Abstract
Background:
Different species of Croton are used in traditional Amazonian medicine. Among the popular uses are treatment of bacterial diseases, poorly healing wounds and fevers.
Objective:
This study evaluated the antileishmanial, antiplasmodial and antimicrobial activities of the extracts and diterpenes of Croton palanostigma Klotzsch (Euphorbiaceae).
Materials and Methods:
Leaves and bark were extracted with dichloromethane and methanol. The bark dichloromethane extract (BDE) was chromatographed on a column, obtaining cordatin and aparisthman. The extracts and diterpenes were assayed thought agar disk diffusion method and their bactericidal or fungicidal effects were evaluated by minimum bactericidal or fungicidal concentration. The antiplasmodial activity was evaluated after 24 and 72 h of exposition. The antileishmanial activity was performed on promastigotes forms of Leishmania amazonensis.
Results:
The bark methanol extract (BME) and cordatin were not active against any microbial strains tested; BDE and leaves methanol extract (LME) were positive for Pseudomonas aeruginosa and aparisthman was positive for Candida albicans. In the determination of the minimum bactericidal concentration, neither of them were active in the highest concentration tested. The extracts and diterpenes were inactive in Plasmodium falciparum, except the LME in 72 h. Any extract was shown to be active in promastigote forms of L. amazonensis.
Conclusion:
These results indicate that the BDE and LME did not inhibit the bacterial growth, then they probably had bacteriostatic effect. LME presented activity in P. falciparum.
Keywords: Antileishmanial, antimicrobial, antiplasmodial, Croton palanostigma, diterpenes, Euphorbiaceae
INTRODUCTION
Croton palanostigma, scientific synonym Croton benthamianus[1,2] is a medium-sized tree, which is native to the north region of Brazil[3] and another parts of the amazon region like the Peru. This specie is known as a “balsa-rana,” “marmeleiro or mameleiro” and “sangre de grado.”
Cordatin and aparisthman were isolated of C. palanostigma.[4,5,6] Other diterpenes, triterpenes, steroids, aromatic derivatives, lignan, and cromone were isolated too.[6] Aparisthman and cordatin have been shown a considerable antiulcerogenic activity,[7,8] but the antimicrobial properties of the extracts and these diterpenes remain unknown.
In this study, we are reporting the in vitro evaluation of antimicrobial, antiplasmodial and antileishmanial activities of the extracts and diterpenes from C. palanostigma.
MATERIALS AND METHODS
Plant material
Leaves and bark of C. palanostigma were collected at Terra Alta, State of Pará, Brazil. The plant was identified by Dr. Ricardo de Souza Seco from Botanic Department of the Museu Paraense Emílio Goeldi. The voucher specimen was deposited (MG n°182.822).
Extract preparation and isolation of the diterpene
Leaves and bark of C. palanostigma were dried at room temperature for 7 days. After that, the dried material was powdered (bark: 10 kg; leaves: 590 g) and extracted by percolation with dichloromethane and methanol successively. The resultant solutions were concentrated in a rotary evaporator in order to obtain the dichloromethane and methanol extracts.[9]
The bark dichloromethane extract (BDE) and bark methanol extract (BME) were chromatographed on a silica gel column eluted with solvents of increasing polarity. Aparisthman and cordatin were crystallized and separated by filtration [Figure 1]. All the process was monitored by thin layer chromatograph using aparisthman and cordatin standards and identified by hydrogen-1 and Carbon-13 nuclear magnetic resonance (NMR) data.[9]
Figure 1.
