Abstract
Background:
Inflammation of adipocytes has been a therapeutic target for treatment of obesity and metabolic disorders which cause insulin resistance and hence lead to type II diabetes. Luteolin is a bioflavonoid with many beneficial properties such as antioxidant, antiproliferative, and anti-cancer.
Objectives:
To elucidate the potential anti-inflammatory response and the underlying mechanism of luteolin in 3T3-L1 adipocytes.
Materials and Methods:
We stimulated 3T3-L1 adipocytes with the mixture of tumor necrosis factor-α, lipopolysaccharide, and interferon-γ (TLI) in the presence or absence of luteolin. We performed Griess’ method for nitric oxide (NO) production and measure mRNA and protein expressions by real-time polymerase chain reaction and western blotting, respectively.
Results:
Luteolin opposed the stimulation of inducible nitric oxide synthase and NO production by simultaneous treatment of adipocytes with TLI. Furthermore, it reduced the pro-inflammatory genes such as cyclooxygenase-2, interleukin-6, resistin, and monocyte chemoattractant protein-1. Furthermore, luteolin improved the insulin sensitivity by enhancing the expression of insulin receptor substrates (IRS1/2) and glucose transporter-4 via phosphatidylinositol-3K signaling pathway. This inhibition was associated with suppression of Iκ-B-α degradation and subsequent inhibition of nuclear factor-κB (NF-κB) p65 translocation to the nucleus. In addition, luteolin blocked the phosphorylation of ERK1/2, c-Jun N-terminal Kinases and also p38 mitogen-activated protein kinases (MAPKs).
Conclusions:
These results illustrate that luteolin attenuates inflammatory responses in the adipocytes through suppression of NF-κB and MAPKs activation, and also improves insulin sensitivity in 3T3-L1 cells, suggesting that luteolin may represent a therapeutic agent to prevent obesity-associated inflammation and insulin resistance.
Keywords: 3T3-L1 adipocytes, inflammation, luteolin, mitogen-activated protein kinases, nuclear factor-κB, obesity
INTRODUCTION
The prevalence of obesity has been increased due to various factors such as genetic, metabolic, behavioral and environmental. It increases the risk factor for metabolic diseases like type II diabetes, cardiovascular diseases, fatty liver diseases, atherosclerosis, multiple sclerosis, and even some forms of cancer.[1] Obesity is characterized by the low-grade chronic inflammation of adipocytes and such inflammation is the important mechanism of causing insulin resistance.[1,2] Several reports have indicated that chronic low grade inflammation is characterized by the dysregulated cytokine production, enhanced inflammatory mediators and activation of signaling associated with obesity, insulin resistance and hence type II diabetes.[3] Adipose tissue is important not only for storage of energy but is also a site of increased macrophage infiltration and the inflammatory response in obesity. The well-known adipokines/cytokines secreted by adipocytes are tumor necrosis factor (TNF)-α, interleukin (IL)-6, monocyte chemoattractant protein (MCP)-1, resistin, leptin, adiponectin, and serum amyloid A.[1,4] These adipokines play an important role in the adipocyte inflammation and regulation of insulin. Furthermore, adipose tissue is an important site of increased nitric oxide (NO) production by inducible nitric oxide synthase (iNOS) upon lipopolysaccharide (LPS) stimulation and its production has been shown to be critical in the generation of insulin resistance in adipose tissues.[5,6] It has been shown that NOS2-/-knockout mice, which are unable to produce NO, are protected from obesity-induced insulin resistance.[7]
In the context, obesity-induced insulin resistance, the nuclear factor-κB (NF-κB) and mitogen-activated protein kinases (MAPKs) pathways have been reported to play a vital role.[8,9] NF-κB is sequestered in the cytoplasm in an inactive form by inhibitory protein IKB. In response to various stimuli, IκB is phosphorylated by IkB kinases and degraded by proteasomes. Subsequently, NF-κB is translocated to the nucleus in an active form and promotes the expression of several target genes whose products induce insulin resistance.[10] On the other hand, c-Jun N-terminal Kinases (JNK), component of the MAPKs signaling, is shown to promote insulin resistance through the serine phosphorylation of insulin receptor substrate-1 (IRS-1).[4] Recent studies have shown that when insulin-resistant or type II diabetic patients were administered aspirin or salsalate, the glycemic control of the patients improved, along with the inhibition of NF-κB activity.[11] Similarly, the deletion of JNK improved obesity-associated insulin resistance.[12] Insulin signaling is a cascade of events initiated by the activation of IRS. IRS-1 and IRS-2 are related with phosphatidylinositol 3-kinase (PI3K), which is a central pathway for stimulation of glucose transporter (GLUT)-4-mediated increase in glucose transport.[13] Then translocation of GLUT-4 from the intracellular compartment to the plasma membrane causes the entry of glucose into the fat cells.[11,12]
In the recent years, many inventions are done on the obesity-induced inflammation and insulin resistance to improve type II diabetes. Among bioactive compounds, flavonoids are naturally occurring compounds with anti-inflammatory and anti-adipogenic properties. Luteolin (3, 4, 5, 7-tetrahydroxy flavone) is a flavonoid found in various plants like Terminalia chebula, celery, broccoli, green pepper, perilla leaf and seed, carrots, olive oil, and medical herbs etc.[14] Luteolin has many biological effects such as anti-oxidant, anti-inflammation, anti-allergy, and anti-cancer.[15,16] Its anti-inflammatory effect is partly by its antioxidant capacity in macrophages and synoviocytes.[17,18] The study also reported that luteolin improves insulin resistance through activation of PPARγ transcription activity and inhibits adipogenesis in 3T3-L1 adipocytes.[19,20] However, the role of luteolin in the inhibition of obesity-induced inflammation responses in adipocytes has not been studied.
