Abstract
Background:
The tubers of Dioscorea zingiberensis, is the most favorable plant material for the production of diosgenin, an important bioactive steroidal sapogenin and requisite precursor of cortin, contraceptive and sex hormone, which is the only desired product after steroidal saponins from the tubers are hydrolyzed.
Objective:
A novel technology, in situ pressurized biphase acid hydrolysis was constructed for the first time to simplify extraction process, increase extraction yield and decrease the consumption of mineral acids.
Materials and Methods:
The method developed in this study has been optimized and verified through orthogonal design for experiments, in which the effect and their significance of four factors including molarity of acid, temperature, extraction duration and sample quantity have been investigated. Then, the comparison was conducted among the newly developed method and other reported methods. The diosgenin was also isolated by column chromatography, followed by mass spectrometry and nuclear magnetic resonance analysis for structural confirmation.
Results:
It was found that temperature is the factor of the most influence and the highest extraction yield at 2.21% has been achieved while the hydrolysis was performed at 140°C for 1.5 h in 0.20M H2SO4 solution with petroleum ether under an uncontrolled pressurized condition. And, compared to the others, the increment in the extraction yield of new method was 20.8 ~ 74.0%, and the consumption of H2SO4 was reduced by 17 times at most.
Conclusion:
This method is a much cleaner and more efficient approach for extraction of diosgenin from the tubers, and is promising to be applied in pharmaceutical industry.
Keywords: Dioscorea zingiberensis, diosgenin, in situ pressurized biphase acid hydrolysis, reverse-phase high performance liquid chromatography
INTRODUCTION
The tubers of Dioscorea zingiberensis, is a tuberous herbaceous perennial liana endemic to Asia, although most of species such as Dioscorea alata (purple yam), Dioscorea opposita (Chinese yam), Dioscorea japonica (Japanese mountain yam) and Dioscorea nipponica (Japanese yam) in the Dioscorea genus (Dioscoreaceae family) are native throughout the tropical and warm temperate regions of the world. Like other yams, the tuber is a popular healthy food rich in starch of high quality and dietary cellulose, however more and more interestingly to researchers and industries, it has most abundant various glycosides of an important chiral steroidal sapogenin, diosgenin [Figure 1] among the plants[1] and recent explorations have revealed that this steriod has many attracting bioactivities, such as anti-cancer, anti-inflammation, anti-thrombosis, inhibiting osteoclastogenesis, invasion, and proliferation, and relieving goiter.[2,3,4,5,6] Moreover, it's the common starting substance for partial synthesis of cortin, oral contraceptives, sex hormones and other steroids in pharmaceutical industry, but it usually occurs in the form of saponins in which either glucose or rhamnose or both sugars were attached to aglycone by C-O glucosidic bonds, and hence an acid hydrolysis procedure was usually required to release this bioactive compound.[7]
Figure 1.
