Abstract
Background:
Eschweilera nana Miers is a tree widely distributed in Cerrado, Brazil.
Objective:
In this study, we aimed to describe its phytochemical properties and antioxidant and topical anti-inflammatory effects for the first time, as well validate an high performance liquid chromatography with ultraviolet/visible (HPLC-UV-Vis) method for the separation and quantification of the main components (hyperoside and rutin) in the hydroalcoholic extract of E. nana leaves.
Materials and Methods:
Structural identification of compounds in E. nana extract was performed by analysis of spectral data by 1H nuclear magnetic resonance, 13C nuclear magnetic resonance and/or ESI/EM. The HPLC-UV-Vis method was validated according International Conference on Harmonization (ICH) parameters. The 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) method were used for determination of in vitro antioxidant activities and the croton oil-induced inflammation for evaluation of in vivo anti-inflammatory effects.
Results:
Hyperoside, rutin, α-amirin, β-amirin, β-sitosterol, and stigmasterol were identified in the hydroalcoholic extract of E. nana leaves. HPLC-UV-Vis was validated according to ICH parameters. Furthermore, in vitro and in vivo assays demonstrated that the hydroalcoholic extract and methanol fraction showed significant antioxidant and topical anti-inflammatory effects, as they were able to reduce ear edema induced by croton-oil application.
Conclusions:
This research showed the first phytochemical study of E. nana extract and their biological activities may be associated with the presence of flavonoids in the extracts.
Keywords: Antioxidant activity, Eschweilera nana Miers, flavonoids, phytochemical composition, topic anti-inflammatory
INTRODUCTION
Eschweilera nana Miers is a member of the family Lecythidacea, and it is widely distributed in Cerrado, the region of Atlantic Forest and Amazonian, Brazil. It is popularly known as “ovo frito” referring to the aspect of the flower, however, there are regions that are also known as “tucari,” “tucari-do-campo,” “sapucaia” and “sapucainha.” In traditional medicine, this species have been administered for colic and dysentery.[1,2]
Phytochemical studies have identified triterpenes,[3,4] saponins,[4] active and inactive ellagic acid derivatives, flavonoids,[5] and sterols[3] as chemical constituents present in members of the genus Eschweilera. However, until the moment, there are no phytochemical studies and no evaluation of some pharmacological properties of E. nana.
So by virtue of shortage studies about this species, in this study, we performed a phytochemical study to identify the main active components of and evaluate the topical anti-inflammatory activity and antioxidant capacity of extracts of E. nana leaves and validate an High performance liquid chromatography with ultraviolet/visible (HPLC-UV-Vis) methodology for the separation and quantification of the main compounds of E. nana extract in order to ensure chemical integrity and hence its biological effects.
MATERIALS AND METHODS
Chemicals and reagents
Silica gel 60 (70–230 mesh), silica gel 60 (230–400 mesh), and silica gel plates F254 (0.25-mm thick) were obtained from Merck®. Sephadex LH-20 was purchased from Pharmacia Fine Chemicals®. 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), 1,1-diphenyl-2-picrylhydrazyl (DPPH), Trolox (purity ≥98%), l-ascorbic acid (purity ≥99%), rutin (purity ≥95%), hyperoside (purity ≥97%), croton oil and dexamethasone were obtained from Sigma-Aldrich®. Potassium persulfate P.A was obtained from Vetec®. All HPLC grade solvents were purchased from J. T. Baker®. Purified water, produced by a Milli-Q purification system, was used throughout the study.
Plant material
Eschweilera nana Miers leaves were collected from Nova Mutum, Mato Grosso, Brazil, in August 2007. The species was identified by Dra. Cássia Mônica Sakuragui from the Botanical Garden of Rio de Janeiro, and the voucher specimen was deposited in the Herbarium of the State University of Maringá, under the registration number HUEM - 13323.
Extraction and fractionation
Dried and milled leaves of E. nana (691.2 g) were extracted with ethanol: water (9:1 v/v), in the proportion of 10% (w/v), by maceration for dynamic stirring until exhaustion. The filtrate was concentrated in a vacuum evaporator at 40°C and then lyophilized to yield 132.63 g of E. nana dry extract (EE). EE (40.75 g) was used for chromatography in a column with silica gel 60 (70–230 mesh, ASTM) eluted with hexane, dichloromethane, dichloromethane: ethylacetate (1:1), ethyl acetate, and methanol, and 5 fractions were obtained: Hexane fraction (HF) (0.7 g), dichloromethane fraction (DF) (4.83 g), dichloromethane:ethyl acetate fraction (DEF) (2.51 g), ethyl acetate fraction (EF) (3.29 g), and methanol fraction (MF) (29.4 g), respectively. The fractions were analyzed by thin-layer chromatography, visualized with UV light, and developed using Godin reactive.