Herein, we identified the potential anti-inflammatory properties of luteolin on obesity-associated inflammatory response and elucidate important molecular mechanisms that underlie its regulation of obesity-induced inflammation. This study is the first approach regarding luteolin inhibits inflammation in adipocytes stimulated with tumor necrosis factor-α, lipopolysaccharide, and interferon-γ (TLI) which resembled with obesity-induced inflammation.
MATERIALS AND METHODS
Materials and reagents
Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were purchased from hyclone. Luteolin, recombinant mouse TNF-α and interferon (IFN γ), dexamethasone (Dex), insulin, 3-isobutyl-1-methylxanthine (IBMX) and RNase A were bought from Sigma. Trizol reagent and Superscript III kit were obtained from Invitrogen. Cell counting kit-8 (CCK-8) was purchased from Dojindo Molecular Technologies (Rockville, MD). Protein assay kit (RIPA buffer), rabbit and mouse secondary antibodies, and anti-β-actin antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
3T3-L1 Cell culture and treatment
Murine 3T3-L1 preadipocytes were obtained from American Type Culture Collection and maintained in DMEM supplemented with 10% FBS and 1% of 100 units/ml penicillin and 100 units/ml streptomycin (P/S) and incubated at 37°C in 5% CO2 incubator in a humidified condition. Cells were induced to differentiate 2 days post-confluence (day 0) by adding 0.5 mM IBMX, 1 μM Dex, and 10 μg/ml insulin (MDI) for 48 h.[21] Then culture medium was changed to DMEM/10% FBS containing insulin and medium was replaced in every 2 days interval. After 8 days of differentiation, cells were pretreated with luteolin 1 h and then stimulated with TLI until the time required for the following tests.
Cell viability
The matured adipocytes were treated with various concentrations of the luteolin 24 h and then cell viability was measured using CCK-8 kit, according to the manufacturer's recommendation. Absorbance was measured at 450 nm on a microplate reader (Biochrom Anthos Zenyth 200, UK).
Nitric oxide assay
The production of NO was estimated by measuring a stable nitrite using Griess reagent as described.[22] Briefly, 3T3-L1 adipocytes were pretreated with the indicated concentration of luteolin for 1 h before stimulation with TLI. Then cells were stimulated with TLI for 24 h, 100 μl of supernatant was mixed with 50 μl of 1% sulfanilamide in 5% phosphoric acid in 96-well plate and incubated for 10 min. Then 0.1% N-(1-naphyl) ethylenediamine dihydrochloride was added in the moisture and incubated for further 10 min at room temperature in the dark. The absorbance was measured in 550 nm using a spectrophotometer and the amount of NO was measured by calculating standard curve given by sodium nitrite.
Enzyme-linked immunoassay
3T3-L1 adipocytes were pre-treated with different concentration of luteolin for 1 h and then stimulated by TLI for 24 h then the supernatant was harvested and kept at −80°C until use. The adipokines such as MCP-1 and IL-6 were assayed by using a mouse MCP-1 enzyme-linked immunoassay (ELISA) kit BD OptEIA TM (Cat. No. 555260, BD Biosciences, San Diego, CA, USA) and IL-6 ELISA MAX TM Deluxe Sets (Cat. No. 31304, BioLegends, San Diego, CA, USA), respectively. The assay was performed according to the manufacturer's protocol.
Real-time polymerase chain reaction
Total RNA was extracted using Trizol reagent, according to the manufacturer's protocol. The total RNA (2 μg) was used for cDNA synthesis using Super Script™ III kit. Then mRNA expression was quantitatively determined by ABI real-time polymerase chain reaction (PCR) system from Applied Biosystem Inc (Forster City, CA) using SYBR green PCR Master Mix (Life technologies). GAPDH was the invariant control. The primer sequences used were: GAPDH, sense (5’-CAT GGC CTT CCG TGT TC-3’) and antisense (5’-CCT GGT CCT CAG TGT AGC-3’); iNOS, sense (5’-CAG CTG GGC TGT ACA AAC-3’) and antisense (5’-CAT TGG AAG TGA AGC GGT TCG-3’); cyclooxygenase (COX2), sense (5’-GAA GTC TTT GGT CTG GTG CCT G-3’) and antisense (5’-GTC TGC TGG TTT GGA ATA GTT GC-3’); IL-6, sense (5’-CCG GAG AGG AGA CTT CAC AG-3’) and antisense (5’-TCC ACG ATT TCC CAG AGA AC-3’); CCL2, sense (5’-CCA AAT GAG TAG GCT GGA GA-3’) and antisense (5’-TCT GGA CCC ATT CCT TCT TG-3’); Resistin, sense (5’-AGC TGT GGG ACA GGA GCT AA-3’) and antisense (5’-AGG AAA AGG AGG GGA AAT GA-3’); IRS-1, sense (5’-TCA ACA GCA GTC CCT ACC AC-3’) and antisense (5’-GCT GTG ATG TCC AGT TAC GC-3’); IRS-2, sense (5’-TCC AGA ACG GCC TCA ACT AT0-3’) and antisense (5’-AGT GAT GGG ACA GGA AGT CG-3’); and Glut 4, sense (5’-CAT GGC TGT CGC TGG TTT-3’) and antisense (5’-AAA CCC ATG CCG ACA ATG-3’).