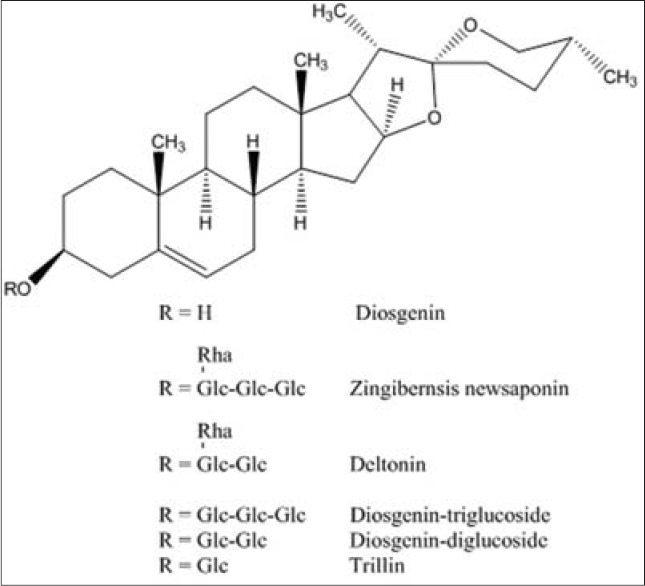
Chemical structure of diosgenin and its glycosides
Conventionally, there are two types of methods for the hydrolysis of saponins to diosgenin, both of which have been widely applied in saponin factories as diagramed [Figure 2]. Although these processes are effective and economic for diosgenin production, they are highly harmful to ambient water bodies due to large consumption of mineral acid, and meanwhile extraction yield is yet to be increased as long-term contact of resulting diosgenin with strong acid during the hydrolysis of plant materials has always caused undesirable side reactions such as dehydration, conversion of configuration (25S → 25R), opening of F ring, and alogenation of hydroxyl. Many effective attempts have been made to develop cleaner and more efficient methods to produce diosgenin in past decades as the demand for the compound is becoming larger and larger, and nowadays approaching up to 6000 tons worldwide. Among those, fermentation or recycling starch and cellulose before acid hydrolysis, and two-phase acid hydrolysis have been well established.[8,9] However, these methods are still leading to severe environmental problems as a great volume of acidic wastewater containing high concentration of SO42− is produced and the production yield of diosgenin has not been increased to a satisfactory extend.[10,11] In this study, a new approach was proposed and established for cleaner and more efficient extraction of diosgenin from the edible tubers of D. zingiberensis.
Figure 2.
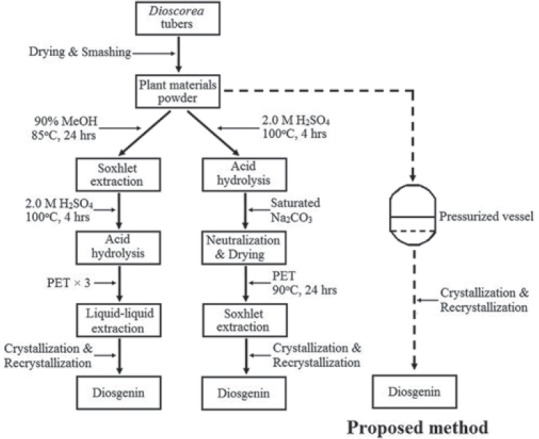
Flow chart of conventional methods and newly proposed method
MATERIALS AND METHODS
Plant material and chemicals
The tubers of D. zingiberensis were purchased from the market in the city of Danjiangkou, Hubei Province, China, and authenticated by Associated Professor Hong-Xia Chen, Pharmacognosy Research Facility, the School of Pharmacy, Jiangsu University. A voucher specimen (DZ130901) has been then deposited in the school. The tubers were dried in an oven below 80°C to remove most of moisture prior to complete lyophilisation for 1 d. The dried tubers were then grounded into powder form, and passed through a 40 mesh sieve. Fine powder was collected and stored in an electronic dry cabinet (RH <30%) at room temperature. Diosgenin standard (purity >98.0% by high performance liquid chromatography-ultraviolet [HPLC-UV], B/N: 20120828) was purchased from Gold Wheat Biotechnology Co., Ltd., China.
1H-NMR and 13C-NMR spectra were obtained by a Bruker AV-400 nuclear magnetic resonance, Switzerland, with TMS as internal standard. Glutathione-0.5 high-pressure reaction kettle was supplied by Weihai Kun Chang Chemical Machinery Co., Ltd., China. Ultrapure water was produced by Millipore Milli-Q Biocel purification systems, France. Silica gel (200–300 mesh) was purchased from Shanghai Sanpont Co., Ltd., China. Acetonitrile (ACN) of HPLC grade was purchased from Honeywell, Korea. And, all other chemicals used in the study were of analytical grade and purchased from Sinopharm Chemical Reagent Co., Ltd., China.