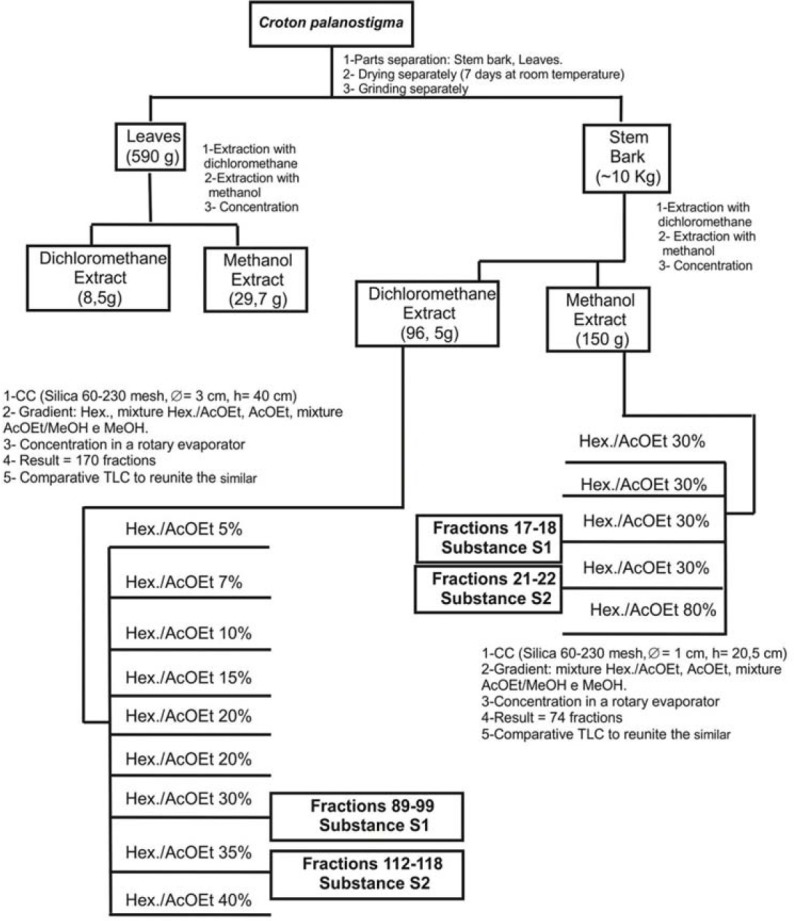
Obtention of organic rough extracts, chromatographic fractionation of dichloromethane stem bark extract and methanol stem bark extract of Croton palanostigma. S1: Aparisthman; S2: Cordatin
Our research group has published several papers describing studies and structural identification of aparisthman and cordatin involving analysis of NMR, infrared and mass spectrometry.[4,5,10,11,12]
Microbial strains
The bacterial and fungal strains used in this study were the American Type Culture Collection (ATCC) of Staphylococcus aureus (ATCC 29213); Escherichia coli (ATCC 25922); Pseudomonas aeruginosa (ATCC 25853) and Candida albicans (ATCC 40175).
Agar disk diffusion
Three or four isolated colonies of each microorganism were taken and diluted in Muller-Hinton broth and the suspensions were adjusted to 0, 5 of Mac-Farland scale (~108 for bacteria and ~106 for fungi colony forming unit/mL).[13]
Each microorganism suspension was spread in plates, in triplicate. After that, the filter paper disks (Watman-type 3) were impregnate with 10 μL of the extracts and diterpenes (500 μg/disk). The plates were incubated for 24 h for bacteria and 48 h for fungus. The result was considered active or inactive by the presence or absence of clear zones around the disks.
Minimum bactericidal concentration
The active extracts or diterpenes in the disk diffusion test were tested through broth microdilution technique.[14,15] All the experiments were carried out in triplicate.
An aliquot of 10 μL of the extracts or diterpenes (1000, 500, 250, 125, 62.5 e 31.25 μg/L/well) and 10 μL of the microorganism suspension were added in 180 μL Mueller-Hinton agar. As positive controls, gentamicin and nystatin were used in the same concentrations of the extracts and diterpenes. The plates were incubated during 24 h.
To determine the minimum bactericidal concentration (MBC) and minimum fungicidal concentration (MFC), subcultures of the 96 well plates test were carried out taken an aliquot of 10 μL of each concentration and the control, which were spread using a disposable loop on the surface of Muller-Hinton agar plates contained on Petri plates 90 × 15. The plates were incubated during 24 and 48 h for bacteria and fungus, respectively. After the incubation, it was verified the lowest concentration of the extracts or diterpenes witch had totally inhibited the growth of the microorganisms tested.