The DF (1.19 g) was purified on a silica gel 60 (70–230 mesh ASTM) eluted with hexane, hexane: dichloromethane (8:2; 1:1; 3:7), dichloromethane, dichloromethane:ethyl acetate (1:1), ethyl acetate: methanol (1:1), and methanol, to yield two subfractions, A (12.5 mg) and B (3 mg). The MF (0.99 g) was purified on a sephadex LH-20 gel eluted with methanol: acetone (1:1), and 65 subfractions were obtained. Fraction 34 was purified using adsorption chromatography under pressure on silica gel (230–400 mesh ASTM) eluted with ethyl acetate: methanol (7,5:2,5; 6:4; 1:1; 4:6), methanol: acetone (1:1), and methanol, to yield two subfractions, C (8.9 mg) and D (6.4 mg).
The chemical structure of the compounds in the subfractions were identified by 1H nuclear magnetic resonance (1H NMR; 300 MHz), 13C nuclear magnetic resonance (13C NMR; 75 MHz), both with Varian® Gemini 2000 BB spectrometers in CDCl3, internal standard TMS (1H NMR) and solvent signal (13C NMR), and/or the mass spectrometry of ESI/EM, negative ion mode, Micromass® Quattro LC. The results were analyzed and compared with data from the literature. The compounds present in subfraction A were identified as a mixture of α-amirin and β-amirin, and in subfraction B, as a mixture of β-sitosterol and stigmasterol. In subfraction C and D, the compounds were identified as quercetin 3-O-galactoside (hyperoside) and quercetin 3-O-rutinoside (rutin), respectively.
High performance liquid chromatography with ultraviolet/visible analysis
The chromatographic conditions were in accordance with the methodology described by Wang et al.[6] The analyses were performed using HPLC (Shimadzu® LC20AT) with a UV-Vis detector (Shimadzu® SPD-20A), C-18 column (250 × 4.60 mm i.d., 5 μm) (Phenomenex® Luna), and 100 RP-18/5 μm guard column (4.0 × 3.0 mm i.d., 5 μm) (Phenomenex®). The isocratic mobile phase consisted of a tetrahydrofuran/acetonitrile/0.05% phosphoric acid solution (20/3/77, v/v/v), with a flow rate of 1 mL/min. The analysis time was 25 min for the rutin and hyperoside standards and 35 min for EE. UV detection was performed at a wavelength of 360 nm, and the column temperature was maintained at 25°C. Data integration was done using LC-solution software (Shimadzu®). All samples were filtered through a membrane of 0.45-μm FH (Millipore®) before injection, which was performed using a 20-μL loop.
Method validation
The analytical method was evaluated using the parameters recommended by ICH.[7] Statistical analysis was performed using Microsoft Excel® (Microsoft Corporation) with a significance level of 5%, and the results were analyzed according to AOAC.[8] A mixture of rutin (50%) and hyperoside (50%) was used as the standard solution. All analyses were performed in triplicate.
Specificity
The methanolic solution of EE (1 mg/mL) was subjected to the UV absorption spectrum, in the range of 200-400 nm, by using HPLC (Waters® 600E) with a photodiode array (PDA) detector to verify if other compounds coeluted with rutin and hyperoside.
Linearity
The methanolic solution of the mixture of rutin and hyperoside standards was diluted to six solutions with concentrations of 1.25–50 mg/mL. Analysis of variance (ANOVA) of the linear regression and testing of the validity and lack of fit of the analytical equation was determined by the Fisher method, with a significance level of 5%.
Limits of detection and quantification
The methanolic solution of the mixture of rutin and hyperoside standards was diluted to a series of appropriated concentrations with methanol, and an aliquot was used for HPLC analysis. Limits of detection (LD) and quantification (LQ) were determined at signal/noise (S/N) of 3 and 10, respectively.
Precision
A methanolic stock solution of EE was prepared and diluted to three concentrations (0.5, 1, and 1.5 mg/mL). The repeatability was evaluated in terms of concentration of rutin and hyperoside obtained at each level in a short period. After 7 days, the procedure was repeated for evaluation of the intermediate precision. Relative standard deviation (RSD) was calculated for repeatability and intermediate precision.