In situ pressurized biphase acid hydrolysis and optimization of the extraction conditions
The newly proposed approach was designed with conformation to imperative need for decreasing the discharge of acidic wastewater and increasing the production yield of diosgenin. As illustrated in Figure 3, an in situ pressurized biphase acid hydrolysis takes place under heating and stirring in a commonly used high-pressure reaction kettle under an uncontrolled pressurized condition. Initially, steroidal saponins were extracted from plant material, and immediately subject to acid hydrolysis in heavy aqueous phase, and then the resulting diosgenin was promptly transferred into light organic phase containing no acid. It can be seen that the production process is much enhanced and simplified as this system has not only effectively protected the chemical group of diosgenin by detaching the product from acid, but combined the extraction and hydrolysis of saponins and the separation of diosgenin into one step.
Figure 3.
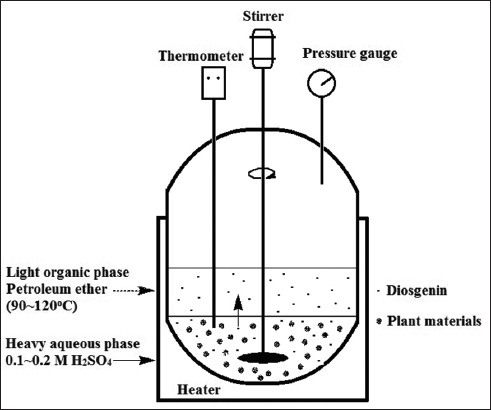
In situ pressurized biphase acid hydrolysis
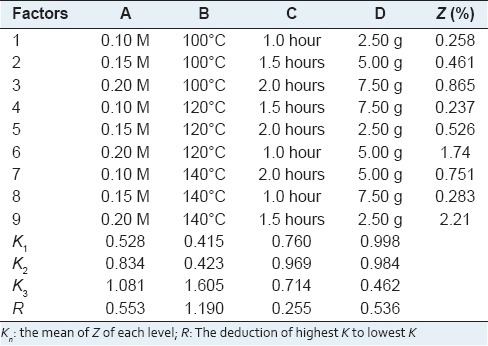
To optimize the condition of in situ pressurized biphase acid hydrolysis, L9 (3)4 orthogonal experiment[12] was designed as shown in Table 1 and carried out according to Table 2. In each experiment, a certain quantity of sample, 50 mL sulfuric acid aqueous solution and 50 mL petroleum ether (90–120°C, PET) were added into the kettle to form two immiscible phases. The solvents were then heated up above 100°C under stirring at constant speed of 100 rpm. After reaction for a certain time, the kettle was cooled down by cold tap water and the organic portion was then transferred into a separating funnel and washed with distilled water twice to completely remove little of sulfuric acid remained in the solution for subsequent preparation.
Table 1.
Factors and levels of L9(3)4 orthogonal experiment design
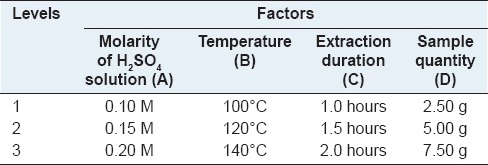
Table 2.