Preparation of whole cell and nuclear extract
3T3-L1 adipocytes were pretreated with different concentration of luteolin for 24 h then stimulated with TNFα for 30 min. Cells were harvested and washed with ice-cold phosphate-buffered saline (PBS) for two times. The pellet was resuspended with 30 μl of RIPA lysis buffer (Chem Cruz) for whole cell lysate and then incubated for 40 min in ice. Then it was centrifuged to 12,000 rpm for 20 min at 4°C and then after supernatant was collected and stored at − 80°C. For nuclear extract, after washing cell pellet with PBS, cytoplasmic buffer (10 mM HEPES, pH 7.9, 10 mM KCl, 0.1 mM EDTA, 1 mM DTT and protease inhibitor) was added and incubated for 10 min. Then 10 μl of 10% NP-40 was mixed and vertex vigorously, followed by centrifugation at 6,000 rpm at 4°C for 4 min to pellet nuclei. Then the pellet was suspended with nuclear buffer containing 20 mM HEPES (pH 7.9), 0.4 M NaCl, 1 mM EDTA, 1 mM DTT and protease inhibitors. After incubation on ice for 15 min, nuclear protein was separated by centrifugation at 14,000 rpm for 5 min at 4°C and stored at −80°C.
Western blot analysis
Cells were lysed in ice-cold RIPA buffer for 40 min and centrifuged (12,000 g) for 20 min at 4°C.[23] Protein concentration was measured using a bicinchoninic acid method. Total 30 μg lysate were run on sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Amersham Pharmacia Biotech). Then, blocking was performed with 5% skimmed milk in tris-buffered saline containing 0.1% tween-20 (TBST) for 1 h at room temperature, and the membranes were probed with primary antibodies as indicated at 4°C overnight, washed with TBST for four times, and subsequently incubated with horseradish peroxidase-conjugated secondary antibody for 45 min. Again washed with TBST for three times and proteins were visualized using an enhanced chemiluminescence detection kit (Millipore, MA, USA).
Statistical analysis
All values were expressed as means ± standard error of the mean statistical significance was determined using the Student's t-test. The P < 0.05 were considered statistically significant.
RESULTS
Effect of luteolin on viability of 3T3-L1 adipocytes
Although luteolin has been reported to have anti-inflammatory effects in macrophages.[16,24] role of luteolin in obesity-linked inflammation has not been studied. Thus, we determined the effect of luteolin in inflammation associated with obesity. We first examined the viability 3T3-L1 adipocytes upon treatment with different concentrations of luteolin. Figure 1 shows that luteolin up to 10 μM did not affect adipocytes viability. Thus, further experiments were performed in the presence of (2.5–10) μM of luteolin.
Figure 1.
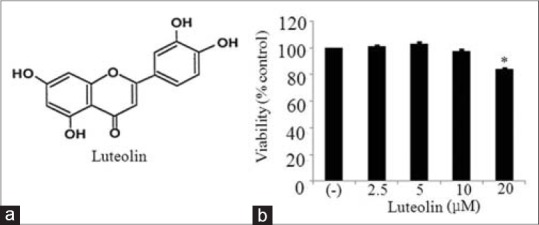
(a) Chemical structure of luteolin and (b) the effect of luteolin on the viability in 3T3-L1 adipocytes. 3T3-L1 cells were treated with different concentration of luteolin for 24 h and subjected to cell counting kit-8 assay. The results are expressed as the mean ± standard error of the mean (n = 3) (*P < 0.05)
Tumor necrosis factor-α, lipopolysaccharide and interferon-γ induced the expression of adipokines in 3T3-L1 adipocytes
We investigated the maximum expression of pro-inflammatory mediators (iNOS, COX2, IL-6, CCL2, and resistin) by adipocytes in the presence of single component or in combination of TNFα (10 ng/ml), LPS (10 ng/ml), and IFNγ (10 ng/ml), collectively called as TLI. Result indicated that the combination of all three components showed maximum expression of the inflammatory mediators when compared to the cells treated singly with TNFα or TNFα/LPS as shown in Figure 2. Thus, we chose TLI combination treatment in all further experiments.
Figure 2.
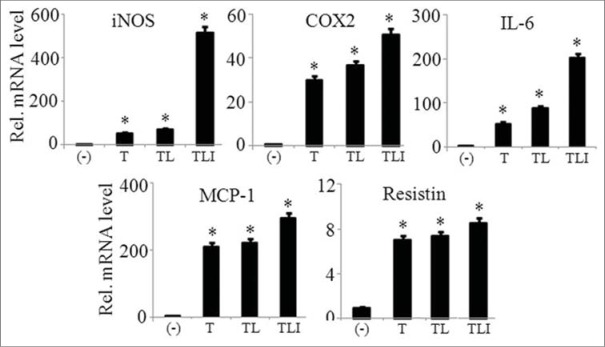
TLI significantly induced the mRNA expression of adipokines in 3T3-L1 adipocytes. 3T3-L1 adipocytes were stimulated with tumor necrosis factor (TNF)-α (10 ng/ml) alone or in combination with lipopolysaccharide (LPS) (10 ng/ml), or LPS and interferon (IFNγ) (10 ng/ml) for 6 h. Then mRNA expression of inducible nitric oxide synthase, cyclooxygenase-2, interleukin-6 MCP-1, and resistin was evaluated. The results are expressed as the mean ± standard error of the mean (n = 3). *P < 0.05. T: TNF-α, TL: TNF-α +LPS and TLI: Tumor necrosis factor-α + lipopolysaccharide + interferon-γ 10 ng/ml
Luteolin inhibited inducible nitric oxide synthase expression and nitric oxide production in tumor necrosis factor-α, lipopolysaccharide, and interferon-γ treated 3T3-L1 adipocytes
To determine the anti-inflammatory effect of luteolin in adipocytes, we determined the mRNA and protein expression of iNOS and production of NO in TLI stimulated adipocytes. Luteolin inhibited mRNA expression of iNOS in a concentration-dependent manner as illustrated in Figure 3a. At 10 μM, the protein expression of iNOS was suppressed by 85% as shown in Figure 3b and c. Furthermore, the production of NO was measured after 24 h of treatment of luteolin by Griess’ method. TLI significantly induced the NO production in matured adipocytes which was inhibited by luteolin in a concentration-dependent manner as indicated in Figure 3d.