Accuracy
The recovery was evaluated by the standard addition method, adding the mixture of flavonoids in three different levels of known concentrations to the extractive solution of E. nana, before lyophilization. All concentrations were prepared independently in three replicates. The recovery data were determined according to recommendations of ICH.[7]
Robustness
Robustness was assessed by changing the HPLC-UV-Vis initially used (Shimadzu®) with Waters® 600 E and a PDA detector. The chromatographic profiles were visually compared, taking the retention time of the flavonoids.
In vitro antioxidant activity 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS)
Determination of EE was performed by capturing the ABTS•+ radical, according to Re et al.[9] with minor modifications. The ABTS radical cation (ABTS•+) was produced and diluted with ethanol to an absorbance of 0.7 (±0.02) at 734 nm. The ethanolic solutions of EE, ascorbic acid, and Trolox were prepared at different concentrations. The sample solution (30 μL) was added to 3 mL of diluted ABTS•+ solution and allowed to react at room temperature in the dark. After 6 min, the absorbance values were measured at 734 nm by using the UV-Vis spectrophotometer (Shimadzu® UV1650) against a blank. Ascorbic acid and Trolox served as the positive control and standard, respectively. The activity results were expressed as Trolox equivalent antioxidant capacity (TEAC) units, which are calculated by dividing the gradient of the plot of percentage inhibition of absorbance versus the final concentration plot for the antioxidant in question by the gradient of plot for Trolox.
1,1-diphenyl-2-picrylhydrazyl (DPPH)
The antioxidant activity of EE, MF, and subfractions C and D were performed using the stable DPPH radical, according to the procedure previously reported[10] with minor modifications. Methanolic solutions of the concentrations and ascorbic acid (positive control) were prepared and diluted in different concentrations. The methanolic solution of DPPH at 0.3 mM (1 mL) was added to the sample (2 mL) and kept in the dark for 30 min. The absorbance values were measured at 517 nm by using the UV-Vis spectrophotometer against a blank. The scavenging activity of the samples was expressed in IC50, the concentration necessary to scavenge 50% of DPPH radicals.
Topical anti-inflammatory effect of Eschweilera nana
Animals
The experimental procedures were approved by the Ethics Committee of the State University of Maringá (protocol number 032/2007). The topical inflammation was established using male Swiss mice (weight: 25–30 g). The animals were housed at 22°C ± 2°C under a 12-h light/12-h dark cycle with free access to food and water.
Croton oil-induced ear edema in mice
Edema was induced by 20 μL of croton oil (200 μg) diluted in a solution of acetone: water (7:3) applied to the inner surface of both ears, according to the Van Arman method;[11] 20 μL of acetone: water (7:3) solutions of EE (5.0 mg/ear), MF (0.625; 1.25; 2.5 mg/ear), or dexamethasone (0.08 mg/ear) was applied to the inner surface of the left ear. On the right ear, only 20 μL of the vehicle (acetone: water 7:3) was applied (as a control). After 6 h, the animals were sacrificed, and each ear was perforated with a metal punch to provide a 6-mm-diameter disc. Edema (E) was assessed by E = (wc–wt); wc: the weight of the disc from the right control ear, wt: the weight of the disc from the left treated ear), thus determining the percentage of edema inhibition.
Statistics analysis
Data are presented as means ± standard error of the mean (SEM). Results were subjected to ANOVA and Tukey's post-hoc test, using Microsoft Excel® (Microsoft Corporation). P < 0.05 were considered statistically significant.
RESULTS AND DISCUSSION
Characterization of Eschweilera nana extract
Substances such as hyperoside, rutin, α-amirin, β-amirin, β-sitosterol, and stigmasterol were identified from the hydroalcoholic extract of E. nana leaves. Structural identification of these compounds was performed by analysis of spectral data by 1H NMR,13C NMR, and/or ESI/EM, and by comparison with those previously reported in the literature.[3,12,13,14,15,16]
All these substances were already identified in other plant species,[14,16,17,18,19,20,21] however, this is the first report in the E. nana species.
In the Eschweilera genus, the triterpenes α-amirin and β-amirin, the sterols β-sitosterol and stigmasterol were isolated and identified from the leaves and bark of E. longipes, respectively[3] and the β-amirin also identified in the extract of leaves of E. rabeliana.[22] However, the identification of the flavonoids hyperoside and rutin were not reported in this genus at the present moment.