Parasite strain and antiplasmodial assay
The strain used in this study was the W2 clone of Plasmodium falciparum. The parasite strains were cultured continuously in human erythrocytes suspended in RPMI 1640 supplemented with 10% human serum.[16] The antiplasmodial assay was performed in 96-well tissue culture plates as described by Rieckman et al. (1980)[17] modificated by Carvalho et al. (1991).[18] Two fold serial dilutions of test samples dissolved in concentrations of 100; 50; 25; 12.5; 6.25; 3.125; 1.5625 μg/mL. A suspension of parasitized erythrocytes (0.5–1% parasitemia, 2.5% hematocrit) containing mainly trophozoites was added to the wells to give a final volume of 100 μl. Quinine was used as positive control and negative control (parasitized erythrocytes).
The plates were incubated at 37°C and after 24 and 72 h the medium was replaced by fresh medium with or without test samples. Samples were taken 24 and 72 h later, smeared, Giemsa stained and microscopically examined to determine the percentage of parasitemia. The results were expressed as the mean IC50 of three independent experiments for each sample.
For the evaluation of antiplasmodial activity it was adopted the following criteria in accordance with the range of the IC50:IC50 <10.0 μg/mL-actives; IC50 among 10 a 100 μg/mL-moderately actives and IC50 >100 μg/mL-inactive.[19]
Antipromastigotes activity of Leishmania amazonensis
Field isolates from mucocutaneous leishmaniasis (MCL, Leishmania amazonensis MHOM/BR/2009/M26361) were obtained from the Instituto Evandro Chagas, Ananindeua, Brazil.
The promastigote cryostailates were obtained after primary isolation on NNN blood slopes. After the strains were thawed, they were subcultured and after adapted to RPMI consisting of at least three additional passages. In total, about four passages were generally performed before the in vitro susceptibility testing was performed.
The MCL were cultivated at 26ºC in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (Gibco, Grand Island, NY, USA), penicillin (100 U/mL) and streptomycin (100 μg/mL). Culture in logaritme phase promastigote forms were counted in a New Bauer chamber the number was adjusted to 5 × 107 parasites/100 μL. The determination of the susceptibility testing was performed in 96-well plates. The extracts were tested in triplicate in a concentration gradient from 200 μg/mL to 3.125 μg/mL. A negative control was performed with three wells containing only parasites and the incubation medium. The positive control was made with amphotericin B (25–0.3906 μg/mL). After 24 h of incubation at 26ºC, each well was addicted 10 μL of the tetrazolium salt (5 mg/mL), and the parasites were quantified in enzyme-linked immunosorbent-assay plate reader.
The data obtained from this quantification were plotted in a graph using Graph Pad Prism version 5.04 (Confidence interval: 95% and significance level: 0,05). It was used the non-parametric method of Kruskal-Wallis, with multiple comparison of Dunn's and the IC50 was extrapolated from the graph as the concentration of the products that inhibited the parasitic growth at 50% of the values of the negative control.
The in vitro results of antipromastigote activity of the extract were classified as follows: When the IC50 or MIC was 100 μg/mL or less, the antipromastigote activity was considered active, when the IC50 or MIC was 101–200 μg/mL the antipromastigote activity was considered moderate active, when the IC5 or MICwas over 200 μg/mL the extracts were considered to be inactive.
RESULTS
Antibacterial and antifungal activities
In the evaluation of antimicrobial activity on disk diffusion assay, the BME did not show activity against any microorganism tested. The BDE did not inhibit the growth of S. aureus, E. coli, and C. albicans, but showed activity against P. aeruginosa. The leaves methanol extract (LME) was not active against S. aureus, E. coli, and C. albicans, but it showed activity against P. aeruginosa [Table 1].
Table 1.