Figure 3.
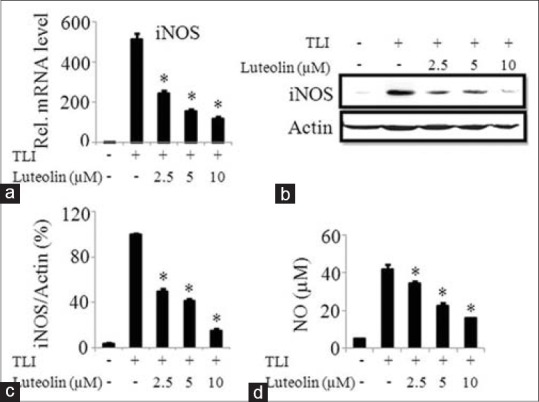
Luteolin inhibited both mRNA and protein expression of inducible nitric oxide synthase (iNOS), and the production of nitric oxide in TLI stimulated 3T3-L1 adipocytes. The cells were pretreated with various concentration of luteolin and then induced with TLI for 6 h for mRNA expression and 24 h to assay protein levels and nitric oxide (NO) production. Effect of luteolin in TLI-induced (a) mRNA and (b) protein expression and (c) band intensity ratio of iNOS and actin and (d) NO production were evaluated. Data are expressed as the mean ± standard error of the mean (n = 3). *P < 0.05. TLI: Tumor necrosis factor-α+ lipopolysaccharide + interferon γ 10 ng/ml, L: Luteolin
Luteolin suppressed the inflammatory mediators in tumor necrosis factor-α, lipopolysaccharide, and interferon-γ treated 3T3-L1 adipocytes
The effect of luteolin on the mRNA expression of inflammatory mediators such as COX2, IL-6, MCP-1, and resistin were investigated in matured adipocytes. Figure 4 demonstrates that luteolin inhibited the mRNA expression of above named inflammatory mediators in a dose-dependent manner. To further confirm the observation that luteolin inhibits inflammatory genes expression, we measured the protein levels of IL-6 and MCP-1 in the cells treated with or without luteolin. Luteolin reduced the production of IL-6 in a significant manner as shown in Figure 5a. Similar result of the inhibitory effect of luteolin was shown with MCP-1 production as illustrated in Figure 5b.
Figure 4.
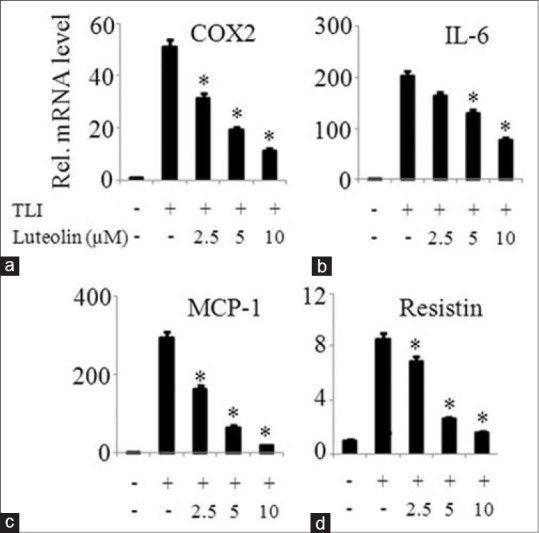
Luteolin inhibited expression of inflammatory mediators in TLI stimulated 3T3-L1 adipocytes. The cells were pretreated with various concentration of luteolin and then induced with TLI for 6 h. The mRNA expression of (a) cyclooxygenase-2, (b) interleukin-6, (c) MCP-1 and (d) resistin were determined. Data are expressed as the mean ± standard error of the mean (n = 3). *P < 0.05. TLI: Tumor necrosis factor-α + lipopolysaccharide + interferon γ 10 ng/ml, L: Luteolin
Figure 5.
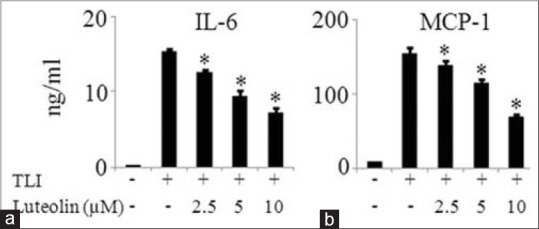
Luteolin inhibited protein expression of MCP-1 and interleukin (IL)-6 in TLI stimulated 3T3-L1 adipocytes. The cells were pretreated with various concentration of luteolin and then induced with TLI for 24 h. Then, cell supernatant was harvested for enzyme-linked immunoassay technique to measure levels (a) IL-6 and (b) MCP-1. Data are expressed as the mean ± standard error of the mean (n = 3). *P < 0.05. TLI: Tumor necrosis factor-α + lipopolysaccharide + interferon γ 10 ng/ml, L: Luteolin
Luteolin regulates the major targets of insulin signaling in tumor necrosis factor-α, lipopolysaccharide, and interferon-γ treated 3T3-L1 adipocytes
Both IRS (1 and 2) along with GLUT-4 expressions are suppressed during insulin resistance occurred by obesity or type 2 diabetes.[11,13] We investigated the mRNA expressions of these mentioned genes in TLI stimulated in presence or absence of luteolin in 3T3-L1 adipocytes. Our results demonstrate that luteolin significantly enhanced the mRNA expressions of IRS1/2 and GLUT-4 genes when compared to non-treated cells in a dose-dependent manner as shown in Figure 6a. To further illustrate the mechanism of insulin signaling in 3T3-L1 adipocytes, we determined the protein level of PI3K by western blotting. PI3K expression was inhibited by TNFα when compared to non-treated cells, but it was significantly upregulated by luteolin in dose-dependent manner as shown in Figure 6b and c. This result illustrates that luteolin enhances insulin sensitivity by the activation of PI3K signaling cascade.