The chromatographic profile of EE [Figure 1a] by using HPLC-UV-Vis, showed the major presence of rutin and hyperoside, with retention times of 12.870 min and 18.851 min, respectively; this result was confirmed by comparison with respective reference patterns in the chromatogram and retention time. Other peaks could not be identified.
Figure 1.
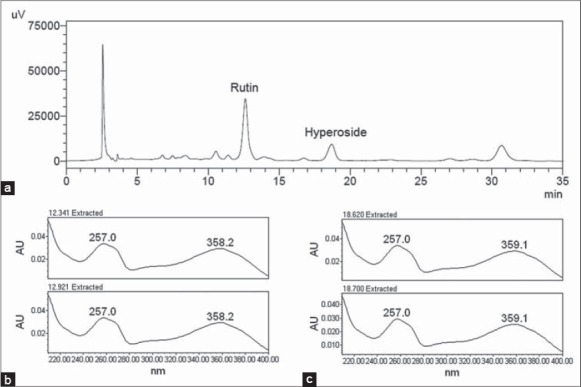
Chromatographic profile of hydroalcoholic extract of Eschweilera nana leaves (rutin: retention time of 12.870 min and hyperoside: retention time of 18.851 min) by High performance liquid chromatography with ultraviolet/visible method (a), and the absorption spectra by photodiode array detector of rutin (b) and hyperoside (c)
The quantitative analysis was performed, indicating that EE has 1.51% ± 0.01% (w/w) of rutin and 0.39% ± 0.01% (w/w) of hyperoside.
Taking into account that the flavonoids were associated with several pharmacological properties such as antioxidant,[23] anti-inflammatory,[24] antiviral, antimicrobial, antifungal,[25] vasodilator,[26] anticarcinogenic and cardioprotective,[27] the subsequent experiments used EE and/or the MF, since it showed a high yield mass (72.15% w/w) when compared with other fractions and because rutin and hyperoside were identified in this fraction.
Validation of high performance liquid chromatography with ultraviolet/visible method for rutin and hyperoside in Eschweilera nana extract
For quantitative analysis of rutin and hyperoside, validation of analytical methodology was performed according to the requirements of ICH.[7] Specificity was evaluated using a methanolic solution of EE. The markers rutin and hyperoside were subjected to an absorption spectrum by a PDA detector (Waters® 2998), and results show that the absorption spectra remained the same [Figure 1b and c], which indicated that no other compounds were quantified with these flavonoids.
Linearity was investigated by analyzing rutin and hyperoside simultaneously in six concentrations (1.25–50 mg/mL). The calibration curves showed an excellent correlation coefficient (r). To verify the validity of the linear regression and linear adjustment, the F-test was performed at 95% confidence level, and the results indicated that the slope was significant and the linear regression model did not show a lack of fit [Table 1].
Table 1.
Linearity, LD and LQ results of rutin and hyperoside determined by HPLC-UV-Vis method
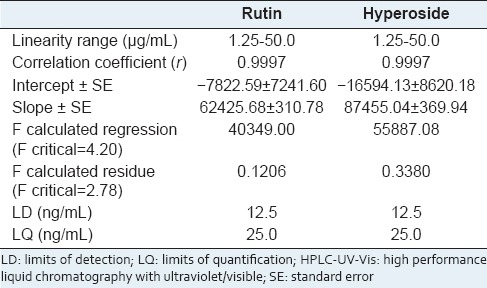
The LD and LQ were determined by the S/N ratio, which was performed by comparing the measured signals of the sample at low concentrations with the blank sample; this established minimum detectable and quantifiable concentrations. The results in Table 1 indicate that the proposed method exhibits a good sensitivity for the quantification of these flavonoids in EE.
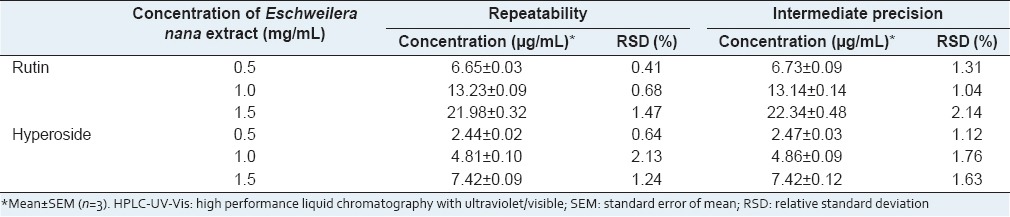
Precision was assessed in terms of repeatability and intermediate precision data at low, medium, and high concentrations and [Table 2] indicated that the method was precise regardless of the concentrations tested, since the RSD values obtained were lower than those established by AOAC,[8] which included data of estimated precision in function of the analyte concentration with an RSD up to 7.3% and 11% for 10 ppm (10 μg/mL) and 1 ppm (1 μg/mL) analyte concentrations, respectively.