Previous evaluation of antimicrobial activity of Croton palanostigma
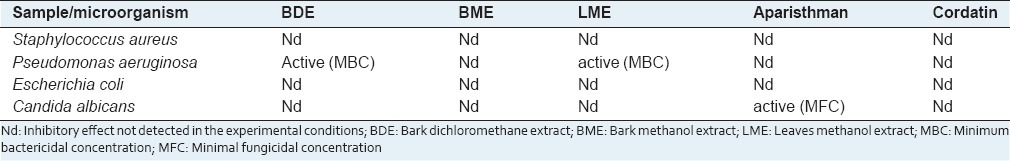
Aparisthman was not active against S. aureus, E. coli, and P. aeruginosa. but it presented activity for C. albicans. Cordatin did not inhibit the growth of any tested microorganism [Table 1].
In the determination of the MBC for the BME and LME, active in the disk diffusion assay against P. aeruginosa, they did not inhibit the bacterial growth in any tested concentration. The number of colonies found on the plates of the two extracts was similar to the ones found in the negative controls of the inoculum and inoculum + dimethyl sulfoxide (DMSO).
In the determination of the MFC for aparisthman, active for C. albicans in the disk diffusion assay, it did not inhibit the fungal growth in any tested concentration. The number of fungal colonies found on the plates was similar to the ones found in the negative controls of the inoculum and inoculum + DMSO.
Antileishmanial and antiplasmodial activities
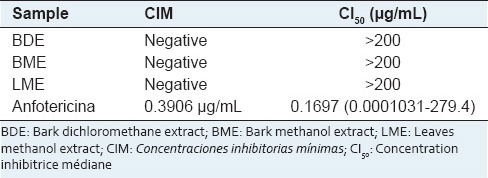
The BDE, BME, and LME did not inhibit the promastigote growth after 24 h of exposition in any tested concentration. The IC50 was higher than 200 μg/mL, and considered inactive [Table 2].
Table 2.
Antileishmanial activity of extracts of Croton palanostigma
The BDE and BME after 24 h and 72 h of exposition did not inhibited the parasitic growth in any tested concentration and we observed the presence of schizonts (IC50 > 100 μg/mL; Table 3).
Table 3.
Medium inibitory concentration of the extracts and diterpenes
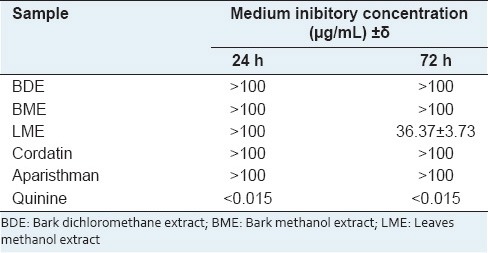
The LME did not inhibit the parasitic growth after 24 h of exposition in any tested concentration. After 72 h it was observed inhibition of parasitic growth. The IC50 values obtained for this extract in 72 h were 36.37 + 3.73 μg/mL [Table 3].
Aparisthman did not reduce the parasitemy after 24 of exposition in any tested concentration. However, it inhibited the parasitic growth after 72 h with an inhibition rate of 71.78% only in the concentration of 100 μg/mL. Cordatin did not inhibit the parasite growth in any time and tested concentrations [Table 3].
In the positive control of quinine it was observed inhibition of parasitic growth in all the tested concentrations, even though the exposition time did not interfere significantly in the inhibition of the parasitic growth (P > 0.05). In the lower concentration tested, the inhibition rate was 71% after 24 h and 75% after 72 h of exposition [Table 3]. The IC50 value for quinine was not calculated due to its high inhibition rate.
DISCUSSION
In our study, the BME of C. palanostigma did not show activity on the disk diffusion assay against any tested microorganism. However, the steam BME of C. zambesicus had been showed a wide spectrum activity against bacterial and fungal strains (>300 mg/mL). The results are suggesting that BME of Croton species has pharmacological potential against bacteria and fungus.[20]
The BDE showed activity against P. aeruginosa on the disk diffusion assay. The BME and cordatin were inactive against P. aeruginosa (MBC > 1.000 mg/L). The BDE of C. urucurana was active for this pathogen. This extract also presented activity against other bacterial strains like S. aureus and S. epidermidis.[21] It is noteworthy that these species may contain different diterpenes.
