi: 10.1124/mol.107.037127. [DOI] [PubMed] [Google Scholar]
- 9.Fachim HA, Cunha AO, Pereira AC, Beleboni RO, Gobbo-Neto L, Lopes NP, et al. Neurobiological activity of Parawixin 10, a novel anticonvulsant compound isolated from Parawixia bistriata spider venom (Araneidae: Araneae) Epilepsy Behav. 2011;22:158–64. doi: 10.1016/j.yebeh.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Löscher W. Basic pharmacology of valproate: A review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs. 2002;16:669–94. doi: 10.2165/00023210-200216100-00003. [DOI] [PubMed] [Google Scholar]
- 11.Pitkänen A, Sutula TP. Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. Lancet Neurol. 2002;1:173–81. doi: 10.1016/s1474-4422(02)00073-x. [DOI] [PubMed] [Google Scholar]
- 12.Meinwald J, Eisner T, editors. The Chemistry of Phyletic Dominance. Proceedings of the National Academy of Sciences of the United States of America. [Last accessed on 2014 Dec];National Academy of Sciences. 1995 92:14–8. doi: 10.1073/pnas.92.1.14. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC42809/pdf/pnas01479-0030.pdf . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rash LD, Hodgson WC. Pharmacology and biochemistry of spider venoms. Toxicon. 2002;40:225–54. doi: 10.1016/s0041-0101(01)00199-4. [DOI] [PubMed] [Google Scholar]
- 14.do Nascimento AL, Dos Santos NF, Campos Pelágio F, Aparecida Teixeira S, de Moraes Ferrari EA, Langone F. Neuronal degeneration and gliosis time-course in the mouse hippocampal formation after pilocarpine-induced status epilepticus. Brain Res. 2012;1470:98–110. doi: 10.1016/j.brainres.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Fujikawa DG. The temporal evolution of neuronal damage from pilocarpine-induced status epilepticus. Brain Res. 1996;725:11–22. doi: 10.1016/0006-8993(96)00203-x. [DOI] [PubMed] [Google Scholar]
- 16.Moe ST, Smith DL, Chien Y, Raszkiewicz JL, Artman LD, Mueller AL. Design, synthesis, and biological evaluation of spider toxin (argiotoxin-636) analogs as NMDA receptor antagonists. Pharm Res. 1998;15:31–8. doi: 10.1023/a:1011988317683. [DOI] [PubMed] [Google Scholar]
- 17.Salamoni SD, da Costa JC, Palma MS, Konno K, Nihei K, Azambuja NA, et al. The antiepileptic activity of JSTX-3 is mediated by N-methyl-D aspartate receptors in human hippocampal neurons. Neuroreport. 2005;16:1869–73. doi: 10.1097/01.wnr.0000185012.98821.2b. [DOI] [PubMed] [Google Scholar]
- 18.Agostini RM, do Nascimento Pinheiro AC, Binda NS, Romano Silva MA, do Nascimento Cordeiro M, Richardson M, et al. Phoneutria spider toxins block ischemia-induced glutamate release and neuronal death of cell layers of the retina. Retina. 2011;31:1392–9. doi: 10.1097/IAE.0b013e318205b249. [DOI] [PubMed] [Google Scholar]
- 19.Andersen TF, Vogensen SB, Jensen LS, Knapp KM, Strømgaard K. Design and synthesis of labeled analogs of PhTX-56, a potent and selective AMPA receptor antagonist. Bioorg Med Chem. 2005;13:5104–12. doi: 10.1016/j.bmc.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 20.Niciu MJ, Kelmendi B, Sanacora G. Overview of glutamatergic neurotransmission in the nervous system. Pharmacol Biochem Behav. 2012;100:656–64. doi: 10.1016/j.pbb.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang PK, Verbich D, McKinney RA. AMPA receptors as drug targets in neurological disease – Advantages, caveats, and future outlook. Eur J Neurosci. 2012;35:1908–16. doi: 10.1111/j.1460-9568.2012.08165.x. [DOI] [PubMed] [Google Scholar]
- 22.Bowie D. Redefining the classification of AMPA-selective ionotropic glutamate receptors. J Physiol. 2012;590:49–61. doi: 10.1113/jphysiol.2011.221689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozawa S, Kamiya H, Tsuzuki K. Glutamate receptors in the mammalian central nervous system. Prog Neurobiol. 1998;54:581–618. doi: 10.1016/s0301-0082(97)00085-3. [DOI] [PubMed] [Google Scholar]
- 24.Strømgaard K. Natural products as tools for studies of ligand-gated ion channels. Chem Rec. 2005;5:229–39. doi: 10.1002/tcr.20048. [DOI] [PubMed] [Google Scholar]
- 25.Mellor IR, Usherwood PN. Targeting ionotropic receptors with polyamine-containing toxins. Toxicon. 2004;43:493–508. doi: 10.1016/j.toxicon.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Kawai N, Niwa A, Abe T. Spider venom contains specific receptor blocker of glutaminergic synapses. Brain Res. 1982;247:169–71. doi: 10.1016/0006-8993(82)91044-7. [DOI] [PubMed] [Google Scholar]
- 27.Hu Y, Jiang L, Chen H, Zhang X. Expression of AMPA receptor subunits in hippocampus after status convulsion. Childs Nerv Syst. 2012;28:911–8. doi: 10.1007/s00381-012-1747-3. [DOI] [PubMed] [Google Scholar]
- 28.Jackson H, Parks TN. Anticonvulsant action of an arylamine-containing fraction from Agelenopsis spider venom. Brain Res. 1990;526:338–41. doi: 10.1016/0006-8993(90)91243-a. [DOI] [PubMed] [Google Scholar]
- 29.Takazawa A, Yamazaki O, Kanai H, Ishida N, Kato N, Yamauchi T. Potent and long-lasting anticonvulsant effects of 1-naphthylacetyl spermine, an analogue of Joro spider toxin, against amygdaloid kindled seizures in rats. Brain Res. 1996;706:173–6. doi: 10.1016/0006-8993(95)01334-2. [DOI] [PubMed] [Google Scholar]
- 30.Bell MR, Belarde JA, Johnson HF, Aizenman CD. A neuroprotective role for polyamines in a Xenopus tadpole model of epilepsy. Nat Neurosci. 2011;14:505–12. doi: 10.1038/nn.2777. [DOI] [PubMed] [Google Scholar]
- 31.Shimada A, Spangler EL, London ED, Ingram DK. Spermidine potentiates dizocilpine-induced impairment of learning performance by rats in a 14-unit T-maze. Eur J Pharmacol. 1994;263:293–300. doi: 10.1016/0014-2999(94)90725-0. [DOI] [PubMed] [Google Scholar]