Results and analysis of the orthogonal experiments
Two-phase acid hydrolysis after fermentation
2.5 g of fine powder of the tubers and 50 mL of water were mixed and fermented at 30°C for 72 h under stirring at 500 rpm. Then, 5 mL of H2SO4 solution, 5 mL of MeOH and 50 mL of PET were added into the suspension, respectively, to form a two-phase immiscible reaction system, which was kept at 120°C in oil bath for 4 h to promote the hydrolysis and diosgenin was extracted into PET layer.[13]
Conventional methods
Conventional method 1
2.5 g of fine powder was extracted continuously in Soxhlet extractor for 24 h with 100 mL of 90% MeOH at 85°C, and the solvent was removed from extraction solution under reduced pressure at 60°C. Then, 50 mL of 2.0M H2SO4 solution was mixed with the residue followed by acid hydrolysis for 4 h under boiling water bath. After cooling down, 3 × 100 mL of PET was poured into the suspension for sequential extraction of diosgenin, and all organic solutions were combined.[14]
Conventional method 2
2.5 g of fine powder was hydrolyzed in 50 mL of 2.0 M H2SO4 solution for 4 h at 100°C, and saturated Na2CO3 aqueous solution was added to neutralize solution after cooling down. Then, the resulting suspension was subject to suction filtration, and the residue was washed by ultrapure water until the filtrate reached neutral. It was dried at 60°C for 12 h under reduced pressure, and was subsequently extracted in Soxhlet extractor for another 24 h by PET in 90°C water bath.[15]
High performance liquid chromatography conditions and quantitative analysis of diosgenin
A Shimadzu prominence HPLC instrument, Japan, was equipped with DGU-20A5 degasser, liquid chromatography-20 AT pump, CTO-10AS column oven, FCV-10AL mixer SUS and SPD-20A UV-Vis detector, and data was acquired and processed using the N2000 SP1 software for chromatographic analysis (Zhejiang University, China). The separation was performed on a Waters Sun Fire RP8 column (150 mm × 4.6 mm, 5 μm, Ireland) kept at 35.0°C, and mobile phases consisting of ACN and water (70:30, v/v) were constantly pumped at the flow rate 1.0 mL/min throughout the analysis. 20 μL of sample solution or standard solution were injected into the HPLC system and 203 nm was selected to monitor the separation.[16]
Diosgenin of 10.48 mg was dissolved in EtOH and scaled to 10 mL as stock solution at 1.048 mg/mL, and a series of dilutions were performed to prepare standard solutions ranging from 2.047 × 10− 3 mg/mL to 0.5240 mg/mL. Prior to injection, all the solutions were filtered through 0.45 μm PTFE membrane, respectively. Peak area (Y) versus concentration of diosgenin (X) was plotted to construct regression equation for subsequent calculations.
To prepare sample solution for HPLC analysis, the organic layer cleaned after acid hydrolysis was transferred into a 100 mL volumetric flask and topped up using petroleum ether. Then, 10 mL of the solution was pipetted onto a watch glass and dried completely above boiling waterbath. The residue was reconstituted in EtOH and scaled up to 10 mL of sample solution, which was filtered through 0.45 μm syringe membrane. The initial 2 mL of the solution was discarded and the subsequent 1 mL was collected into HPLC vial for analysis.
Calculation of extraction yield (Z%) and its increment (I%)
The concentration of diosgenin (C) in sample solution was calculated according to the calibration curve simulated and extraction yield of diosgenin (Z%) and its increment (I) were calculated by the following two equations:
Z% = (C × 100 mL/sample quantity) ×100%
I% = (Z1 – Z2)/Z2 × 100%
Z1: Z of in situ pressurized biphase acid hydrolysis
Z2: Z of other methods.
Isolation and elucidation of chemical structure of diosgenin
The organic phase was condensed to dryness under reduced pressure, and the residue was subjected to silica gel column chromatography constantly eluted with PET-EtOAc (7:1, v/v). The eluate was collected and monitored by TLC, and then the solution containing compound of interest was combined and solvent was recycled. The resulting residue was dissolved in hot EtOH at about 50°C with some water added after, and then the mixture was sealed and stored at 4°C in refrigerator to allow crystallization overnight. After crystals were formed, they were collected by suction filtration and flushed with water, and dried in an oven at 105°C for 2 h. The compound isolated above was subject to purity determination and spectrometric analysis, and the chemical structure was then elucidated on the basis of electrospray ionization-mass spectrometry (ESI-MS),1H-NMR and 13C-NMR spectrum and compared to literature data.
RESULTS
High performance liquid chromatography chromatograms and calibration curve of diosgenin
The calibration curve was plotted to be Y = 7379498.8950X + 47977.0833, and correlation coefficient (r2 = 0.9997) was calculated, indicating a good linearity within the range from 2.047 × 10−3 mg/mL to 0.5240 mg/mL. HPLC chromatograms of diosgenin and sample solution were shown as Figure 4.
Figure 4.