Figure 6.
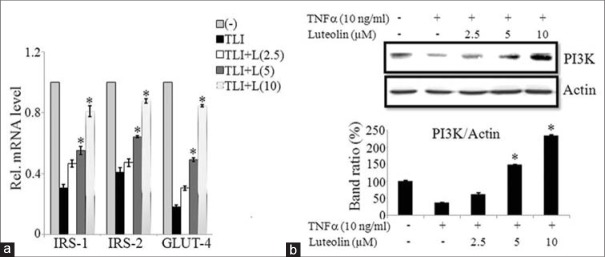
Luteolin improves insulin sensitivity in 3T3-L1 cells via phosphatidylinosito-3K (PI3K) signaling. (a) Luteolin increased the expression of targets of insulin signaling genes insulin receptor substrates (IRS)-1, IRS-2 and glucose transporter-4 in TLI stimulated 3T3-L1 cells. (b) Luteolin enhanced the tumor necrosis factor (TNF) a-induced activation of PI3K signaling pathway in 3T3-L1 adipocytes. (c) Bar diagram represents relative band intensity of the blots from three experiments. Data are expressed as the mean ± standard error of the mean (n = 3). *P < 0.05. TLI: TNFα+ lipopolysaccharide + interferon γ 10 ng/ml, L: Luteolin
Luteolin suppressed nuclear factor-κB signaling activated by tumor necrosis factor-α in 3T3-L1 adipocytes
Several studies have reported that luteolin exhibits anti-inflammatory effects through NF-κB pathway.[16,18] To elucidate the anti-inflammatory mechanisms of luteolin in 3T3-L1 adipocytes, we investigated the effect of luteolin on NF-κB activation induced by TNFα. For this purpose, cells were treated with luteolin for 24 h and then stimulated with TNFα for 30 min. Then cytoplasmic and nuclear p65 levels were determined by western blot. Results showed that luteolin inhibited the downregulation of IκBα when compared to control cells. Furthermore, luteolin prevented the translocation of p65 subunits of NF-κB from cytosol into the nucleus as demonstrated by reduced levels of p65 in the nuclear fraction and enhanced levels of it in cytoplasmic fraction as shown in Figure 7a and b.
Figure 7.
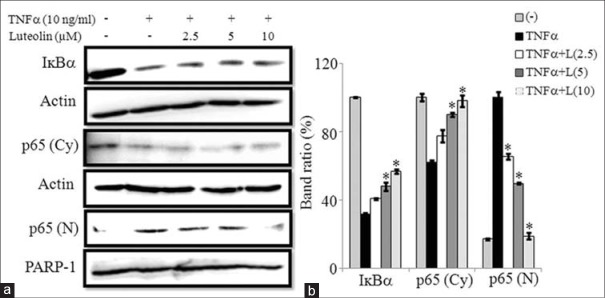
Luteolin inhibited the tumor necrosis factor (TNF) α-induced activation of nuclear factor-κB (NF-κB) in 3T3-L1 adipocytes. The cells were pretreated with different concentration of luteolin for 24 h and then stimulated with TNF-α for 30 min. (a) Immunoblot analysis for NF-κB. (b) Bar diagram represents relative band intensity of the blots from three experiments. Data are expressed as the mean ± standard error of the mean (n = 3). *P < 0.05. T: TNFα 10 ng/ml, L: Luteolin
Luteolin attenuated mitogen-activated protein kinases signaling activated by tumor necrosis factor-α in 3T3-L1 adipocytes
We tested whether luteolin inhibits MAPKs activation in 3T3-L1 adipocytes. Various concentrations of luteolin were treated in matured adipocytes for 24 h and then induced by TNFα for 30 min. The western blot results showed that luteolin inhibits the phosphorylation of ERK1/2, JNK, and p38 MAPKs in concentration-dependent manner. These findings suggested that luteolin inhibits activation of MAPKs signaling pathway in adipocytes to suppress obesity-linked inflammation as illustrated in Figure 8a and b.
Figure 8.
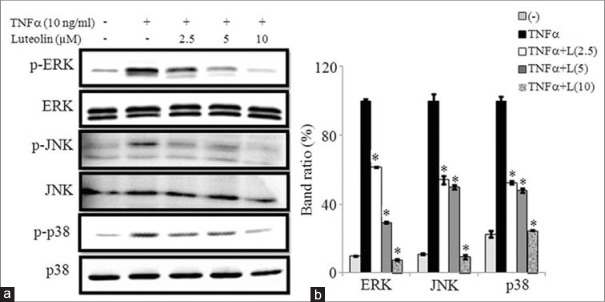
Luteolin inhibited the tumor necrosis factor (TNF)-α-induced activation of mitogen-activated protein kinases (MAPKs) in 3T3-L1 adipocytes. The cells were pretreated with different concentration of luteolin for 24 h and then stimulated with TNF-α for 30 min. (a) Immunoblot analysis for the phosphorylation and total forms of ERK, c-Jun N-terminal Kinases and p38 MAPK. (b) Bar diagram represents relative band intensity of the blots from three experiments. Data are expressed as the mean ± standard error of the mean (n = 3). *P < 0.05. T: TNF-α 10 ng/ml, L: Luteolin
DISCUSSION
Obesity induces chronic inflammation, characterized by the proinflammatory polarization of adipose tissue innate and adaptive resident and recruited immune cells eventually leading to insulin resistance.[25] The proinflammatory cytokines such as iNOS, COX2, IL-6, MCP-1, and TNFα participate in the induction and maintenance of the chronic inflammatory responses and are perceived in obesity-induced diseases.[26,27] The obesity-induced inflammation leads to various metabolic disorders like type II diabetes, atherosclerosis, cardiovascular diseases, fatty liver diseases, multiple sclerosis, some types of cancer.[1]
There are many drugs for the treatment of obesity-induced inflammation like non-steroidal anti-inflammatory drug, immune suppressant and IL-6 signaling inhibitor like tocilizumab.[28] Among these drugs, aspirin is the well-known promising anti-inflammatory drug that decreases inflammation and also improves type II diabetes. Aspirin is an NSAID that inhibits COX enzyme and prostaglandin synthesis, also IL-1 and TNFα.[28,29,30] The study had showed that low-dose of aspirin reduced pro-inflammatory genes such as IL-6 and MCP-1 in adipose tissue of obese mice.[7] However, regular uptake of aspirin for a longer time increased the risk of gastrointestinal and cerebral bleeding. Other NSAIDs can increase the risk of myocardial infarction and also damage kidney. Therefore, it is necessary to develop novel therapeutic agent using a natural product molecules with anti-inflammatory effects.