Aparisthman showed activity for C. albicans in the disk diffusion assay, but cordatin was inactive. The results obtained for aparisthman corroborate with previous studies about diterpenes, which evaluated three novel clerodane diterpenes from Casearia sylvestris, whose were actives against Aspergillus niger.[22]
Some diterpenes proved to be active in promastigote and amastigote forms of Leishmania.[23] Rich in diterpenes extracts were inactive on the promastigote form.
Some substances are inactive in promastigote and amastigote inactive. Other times, the sample is inactive in L. amazonensis and active in other Leishmania. Hence, if I suggested that these extracts are tested.
Croton species have considerable antiplasmodial activity such in vitro[24,25] as in vivo[26] and from them were isolated diterpenes with high pharmacological potential.[24,25,27]
The BDE and BME were not active against P. falciparum at any tested concentration [Table 3]. Some extracts obtained from plants were inactive or presented moderate activity on chloroquine resistant clone of P. falciparum (W2), but when evaluated on sensitive clone of P. falciparum (3D7) the activity profile was better.[19] Nowadays the most infections caused by P. falciparum are chloroquine resistant[28,29] and because of that we had chosen chloroquine resistant clone W2 of P. falciparum to evaluate the antiplasmodial potential.
The LME presented moderate antiplasmodial activity after 72 h (IC50 =36.37 + 3.73 μg/mL); then the antiplasmodial effect was time dependent. This activity can be amplified if this extract is submitted to the fractionalization and Obtention of pure substances. This fact was shown for the bark ethanol extract of Aspidosperma parvifolium.[19]
The results obtained for the LME show that it was less active when compared with the methanol extract (tannins free) and methylene chlorhydrate of C. lobatus, which presented very good antiplasmodial activity on 3d7 and K1 clones with IC50 values varying between 0.38 and 6.56 μg/mL, been the methanol extract (tannins free) of aerial parts the most active (IC50 =0.38 μg/mL).[30]
The differences among the antiplasmodial responses of the LME of C. palanostigma and C. lobatus can be explained partially in function of their chemical compounds. From bark and leaves of C. lobatus were isolated different class of terpenes[27] and from the bark of C. palanostigma were isolated diterpenes, triterpenes, lignan, steroids. Furthermore, the LME was not fractionalized.[6,9]
As the diterpene cordatin did not inhibit the parasitic growth at any tested concentrations and exposition times, it was considered inactive. Whereas aparisthman presented inhibition of parasitic growth in 71.78%, after 72 h of exposition, only in the concentration of 100 μg/mL, then the absence of activity in the lowest concentrations did not allowed us to determine the CI50 value for this diterpene. Other clerodane diterpenes showed inhibition effects in P. falciparum chloroquine sensible clone (F-32) and chloroquine resistant clone (FcB1).[31] These data reinforce the idea that this class of diterpenes are promising as antimalarial chemotherapy.
CONCLUSION
Summarizing, we can conclude that the BME and cordatin do not present antibacterial and antifungal activity. The bark dichloromethane and LME of C. palanostigma did not inhibit the bacterial growth and probably have a bacteriostatic effect against P. aeruginosa. The activity of the BDE for P. aeruginosa is probably related with other constituents and not with aparisthman and cordatin. Aparisthman probably have fungistatic effect against C. albicans. The BME and BDE and cordatin does not have antiplasmodial activity against chloroquine resistant P. falciparum. The LME presented moderate antiplasmodial activity and needs to be fractionated in order to isolate the possible actives compounds. Once aparisthman showed weak activity, maybe it does not have antimalarial potential. No sample inhibited the growth of L. amazonensis promastigote forms.
ACKNOWLEDGMENT
The authors thank to Capes, CNPq and Fundação de Amparo a Pesquisa do Pará and PADRC-UFPA by funding support our research.
Footnotes
Source of Support: The authors thank to Capes, CNPq and Fundação de Amparo a Pesquisa do Pará and PADRC-UFPA by funding support our research.
Conflict of Interest: None declared.