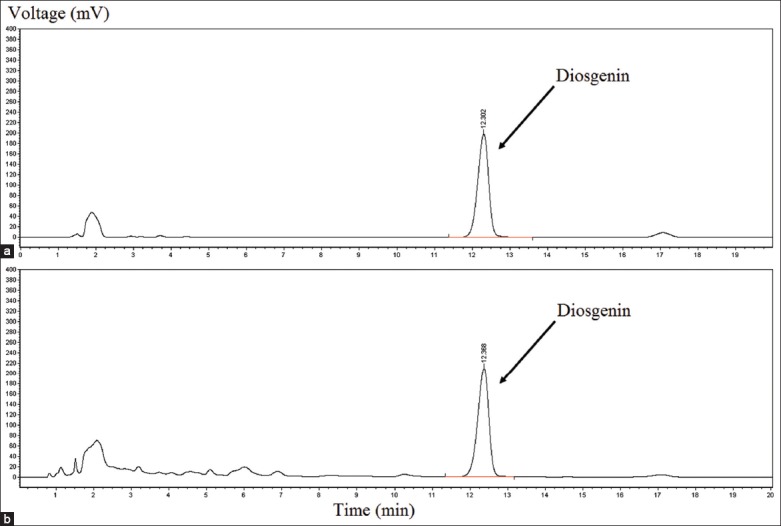
HPLC chromatograms of diosgenin reference (A) and sample solution (B)
Identification of diosgenin
ESI-MS m/z: 415.3 [M + H]+;1H-NMR (400 MHz, CDCl3) δ: 0.80 (3H, s, H-19), 0.81 (3H, d, J = 6.0 Hz, H-27), 0.97 (3H, d, J = 7.0 Hz, H-21), 1.02 (3H, s, H-18), 4.40 (1H, dd, H-16), 5.36 (1H, t, H-6), 3.30–3.60 (4H, m, H-26α, H-26β, H-3, H-5); 13C-NMR (100 MHz, CDCl3) δ: 37.24 (C-1), 31.45 (C-2), 71.67 (C-3), 42.27 (C-4), 140.83 (C-5), 121.37 (C-6), 32.05 (C-7), 31.60 (C-8), 50.08 (C-9), 36.64 (C-10), 20.87 (C-11), 39.79 (C-12), 40.26 (C-13), 56.53 (C-14), 31.39 (C-15), 80.82 (C-16), 62.12 (C-17), 16.26 (C-18), 19.40 (C-19), 41.61 (C-20), 14.50 (C-21), 109.27 (C-22), 31.83 (C-23), 28.80 (C-24), 30.28 (C-25), 66.83 (C-26), 17.11 (C-27). The chemical structure was identified as diosgenin by comparison of spectral data with those reported in literature.[17,18]
Optimization of the extraction conditions of diosgenin from the tubers of Dioscorea zingiberensis
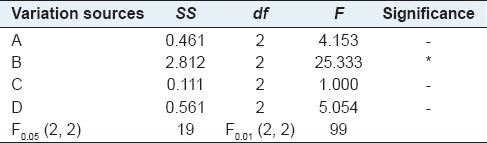
Diosgenin in the extract obtained from each acid hydrolysis experiment was quantitatively analyzed and then the extraction yields of diosgenin were calculated according to the above equations. The results of L9 (3)4 orthogonal experiments were summarized in Table 2, showing that the highest extraction yield of diosgenin was obtained at 2.21% among nine experiments performed under different conditions. However, the best extraction conditions could not be selected only based on these outcomes, and a further orthogonal analysis was necessary. Thus, K and R values were calculated and listed in the table, which has indicated that temperature is the factor of the most influence on extraction yield. It also can be seen that the influence to the mean extraction yields of diosgenin decreases in the order: B >A >D >C according to the R values of each factor. In addition, it was predicted that the highest extraction yield would be achieved under the optimal conditions, namely, A3B3C2D1. Moreover, the variance analysis as Table 3 was used to verify the result of the orthogonal experiments design, which has showed that they were in good agreement with each other and the temperature has affected on the yield significantly.
Table 3.