Table 2.
Repeatability and intermediate precision results of the HPLC-UV-Vis method
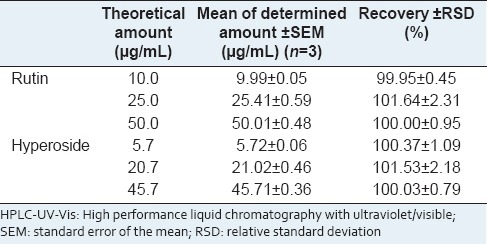
Accuracy was evaluated using the standard addition method. According to AOAC[8] recovery values of 90–107% and 80–110% for analyte concentrations of 100 ppm (100 μg/mL) and 10 ppm (10 μg/mL), respectively, are acceptable. The results obtained [Table 3] show that the analytical method applied is satisfactory.
Table 3.
Accuracy results of the HPLC-UV-Vis method
Despite changing the analysis equipment, the chromatographic profiles of rutin and hyperoside methanolic solutions, obtained by HPLC, were similar, with little variation in retention time; this indicated that the analytical method was robust.
In vitro antioxidant activity
Among the chemical methods available to detect antioxidant capacity, ABTS and DPPH are the most popular because of their simplicity and speed.[28] The ABTS method measures the total antioxidant capacity of hydrophilic and lipophilic substances,[29] and thus was chosen initially to evaluate the activity of EE. Trolox, a water-soluble analogue of Vitamin E,[29] was used as the standard to report the results in TEAC and the higher this value, the greater the activity of the sample. The TEAC means obtained for EE and ascorbic acid (the positive control), were 0.77 ± 0.01 and 1.43 ± 0.05, respectively. When comparing these values, the antioxidant activity of EE does not seem high; however, compared with the TEAC values in several other studies,[30,31,32] this plant can be seen to possess significant activity.
After verification of the high antioxidant capacity of EE, the antiradical capacity of this extract, MF and subfractions C and D were determined using the DPPH method. The results obtained were compared with each other and the reference standard, ascorbic acid. The results were expressed in IC50 (the lower the value, greater the antioxidant capacity of the compound) as shown in Table 4. All samples presented antioxidant activity with significant differences at P < 0.05. EE, despite being a mixture of compounds, showed good antioxidant activity, with values close to the reference standard.
Table 4.
Comparison of IC50 value obtained of hydroalcoholic EE leaves, MF, subfraction C, subfraction D and ascorbic acid by DPPH method

The results published previously[33] also demonstrate that EE has significant activity when compared with the IC50 values of the crude extracts of other species. The MF and subfractions C and D also presented good antioxidant potential, with IC50 values up to 17.06 μg/mL. Subfraction C showed better antioxidant activity than subfraction D, in which contained hyperoside and rutin, respectively. Subfraction C also presented a higher capacity than the positive control, ascorbic acid.
According Seyoum et al.,[34] flavonoids and other polyphenols are great scavengers of free radicals because they easily donate hydrogen atoms due to the presence of OH grouping. Thus, the antioxidant activity present in EE, MF, and subfraction C and D was hypothesized to be due to the presence of flavonoids, including hyperoside and rutin, since they possess hydroxyl groups.
Topical anti-inflammatory effect of Eschweilera nana
Evaluation of the topical anti-inflammatory activity of EE and MF was performed using croton oil-induced inflammation, which increases phospholipase A2 activity,[35] which results in the release of arachidonic acid and biosynthesis of prostaglandins and leukotrienes.[36,37]
The data [Figure 2] showed that EE at a dose of 5 mg/ear significantly inhibited swelling, probably due to the decrease of vascular permeability. Despite being a mixture of compounds, EE inhibited 45% of the edema in 6 h after application of the inflammatory agent. MF at doses of 0.625, 1.25, and 2.5 mg/ear also showed significant inhibition of inflammation [Figure 2], with mean percentages of 41, 52, and 67%, respectively. The positive control, dexamethasone, at a dose of 0.1 mg/ear presented with 93% edema inhibition.
Figure 2.