Despite a variety of therapeutic properties of luteolin discovered so far, the role of it in obesity-associated inflammation has not been investigated so far. In the current study, we determined whether luteolin attenuates inflammatory responses in 3T3-L1 adipocytes. In our model of obesity-induced inflammation, we treated the matured adipocytes in the presence of TLI, singly or in combination of each molecule, to know maximum activation of adipocytes to enhance adipokines expression. Consistent to the previous report,[31] our data showed that the combination of three molecules contributed in achieving maximum expression of the adipokines, suggesting that TLI could maximally activate adipocytes when compared to single molecules treatment. Thus, we chose TLI stimulation in all our further experiments.
Luteolin up to 10 μM did not affect the viability of 3T3-L1 adipocytes. Thus, we chose the highest concentration of luteolin up to this level to rule out the possibility that luteolin-induced cytotoxicity results in suppression of adipocytes inflammation.
It has been reported that iNOS is a key mediator of inflammation in obesity-induced insulin resistance.[4,32] The expression of iNOS and its product NO is increased adipose tissue, skeletal muscle, and liver, and contribute to inflammation in obesity.[33,34] Our data showed that luteolin significantly inhibits the expression of iNOS and NO in TLI-induced adipocytes. Similar to our results, a study reported that butein, a polyphenolic compound, inhibits the expression of INOS and NO in adipocytes treated with TLI.[31] Furthermore, luteolin also inhibited the expression of COX-2, an enzyme which is responsible for inflammation and acts downstream of NO signaling. Many immunological phenomena during obesity-associated inflammation are mediated by cytokines and other bioactive molecules, such as IL-6, MCP-1, and resistin.[4] Our data demonstrated that adipocytes treated with luteolin showed lowered expression of IL-6, MCP-1, and resistin, when compared to control. In line with our data, the previous report have indicated that caffeic acid phenethyl ester, an active component of propolis from honeybee hives, exerts an anti-inflammatory effect on adipocytes through inhibition of IL-6 and MCP-1 expression.[35] Similarly, a recent study showed that paeoniflorin, plant derived glucoside, inhibits IL-6 and MCP-1 expression in adipocytes and contributes to reduced inflammation when compared to control.[36] Further, we sought to dissect a signaling mechanism associated with inhibition of obesity-induced inflammation by luteolin in 3T3-L1 adipocytes. We speculated that luteolin may inhibit a regulator that activates the proinflammatory genes expression. In addition, we evaluated the potential role of luteolin in improving the insulin sensitivity in adipocytes. For this, we measured the major targets of insulin signaling cascade in the cells. IRS1/2 and PI3K are important molecules in insulin signaling cascade.[11,12,13] Our result showed that luteolin increased the expression of IRS-1/2 mRNA in a dose-dependent manner when compared to the non-treated cells. Furthermore, luteolin increased the protein levels of PI3K significantly. These results were similar to previous reports which showed that a plant saponin, Ginsenoside Re, improves the insulin sensitivity via IRS-1/2 and PI3K pathway in adipocytes.[13]
Previous reports have shown that the anti-inflammatory effect of luteolin in mouse alveolar macrophages and synoviocytes was due to inactivation of NF-κB pathway.[17,18] In addition, obesity-associated inflammation has been correlated with activation of NF-κB signaling.[12] After activating stimuli, an inhibitory molecule, IκBα, is phosphorylated, and NF-κB p65 component translocates to the nucleus and regulates transcription of several pro-inflammatory genes.[11] Our result showed that luteolin inhibited the downregulation of IκBα in a dose-dependent manner when compared to control adipocytes. We also determined p65 level in the cytoplasm and nuclear extract by western blotting. Our result showed that luteolin blocked the translocation of p65 from cytosol to the nucleus, suggesting that luteolin inhibits adipocytes inflammation by blocking the activation of NF-κB. These findings were similar to a report showing the anti-inflammatory role of butein via inhibition of NF-κB activation in adipocytes.[31]
Furthermore, previous reports have suggested that anti-inflammatory actions of plant flavonoids could be mediated through inhibition of MAPKs activation.[37,38] Furthermore, the activation of MAPKs is related with an enhanced expression of iNOS and NO in macrophages.[38] In addition, MAPKs pathway has also been shown to play a crucial role in adipocytes-associated inflammation process.[9] Hence, we sought to investigate the role of MAPKs in adipocytes treated with or without luteolin. Our data showed that luteolin significantly suppressed phosphorylation of ERK, JNK, and p38 MAPKs in a concentration-dependent manner. These data suggested that luteolin inhibits MAPKs signaling pathway activation, which could contribute to abrogation of the adipocyte inflammation. Similar to our results, previous reports have indicated that ERK, JNK, and p38 MAPKs phosphorylation inhibition is important for attenuation of the adipocyte inflammation.[31]
Collectively, these data demonstrate that luteolin attenuates adipocytes inflammatory responses and improves insulin sensitivity in the 3T3-L1 cells. Further studies on the effects of luteolin in vivo will elucidate if it has therapeutic potential for obesity-associated inflammation and insulin resistance.