Variance analysis of the orthogonal experiments
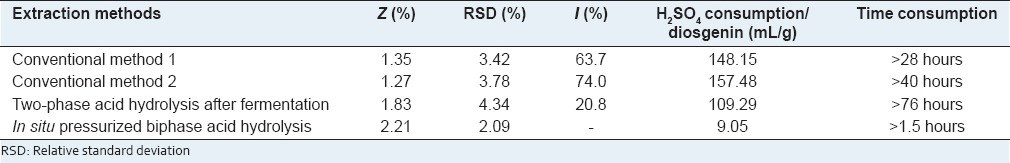
Verification experiments of the optimized extraction conditions and comparison to other methods
According to the results from the L9 (3)4 orthogonal experiments, highest Z would be achieved if 2.50 g of the tubers was hydrolyzed in 50 mL of 0.20 M H2SO4 aqueous solution at 140°C for 1.5 h by using the novel in situ pressurized biphase acid hydrolysis technology. To verify the newly proposed extraction method, three more verification experiments were hence performed and the comparison between the new method and three other methods was made and tabulated in Table 4. It can be seen that compared to other three methods, the consumption of H2SO4 and extraction time was largely decreased in terms of the extraction of diosgenin per gram, the RSD value of the new method optimized was lower and the extraction yield was much higher, which suggested that the proposed new method was greener and more repeatable, and was of greater efficiency as well.
Table 4.
Verification experiments and comparison to other methods
DISCUSSIONS
Diosgenin is an important bioactive chiral compound originated from yams and has many applications in pharmaceutical industries, however severe pollution and low extraction yield during the hydrolysis of plant materials by conventional methods are unfavorable. In this study, a new approach for the cleaner and more efficient extraction of diosgenin from the tubers of D. zingiberensis has been developed and optimized accordingly. It was supposed that several aspects of cause may have contributed to higher extraction yield and efficiency of this method. Firstly, the extraction of glycosides from plant materials and their hydrolysis were combined with the extraction of diosgenin into one step, which has greatly simplified the process. Secondly, biphase immiscible solvent system composed of non-polar PET can maintain the stability of diosgenin by quickly transferring the compound into the organic phase after it was produced during the hydrolysis in strongly acidic solution. Thirdly, acidic aqueous solution is much easier to permeate through cell wall and membrane of plant materials under high pressure, which could have been accelerating the extraction and hydrolysis of glycosides as well as the release of diosgenin. Lastly, reaction temperature greatly affecting the extraction yield can reach a higher and more proper level in an uncontrolled pressurized condition as boiling point of solvents was increased.
At the present time, some other yams including the tubers of D. nipponica, D. mexicana and D. bulbifera, as well as the rhizome of Paris polyphylla Smith var. yunnanensis (Franch.) Hand.-Mazz. and the seeds of Trigonella foenum-graecum L. have been used as the plant sources of diosgenin in industries.[19,20,21,22] The application of in situ pressurized biphase acid hydrolysis is also promising in the extraction of diosgenin from these plant materials, and the pilot scale and real bulk product are going to be studied further.
In recent decade, a few approaches such as microbial hydrolysis by Trichoderma reesei[9] or Trichoderma,[23] or multi-enzyme hydrolysis by ⊠-glucosidase, cellulose, pectinase, amylase and glucoamylase,[24] have been studied and proposed to increase the yield or make the process greener, and both of which were considered as promising technologies for diosgenin production from an economic and environment-friendly viewpoint. Upon the exploration of key enzymes to hydrolyze glycosides in the tubers of D. zingiberensis, the covalent immobilization of them on magnetic nanoparticles would be the crucial technology of next generation for this application.
CONCLUSION
In this study, by using the newly developed method in situ pressurized biphase acid hydrolysis, the pollution caused during the hydrolysis and time needed for the extraction of diosgenin have been greatly reduced, and meanwhile the extraction yield of diosgenin was improved largely. It has been well demonstrated that the new method was a cleaner, more reliable and efficient approach for the extraction of diosgenin from the tubers of D. zingiberensis. Furthermore, this established method could also be applied for the extraction of some other aglycones as well.