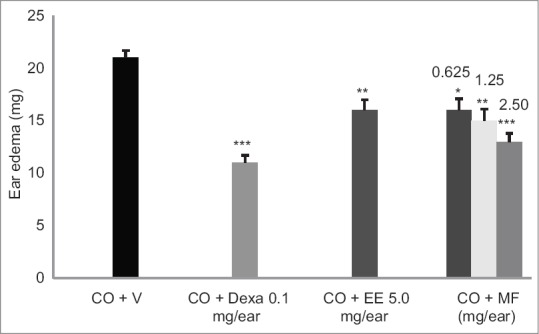
Effect of extract of Eschweilera nana (EE), methanol fraction (MF) and dexamethasone (dexa) on edema of the ear induced by croton oil (CO). (*P < 0.05, **P < 0.01, ***P < 0.001, compared with the control group [CO + V] [analysis of variance, Tukey's post hoc test])
Methanol fraction at a dose of 2.5 mg/ear showed a higher topical anti-inflammatory activity than the EE (an increase of around 49%), indicating that higher the concentration of polar compounds, higher the anti-inflammatory activity. Flavonoids have been correlated with the anti-inflammatory activity of many plant extracts and have been hypothesized to inhibit inflammatory mediators such as cyclooxygenase and/or lipoxygenase, which are involved in arachidonic acid release.[27,38] Furthermore, hyperoside and rutin, the major compounds in EE, demonstrated significant anti-inflammatory activity, which can be related by the inhibition of phospholipase A2 activity, which has an important role in the arachidonic acid cascade.[39,40,41]
Coutinho et al.[42] describes some of the structural factors that positively influencing anti-inflammatory activity of flavonoids, among them are the unsaturation in C-ring (positions 2–3), number and position of the OH groups, carbonyl group at C-4 (B-ring), and the absence glycosylation of the molecule. Most of these items are found in the structure of hyperoside and rutin, which explain the anti-inflammatory activity of them.
The results published previously[43] and obtained in this test indicate that EE and MF had satisfactory inhibitory activity against edema, probably due to the presence of flavonoids, including, the hyperoside and rutin that were identified, previously. The results do not explain the exact pharmacological mechanism involved; however, it may be related to the inhibition of different mediators of the inflammatory response, such as involved in arachidonic acid cascade.
CONCLUSION
We identified hyperoside, rutin, α-amirin, β-amirin, β-sitosterol, and stigmasterol in EE, through the first phytochemical study of this plant species. HPLC-UV-Vis for simultaneous detection and quantification of major compounds, hyperoside and rutin, in EE was validated. Furthermore, in vitro and in vivo assays demonstrated that EE and MF showed significant antioxidant and topical anti-inflammatory effects, possibly associated with flavonoids.
ACKNOWLEDGMENTS
The authors are grateful to CNPq, FINEP, and CAPES for financial support and fellowships.
Footnotes
Source of Support: CNPq, FINEP, and CAPES for financial support and fellowships
Conflict of Interest: None declared.
REFERENCES
- 1.Lorenzi H. 1st ed. Vol. 3. Nova Odessa: Instituto Plantarum; 2000. Brazilian Trees: Identification manual and cultivation of native tree plants in Brazil; p. 187. [Google Scholar]
- 2.Silva Júnior MC, Santos GC, Nogueira PE, Munhoz CB, Ramos AE. BrasÍlia: Cerrado Seed Network; 2005. 100 Cerrado Trees: Field Guide 100; p. 74. [Google Scholar]
- 3.Carvalho MG, Velandia JR, Oliveira LF, Bezerra FB. Triterpene isolated from Eschweilera Longipes Miers (Lecythidaceae) Quím Nova. 1998;21:740–3. [Google Scholar]
- 4.da Costa PM, de Carvalho MG. New triterpene isolated from Eschweilera longipes (Lecythidaceae) An Acad Bras Cienc. 2003;75:21–5. doi: 10.1590/s0001-37652003000100003. [DOI] [PubMed] [Google Scholar]
- 5.Yang SW, Zhou BN, Wisse JH, Evans R, van der Werff H, Miller JS, et al. Three new ellagic acid derivatives from the bark of Eschweilera coriacea from the Suriname rainforest. J Nat Prod. 1998;61:901–6. doi: 10.1021/np980046u. [DOI] [PubMed] [Google Scholar]
- 6.Wang CH, Wang YX, Liu HJ. Validation and application by HPLC for simultaneous determination of vitexin-2“-O-glucoside, vitexin-2”-O-rhamnoside, rutin, vitexin, and hyperoside. J Pharm Anal. 2011;1:291–6. doi: 10.1016/j.jpha.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Topic Q2 (R1) Validation of Analytical Procedures: Text and methodology. International Conference on Harmonisation, ICH. 2005. Available from: http://www.ich.org .