ACKNOWLEDGMENTS
The present study was financially supported by the Ministry of Knowledge Economy (MKE), Korea Institute for Advancement of Technology (KIAT) through the Inter-ER Cooperation Projects (R0002019).
Footnotes
Source of Support: Ministry of Knowledge Economy (MKE), Korea Institute for Advancement of Technology (KIAT) through the inter-ER Cooperation Projects (R0002019).
Conflict of Interest: None declared.
REFERENCES
- 1.Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012;15:635–45. doi: 10.1016/j.cmet.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 3.Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci U S A. 2003;100:7265–70. doi: 10.1073/pnas.1133870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ribiere C, Jaubert AM, Gaudiot N, Sabourault D, Marcus ML, Boucher JL, et al. White adipose tissue nitric oxide synthase: A potential source for NO production. Biochem Biophys Res Commun. 1996;222:706–12. doi: 10.1006/bbrc.1996.0824. [DOI] [PubMed] [Google Scholar]
- 6.Linscheid P, Keller U, Blau N, Schaer DJ, Müller B. Diminished production of nitric oxide synthase cofactor tetrahydrobiopterin by rosiglitazone in adipocytes. Biochem Pharmacol. 2003;65:593–8. doi: 10.1016/s0006-2952(02)01562-9. [DOI] [PubMed] [Google Scholar]
- 7.Perreault M, Marette A. Targeted disruption of inducible nitric oxide synthase protects against obesity-linked insulin resistance in muscle. Nat Med. 2001;7:1138–43. doi: 10.1038/nm1001-1138. [DOI] [PubMed] [Google Scholar]
- 8.Suganami T, Nishida J, Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: Role of free fatty acids and tumor necrosis factor alpha. Arterioscler Thromb Vasc Biol. 2005;25:2062–8. doi: 10.1161/01.ATV.0000183883.72263.13. [DOI] [PubMed] [Google Scholar]
- 9.Park KS. Aucubin, a naturally occurring iridoid glycoside inhibits TNF-α-induced inflammatory responses through suppression of NF-κB activation in 3T3-L1 adipocytes. Cytokine. 2013;62:407–12. doi: 10.1016/j.cyto.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Gu BH, Minh NV, Lee SH, Lim SW, Lee YM, Lee KS, et al. Deoxyschisandrin inhibits H2O2-induced apoptotic cell death in intestinal epithelial cells through nuclear factor-kappaB. Int J Mol Med. 2010;26:401–6. [PubMed] [Google Scholar]
- 11.Lee BC, Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim Biophys Acta. 2014;1842:446–62. doi: 10.1016/j.bbadis.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–6. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 13.Gao Y, Yang MF, Su YP, Jiang HM, You XJ, Yang YJ, et al. Ginsenoside Re reduces insulin resistance through activation of PPAR-γ pathway and inhibition of TNF-α production. J Ethnopharmacol. 2013;147:509–16. doi: 10.1016/j.jep.2013.03.057. [DOI] [PubMed] [Google Scholar]
- 14.Pandurangan AK, Kumar SA, Dharmalingam P, Ganapasam S. Luteolin, a bioflavonoid inhibits azoxymethane-induced colon carcinogenesis: Involvement of iNOS and COX-2. Pharmacogn Mag. 2014;10:S306–10. doi: 10.4103/0973-1296.133285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin Y, Shi R, Wang X, Shen HM. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr Cancer Drug Targets. 2008;8:634–46. doi: 10.2174/156800908786241050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen CY, Peng WH, Tsai KD, Hsu SL. Luteolin suppresses inflammation-associated gene expression by blocking NF-kappaB and AP-1 activation pathway in mouse alveolar macrophages. Life Sci. 2007;81:1602–14. doi: 10.1016/j.lfs.2007.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park CM, Jin KS, Lee YW, Song YS. Luteolin and chicoric acid synergistically inhibited inflammatory responses via inactivation of PI3K-Akt pathway and impairment of NF-κB translocation in LPS stimulated RAW 264.7 cells. Eur J Pharmacol. 2011;660:454–9. doi: 10.1016/j.ejphar.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Choi EM, Lee YS. Luteolin suppresses IL-1beta-induced cytokines and MMPs production via p38 MAPK, JNK, NF-kappaB and AP-1 activation in human synovial sarcoma cell line, SW982. Food Chem Toxicol. 2010;48:2607–11. doi: 10.1016/j.fct.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 19.Ding L, Jin D, Chen X. Luteolin enhances insulin sensitivity via activation of PPARγ transcriptional activity in adipocytes. J Nutr Biochem. 2010;21:941–7. doi: 10.1016/j.jnutbio.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Poudel B, Nepali S, Xin M, Ki HH, Kim YH, Kim DK, et al. Flavonoid from Triticum aestivum inhibits adipogenesis in 3T3-L1 cells through up-regulation of the insig pathway. Mol Med Rep. 2015 doi: 10.3892/mmr.2015.3700. In Press. [DOI] [PubMed] [Google Scholar]