ACKNOWLEDGMENTS
The work was financially supported by the National Natural Science Foundation of China (81303313), the Jiangsu Nature Science Foundation (BK2012290), the Program for Undergraduate's Scientific Research of Jiangsu University (13A064, 13A170), and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institution (PAPD).
Footnotes
Source of Support: National Natural Science Foundation of China (81303313), the Jiangsu Nature Science Foundation (BK2012290), the Program for Undergraduate's Scientific Research of Jiangsu University (13A064, 13A170), and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institution (PAPD)
Conflict of Interest: None declared.
REFERENCES
- 1.Peng YE, Yang ZH, Wang YX, Liu ZY, Bao JG, Hong Y. Pathways for the steroidal saponins conversion to diosgenin during acid hydrolysis of Dioscorea zingiberensis C. H. Wright. Chem Eng Res Des. 2011;89:2620–5. [Google Scholar]
- 2.Mao ZJ, Tang QJ, Zhang CA, Qin ZF, Pang B, Wei PK, et al. Anti-proliferation and anti-invasion effects of diosgenin on gastric cancer BGC-823 cells with HIF-1a shRNAs. Int J Mol Sci. 2012;13:6521–33. doi: 10.3390/ijms13056521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gong G, Qin Y, Huang W. Anti-thrombosis effect of diosgenin extract from Dioscorea zingiberensis C.H. Wright in vitro and in vivo. Phytomedicine. 2011;18:458–63. doi: 10.1016/j.phymed.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 4.Ebrahimi H, Badalzadeh R, Mohammadi M, Yousefi B. Diosgenin attenuates inflammatory response induced by myocardial reperfusion injury: Role of mitochondrial ATP-sensitive potassium channels. J Physiol Biochem. 2014;70:425–32. doi: 10.1007/s13105-014-0320-9. [DOI] [PubMed] [Google Scholar]
- 5.Shishodia S, Aggarwal BB. Diosgenin inhibits osteoclastogenesis, invasion, and proliferation through the downregulation of Akt, I kappa B kinase activation and NF-kappa B-regulated gene expression. Oncogene. 2006;25:1463–73. doi: 10.1038/sj.onc.1209194. [DOI] [PubMed] [Google Scholar]
- 6.Cai H, Wang Z, Zhang HQ, Wang FR, Yu CX, Zhang FX, et al. Diosgenin relieves goiter via the inhibition of thyrocyte proliferation in a mouse model of Graves’ disease. Acta Pharmacol Sin. 2014;35:65–73. doi: 10.1038/aps.2013.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu Y, Zhu H, Qiu M, Zhu T, Ni J. Investigation on the mechanisms for biotransformation of saponins to diosgenin. World J Microbiol Biotechnol. 2014;30:143–52. doi: 10.1007/s11274-013-1429-7. [DOI] [PubMed] [Google Scholar]
- 8.Qiu LL, Niu H, Huang W. Ultrasonic and fermented pretreatment technology for diosgenin production from Dioscorea zingiberensis C. H. Wright. Chem Eng Res Des. 2011;89:239–47. [Google Scholar]
- 9.Zhu YL, Huang W, Ni JR. A promising clean process for production of diosgenin from Dioscorea zingiberensis C. H. Wright. J Clean Prod. 2010;18:242–7. [Google Scholar]
- 10.Cheng P, Zhao H, Zhao B, Ni J. Pilot treatment of wastewater from Dioscorea zingiberensis C.H. Wright production by anaerobic digestion combined with a biological aerated filter. Bioresour Technol. 2009;100:2918–25. doi: 10.1016/j.biortech.2009.01.054. [DOI] [PubMed] [Google Scholar]
- 11.Liang Z, Wang YX. Pretreatment of diosgenin wastewater using polyferric sulfate and cationic polyacrylamide. J Earth Sci China. 2010;21:340–6. [Google Scholar]