- 8.Arlington: VA; 1993. AOAC Peer Verified Methods Program. Manual on Policies and Procedures. [Google Scholar]
- 9.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–7. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 10.Silva CG, Herdeiro RS, Mathias CJ, Panek AD, Silveira CS, Rodrigues VP, et al. Evaluation of antioxidant activity of Brazilian plants. Pharmacol Res. 2005;52:229–33. doi: 10.1016/j.phrs.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Van Arman CF. Anti-inflammatory drugs. Clin Pharmacol Ther. 1974;16:900–4. doi: 10.1002/cpt1974165part2900. [DOI] [PubMed] [Google Scholar]
- 12.Mabry TJ, Markham KR, Thomas MB. New York: Springer-Verlag; 1979. The systematic identification of flavonoids; p. 354. [Google Scholar]
- 13.Mahato SB, Kundu AP. 13C NMR spectra of pentacyclic triterpenoids – A compilation and some salient features. Phytochemistry. 1994;37:1517–75. [Google Scholar]
- 14.Forgo P, Kövér KE. Gradient enhanced selective experiments in the 1H NMR chemical shift assignment of the skeleton and side-chain resonances of stigmasterol, a phytosterol derivative. Steroids. 2004;69:43–50. doi: 10.1016/j.steroids.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 15.March RE, Lewars EG, Stadey CJ, Miao XS, Zhao X, Metcalfe CD. A comparison of flavonoid glycosides by electrospray tandem mass spectrometry. J Mass Spectrom. 2006;248:61–85. [Google Scholar]
- 16.Habib MR, Nikkon F, Rahman M, Haque ME, Karim MR. Isolation of stigmasterol and beta-sitosterol from methanolic extract of root bark of Calotropis gigantea (Linn) Pak J Biol Sci. 2007;10:4174–6. doi: 10.3923/pjbs.2007.4174.4176. [DOI] [PubMed] [Google Scholar]
- 17.Spessoto MA, Ferreira DS, Crotti AE, Silva ML, Cunha WR. Evaluation of the analgesic activity of extracts of Miconia rubiginosa (Melastomataceae) Phytomedicine. 2003;10:606–9. doi: 10.1078/094471103322331629. [DOI] [PubMed] [Google Scholar]
- 18.Krasteva I, Nikolova I, Danchev N, Nikolov S. Phytochemical analysis of ethyl acetate extract from Astragalus corniculatus Bieb. and brain antihypoxic activity. Acta Pharm. 2004;54:151–6. [PubMed] [Google Scholar]
- 19.Santos FV, Colus IM, Silva MA, Vilegas W, Varanda EA. Assessment of DNA damage by extracts and fractions of Strychnos pseudoquina, a Brazilian medicinal plant with antiulcerogenic activity. Food Chem Toxicol. 2006;44:1585–9. doi: 10.1016/j.fct.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Costa HN, Santos MC, Alcântara AF, Silva MC, França RC, Piló-Veloso D. Chemical contituints and antiedematogenic activity of Peltodon radicans (Lamiaceae) Quim Nova. 2008;31:744–50. [Google Scholar]
- 21.Sagratini G, Ricciutelli M, Vittori S, Oztürk N, Oztürk Y, Maggi F. Phytochemical and antioxidant analysis of eight Hypericum taxa from Central Italy. Fitoterapia. 2008;79:210–3. doi: 10.1016/j.fitote.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Carvalho MG, Almeida ME, Hauptli MB, Meleiro LA. Triterpene isolated from Eschweilera rabeliana Mori (Lecythidaceae) Rural Univ Mag, Series of Exact Sciences and Earth. 1995;17:33–6. [Google Scholar]
- 23.Martínez-Flórez S, González-Gallego J, Culebras JM, Tuñón MJ. Flavonoids: Properties and anti-oxidizing action. Nutr Hosp. 2002;17:271–8. [PubMed] [Google Scholar]
- 24.Rotelli AE, Guardia T, Juárez AO, de la Rocha NE, Pelzer LE. Comparative study of flavonoids in experimental models of inflammation. Pharmacol Res. 2003;48:601–6. doi: 10.1016/s1043-6618(03)00225-1. [DOI] [PubMed] [Google Scholar]