- 21.Poudel B, Lim SW, Ki HH, Nepali S, Lee YM, Kim DK. Dioscin inhibits adipogenesis through the AMPK/MAPK pathway in 3T3-L1 cells and modulates fat accumulation in obese mice. Int J Mol Med. 2014;34:1401–8. doi: 10.3892/ijmm.2014.1921. [DOI] [PubMed] [Google Scholar]
- 22.Lee SH, Seo GS, Sohn DH. Inhibition of lippopolysaccharide-induced expression of inducible nitric oxide synthase by butein in RAW 264.7 cells. Biochem Biophys Res Commun. 2004;323:125–32. doi: 10.1016/j.bbrc.2004.08.063. [DOI] [PubMed] [Google Scholar]
- 23.Poudel B, Yoon DS, Lee JH, Lee YM, Kim DK. Collagen I enhances functional activities of human monocyte-derived dendritic cells via discoidin domain receptor 2. Cell Immunol. 2012;278:95–102. doi: 10.1016/j.cellimm.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Jung HA, Jin SE, Min BS, Kim BW, Choi JS. anti-inflammatory activity of Korean thistle Cirsium maackii and its major flavonoid, luteolin 5-O-glucoside. Food Chem Toxicol. 2012;50:2171–9. doi: 10.1016/j.fct.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Varol C, Zvibel I, Spektor L, Mantelmacher FD, Vugman M, Thurm T, et al. Long-acting glucose-dependent insulinotropic polypeptide ameliorates obesity-induced adipose tissue inflammation. J Immunol. 2014;193:4002–9. doi: 10.4049/jimmunol.1401149. [DOI] [PubMed] [Google Scholar]
- 26.Carvalho-Filho MA, Ueno M, Carvalheira JB, Velloso LA, Saad MJ. Targeted disruption of iNOS prevents LPS-induced S-nitrosation of IRbeta/IRS-1 and Akt and insulin resistance in muscle of mice. Am J Physiol Endocrinol Metab. 2006;291:476–482. doi: 10.1152/ajpendo.00422.2005. [DOI] [PubMed] [Google Scholar]
- 27.Hsu CL, Lin YJ, Ho CT, Yen GC. The inhibitory effect of pterostilbene on inflammatory responses during the interaction of 3T3-L1 adipocytes and RAW 264.7 macrophages. J Agric Food Chem. 2013;61:602–10. doi: 10.1021/jf304487v. [DOI] [PubMed] [Google Scholar]
- 28.Ye J, McGuinness OP. Inflammation during obesity is not all bad: Evidence from animal and human studies. Am J Physiol Endocrinol Metab. 2013;304:E466–77. doi: 10.1152/ajpendo.00266.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JK, Kim YJ, Fillmore JJ, Chen Y, Moore I, Lee J, et al. Prevention of fat-induced insulin resistance by salicylate. J Clin Invest. 2001;108:437–46. doi: 10.1172/JCI11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fleischman A, Shoelson SE, Bernier R, Goldfine AB. Salsalate improves glycemia and inflammatory parameters in obese young adults. Diabetes Care. 2008;31:289–94. doi: 10.2337/dc07-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z, Lee Y, Eun JS, Bae EJ. Inhibition of adipocyte inflammation and macrophage chemotaxis by butein. Eur J Pharmacol. 2014;738:40–8. doi: 10.1016/j.ejphar.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 32.Fujimoto M, Shimizu N, Kunii K, Martyn JA, Ueki K, Kaneki M. A role for iNOS in fasting hyperglycemia and impaired insulin signaling in the liver of obese diabetic mice. Diabetes. 2005;54:1340–8. doi: 10.2337/diabetes.54.5.1340. [DOI] [PubMed] [Google Scholar]
- 33.Sugita H, Fujimoto M, Yasukawa T, Shimizu N, Sugita M, Yasuhara S, et al. Inducible nitric-oxide synthase and NO donor induce insulin receptor substrate-1 degradation in skeletal muscle cells. J Biol Chem. 2005;280:14203–11. doi: 10.1074/jbc.M411226200. [DOI] [PubMed] [Google Scholar]
- 34.Salerno L, Sorrenti V, Di Giacomo C, Romeo G, Siracusa MA. Progress in the development of selective nitric oxide synthase (NOS) inhibitors. Curr Pharm Des. 2002;8:177–200. doi: 10.2174/1381612023396375. [DOI] [PubMed] [Google Scholar]
- 35.Juman S, Yasui N, Ikeda K, Ueda A, Sakanaka M, Negishi H, et al. Caffeic acid phenethyl ester suppresses the production of pro-inflammatory cytokines in hypertrophic adipocytes through lipopolysaccharide-stimulated macrophages. Biol Pharm Bull. 2012;35:1941–6. doi: 10.1248/bpb.b12-00317. [DOI] [PubMed] [Google Scholar]
- 36.Kong P, Chi R, Zhang L, Wang N, Lu Y. Effects of paeoniflorin on tumor necrosis factor-α-induced insulin resistance and changes of adipokines in 3T3-L1 adipocytes. Fitoterapia. 2013;91:44–50. doi: 10.1016/j.fitote.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 37.Schmitz ML, Bacher S, Kracht M. I kappa B-independent control of NF-kappa B activity by modulatory phosphorylations. Trends Biochem Sci. 2001;26:186–90. doi: 10.1016/s0968-0004(00)01753-9. [DOI] [PubMed] [Google Scholar]
- 38.Xie C, Kang J, Li Z, Schauss AG, Badger TM, Nagarajan S, et al. The açaí flavonoid velutin is a potent anti-inflammatory agent: Blockade of LPS-mediated TNF-α and IL-6 production through inhibiting NF-κB activation and MAPK pathway. J Nutr Biochem. 2012;23:1184–91. doi: 10.1016/j.jnutbio.2011.06.013. [DOI] [PubMed] [Google Scholar]