REFERENCES
- 1.Brako L, Zarucchi JL. St. Louis, MO: Missouri Botanical Garden; 1993. Catalogue of the Flowering Plants and Gymnosperms of Peru; p. 433. [Google Scholar]
- 2.Missouri Botanical Garden. [Last accessed on 2014 Aug 25]. Available from: http://www.tropicos.org/NameSynonyms.aspx?nameid=12802357 .
- 3.Secco RS. Sinopse das espécies de Croton L. (Euphorbiaceae) na Amazônia brasileira: Um ensaio taxonômico. Belém, Brazil; Museu Paraense Emílio Goeldi. 2008:169. [Google Scholar]
- 4.Müller AH, Oster CB, Schukmann WK, Bartl H. Aparisthman, a seven membered ring diterpenoid from Aparisthmium cordatum. Phytochemistry. 1986;25:1415–7. [Google Scholar]
- 5.Dadoun H, Müller AH, Cesario M, Guilhem J, Cordatin PC. A new diterpene from Aparisthmium cordatum. Phytochemistry. 1987;26:2108–10. [Google Scholar]
- 6.Brasil DS. Chemical contribution for aparisthmium genus. Msc Thesis. Belém, Federal University of Pará-ICEN. 1999:232. [Google Scholar]
- 7.Hiruma-Lima CA, Gracioso JD, Toma W, de Paula AC, de Almeida AB, Brasil DD, et al. Evaluation of the gastroprotective activity of cordatin, a diterpene isolated from Aparisthmium cordatum (Euphorbiaceae) Biol Pharm Bull. 2000;23:1465–9. doi: 10.1248/bpb.23.1465. [DOI] [PubMed] [Google Scholar]
- 8.Hiruma-Lima CA, Gracioso JS, Toma W, Almeida AB, Paula AC, Brasil DS, et al. Gastroprotective effect of aparisthman, a diterpene isolated from Aparisthmium cordatum, on experimental gastric ulcer models in rats and mice. Phytomedicine. 2001;8:94–100. doi: 10.1078/0944-7113-00017. [DOI] [PubMed] [Google Scholar]
- 9.Brasil DS. Study of chemical constituents of Croton palanostigma Klotzsch, using experimental tools and of molecular modeling: Identification of diterpenes with potential anti-ulcer and gastric protection activities Belém: PhD Thesis, Federal University of Pará-ICEN. 2008:241. [Google Scholar]
- 10.Brasil DS, Alves CN, Guilhon GM, Müller AH, Secco RD Peris G, Liusar R. Crystal structure and theoretical study of IR and 1H and 13C NMR spectra of cordatin, a natural product with antiulcerogenic activity. Int J Quantum Chem. 2008;108:2564–75. [Google Scholar]
- 11.Brasil DS, Moreira RY, Müller AH, Alves CN. Theoretical and experimental study of aparisthman: A natural product with anti-ulcer activity. Int J Quantum Chem. 2006;106:2706–13. [Google Scholar]
- 12.Brasil DS, Müller AH, Guilhon GM, Alves CN, Peris G, Liusar R, et al. Isolation, X-ray crystal structure and theoretical calculations of the new compound 8-epicordatin and identification of others terpenes and steroids from the bark and leaves of Croton palanostigma Klotzsch. J Braz Chem Soc. 2010;21:731–9. [Google Scholar]
- 13.Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493–6. [PubMed] [Google Scholar]
- 14.Standard M27-A2 of CLSI. Second edition 2002. CLSI. Reference method for broth dilution antifungal susceptibility testing of yeast; Approved standard. [Google Scholar]
- 15.Wayne, Pennsylvania: Clinical and Laboratory Standards Institute; 2003. CLSI. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: Approved standard. CLSI document M7-A6. [Google Scholar]
- 16.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–5. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 17.Rieckman KH, Sax LJ, Campbell GH, Mrena JF. World Health Organization editor. Geneva: Schwabe and Co. AG; 1980. Susceptibility of cultured parasites of P. falciparum to antimalarial drugs; pp. 35–50. Tropical Diseases Research Series III. The In Vitro Cultivation of Pathogens of Tropical Diseases. [Google Scholar]