- 25.Cushnie TP, Lamb AJ. Antimicrobial activity of flavonoids. Int J Antimicrob Agents. 2005;26:343–56. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu YC, Leung SW, Yeung DK, Hu LH, Chen GH, Che CM, et al. Structure-activity relationships of flavonoids for vascular relaxation in porcine coronary artery. Phytochemistry. 2007;68:1179–88. doi: 10.1016/j.phytochem.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481–504. doi: 10.1016/s0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 28.Antolovich M, Prenzler PD, Patsalides E, McDonald S, Robards K. Methods for testing antioxidant activity. Analyst. 2002;127:183–98. doi: 10.1039/b009171p. [DOI] [PubMed] [Google Scholar]
- 29.Krishnaiah D, Sarbatly R, Nithyanandam R. A review of the antioxidant potential of medicinal plant species. Food Bioprod Process. 2011;89:217–33. [Google Scholar]
- 30.Dey P, Chaudhuri P, Chaudhuri TK, Mandal N. Comparative assessment of the antioxidant activity and free radical scavenging potential of different parts of Nerium indicum. Int J Phytomed. 2012;4:54–69. [Google Scholar]
- 31.McGaw LJ, Steenkamp V, Eloff JN. Evaluation of Athrixia bush tea for cytotoxicity, antioxidant activity, caffeine content and presence of pyrrolizidine alkaloids. J Ethnopharmacol. 2007;110:16–22. doi: 10.1016/j.jep.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 32.Chaudhuri D, Ghate NB, Sarkar R, Mandal N. Phytochemical analysis and evaluation of antioxidant and free radical scavenging activity of withania somnifera root. Asian J Pharm Clin Res. 2012;5:193–9. [Google Scholar]
- 33.Menezes PR, Schwarz EA, Santos CA. In vitro antioxidant activity of species collected in Paraná. Fitoterapia. 2004;75:398–400. doi: 10.1016/j.fitote.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 34.Seyoum A, Asres K, El-Fiky FK. Structure-radical scavenging activity relationships of flavonoids. Phytochemistry. 2006;67:2058–70. doi: 10.1016/j.phytochem.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Kondoh H, Sato Y, Kanoh H. Arachidonic acid metabolism in cultured mouse keratinocytes. J Invest Dermatol. 1985;85:64–9. doi: 10.1111/1523-1747.ep12275349. [DOI] [PubMed] [Google Scholar]
- 36.Fürstenberger G, Marks F. Early prostaglandin E synthesis is an obligatory event in the induction of cell proliferation in mouse epidermis in vivo by the phorbol ester TPA. Biochem Biophys Res Commun. 1980;92:749–56. doi: 10.1016/0006-291x(80)90767-6. [DOI] [PubMed] [Google Scholar]
- 37.McColl SR, Hurst NP, Cleland LG. Modulation by phorbol myristate acetate of arachidonic acid release and leukotriene synthesis by human polymorphonuclear leukocytes stimulated with A23187. Biochem Biophys Res Commun. 1986;141:399–404. doi: 10.1016/s0006-291x(86)80186-3. [DOI] [PubMed] [Google Scholar]
- 38.Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. Flavonoids: A review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74:418–25. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- 39.Selloum L, Bouriche H, Tigrine C, Boudoukha C. Anti-inflammatory effect of rutin on rat paw oedema, and on neutrophils chemotaxis and degranulation. Exp Toxicol Pathol. 2003;54:313–8. doi: 10.1078/0940-2993-00260. [DOI] [PubMed] [Google Scholar]
- 40.Kao ES, Wang CJ, Lin WL, Yin YF, Wang CP, Tseng TH. Anti-nflammatory potential of flavonoid contents from dried fruit of Crataegus pinnatifida in vitro and in vivo. J Agric Food Chem. 2005;53:430–6. doi: 10.1021/jf040231f. [DOI] [PubMed] [Google Scholar]
- 41.Lindahl M, Tagesson C. Flavonoids as phospholipase A2 inhibitors: Importance of their structure for selective inhibition of group II phospholipase A2. Inflammation. 1997;21:347–56. doi: 10.1023/a:1027306118026. [DOI] [PubMed] [Google Scholar]
- 42.Coutinho MA, Muzitano MF, Costa SS. Flavonoids: Potential therapeutic agents to the inflammatory process. Rev Virtual Quim. 2009;1:241–56. [Google Scholar]
- 43.Sosa S, Balick MJ, Arvigo R, Esposito RG, Pizza C, Altinier G, et al. Screening of the topical anti-inflammatory activity of some Central American plants. J Ethnopharmacol. 2002;81:211–5. doi: 10.1016/s0378-8741(02)00080-6. [DOI] [PubMed] [Google Scholar]