- 12.Liang RJ. Orthogonal test design for optimization of the extraction of polysaccharides from Phascolosoma esulenta and evaluation of its immunity activity. Carbohydr Polym. 2008;73:558–63. doi: 10.1016/j.carbpol.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 13.Wang XB, Yang H, Yin HW, Yu JY, Li L, Guo XM. Fermentation pretreatment followed by two-phase combination acid hydrolysis for the extraction of diosgenin. Chin Pharm J. 2014;49:187–90. [Google Scholar]
- 14.Luo X, Liu Y, Yao X. Research of production processes of diosgenin from Dioscorea zingiberensis C. H. Wright. Chin J Ethnomed Ethnopharm. 2010;19:38–40. [Google Scholar]
- 15.Shen YP, Yin HW, Liu YH, Wang XB, Yang H, Jia XB. The research on two-phase acid hydrolysis of extracting diosgenin from the tubers of Dioscorea zingiberensis C. H. Wright. Chin Tradit Pat Med. 2013;35:2272–4. [Google Scholar]
- 16.Yang H, Chen B, Wang XB, Chue PW, Shen YP, Xia GH, et al. Rapid quantitative analysis of diosgenin in the tubers of Dioscorea zingiberensis C.H. Wright by coupling cellulose enzymolysis and two-phase acid hydrolysis in tandem with HPLC-UV. Nat Prod Res. 2013;27:1933–5. doi: 10.1080/14786419.2013.790030. [DOI] [PubMed] [Google Scholar]
- 17.Yang L, Feng F, Gao Y. Chemical constituents from herb of Solanum lyratum. Zhongguo Zhong Yao Za Zhi. 2009;34:1805–8. [PubMed] [Google Scholar]
- 18.Deng S, Yu B, Hui Y, Yu H, Han X. Synthesis of three diosgenyl saponins: Dioscin, polyphyllin D, and balanitin 7. Carbohydr Res. 1999;317:53–62. doi: 10.1016/s0008-6215(99)00066-x. [DOI] [PubMed] [Google Scholar]
- 19.Kaufmann B, Rudaz S, Cherkaoui S, Veuthey JL, Christen P. Influence of plant matrix on microwave-assisted extraction process. The case of diosgenin extracted from fenugreek (Trigonella foenum-graecum L.) Phytochem Anal. 200;18:70–6. doi: 10.1002/pca.954. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh S, More P, Derle A, Patil AB, Markad P, Asok A, et al. Diosgenin from Dioscorea bulbifera: Novel hit for treatment of type II diabetes mellitus with inhibitory activity against a-amylase and a-glucosidase. PLoS One. 2014;9:e106039. doi: 10.1371/journal.pone.0106039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu X, Wang L, Wang H, Dai Y, Ye WC, Li YL. Steroidal saponins from Paris polyphylla var. yunnanensis. Phytochemistry. 2012;81:133–43. doi: 10.1016/j.phytochem.2012.05.034. [DOI] [PubMed] [Google Scholar]
- 22.Yin LH, Xu YW, Qi Y, Han X, Xu LN, Peng JY. A green and efficient protocol for industrial-scale preparation of dioscin from Dioscorea nipponica Makino by two-step macroporous resin column chromatography. Chem Eng J. 2010;165:281–9. [Google Scholar]
- 23.Liu L, Dong YS, Qi SS, Wang H, Xiu ZL. Biotransformation of steriodal saponins in Dioscorea zingiberensis C. H. Wright to diosgenin by Trichoderma harzianum. Appl Microbiol Biotechnol. 2010;85:93–40. doi: 10.1007/s00253-009-2098-1. [DOI] [PubMed] [Google Scholar]
- 24.Huang W, Zhao H, Ni J, Zuo H, Qiu L, Li H, et al. The best utilization of D. zingiberensis C.H. Wright by an eco-friendly process. Bioresour Technol. 2008;99:7407–11. doi: 10.1016/j.biortech.2008.01.015. [DOI] [PubMed] [Google Scholar]