- 18.Carvalho LH, Brandão MG, Santos-Filho D, Lopes JL, Krettli AU. Antimalarial activity of crude extracts from Brazilian plants studied in vivo in Plasmodium berghei-infected mice and in vitro against Plasmodium falciparum in culture. Braz J Med Biol Res. 1991;24:1113–23. [PubMed] [Google Scholar]
- 19.Dolabela MF. Antiplasmodial activity and cytotoxicity of Esenbeckia febrífuga (A.St-Hil.) Juss. ex Mart. (Rutaceae) and of species of the genus Aspidosperma (Apocynaceae). PhD Thesis, Minas Gerais, Federal University of Minas Gerais, Faculty of Pharmacy, Minas Greais, Brazilp. 2007:181. [Google Scholar]
- 20.Reuben KD, Abdulrahman FI, Akan JC, Usman H, Sodipo OA, Egwu GO. Phytochemical screening and In Vitro antimicrobial investigation of the methanolic extract of Croton zambesicus muell ARG. Stem Bark. Eur J Sci Res. 2008;23:134–40. [Google Scholar]
- 21.Oliveira IS, Lima JC, Silva RM, Martins DTO. In vitro screening of antibacterial activity of the latex and extracts from Croton urucurana Baillon. Rev Bras Farmacogn. 2008;18:587–93. [Google Scholar]
- 22.Oberlies NH, Burgess JP, Navarro HA, Pinos RE, Fairchild CR, Peterson RW, et al. Novel bioactive clerodane diterpenoids from the leaves and twigs of Casearia sylvestris. J Nat Prod. 2002;65:95–9. doi: 10.1021/np010459m. [DOI] [PubMed] [Google Scholar]
- 23.Brito S, Crescente O, Fernández A, Coronado A, Rodriguez N. Efficacy of a kaurenic acid extracted from the Venezuelan plant Wedelia trilobata (Asteracea) against Leishmania (Viannia) braziliensis. Rev Biomed. 2012;26:180–7. [PubMed] [Google Scholar]
- 24.Thongtan J, Kittakoop P, Ruangrungsi N, Saenboonrueng J, Thebtaranonth Y. New antimycobacterial and antimalarial 8,9-secokaurane diterpenes from Croton kongensis. J Nat Prod. 2003;66:868–70. doi: 10.1021/np030067a. [DOI] [PubMed] [Google Scholar]
- 25.Adelekan AM, Prozesky EA, Hussein AA, Ureña LD, van Rooyen PH, Liles DC, et al. Bioactive diterpenes and other constituents of Croton Steenkampianus. J Nat Prod. 2008;71:1919–22. doi: 10.1021/np800333r. [DOI] [PubMed] [Google Scholar]
- 26.Okokon JE, Nwafor PA. Antiplasmodial activity of root extract and fractions of Croton zambesicus. J Ethnopharmacol. 2009;121:74–8. doi: 10.1016/j.jep.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 27.Attioua B, Weniger B, Chabert P. Antiplasmodial activity of constituents isolated from C. lobatus. Pharm Biol. 2007;45:263–6. [Google Scholar]
- 28.Marsh K. Malaria disaster in Africa. Lancet. 1998;352:924. doi: 10.1016/S0140-6736(05)61510-3. [DOI] [PubMed] [Google Scholar]
- 29.Samarasekera U. Countries race to contain resistance to key antimalarial. Lancet. 2009;374:277–80. doi: 10.1016/s0140-6736(09)61349-0. [DOI] [PubMed] [Google Scholar]
- 30.Weniger B, Lagnika L, Vonthron-Sénécheau C, Adjobimey T, Gbenou J, Moudachirou M, et al. Evaluation of ethnobotanically selected Benin medicinal plants for their in vitro antiplasmodial activity. J Ethnopharmacol. 2004;90:279–84. doi: 10.1016/j.jep.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Sathe M, Ghorpade R, Srivastava AK, Kaushik MP. In vivo antimalarial evaluation of Gomphostenins. J Ethnopharmacol. 2010;130:171–4. doi: 10.1016/j.jep.2010.04.006. [DOI] [PubMed] [Google Scholar]


