Abstract
Background:
Lespedeza cuneata (Dum. Cours.) G. Don, a perennial legume native to Eastern Asia, has been used therapeutically in traditional Asian medicine to protect the function of liver, kidneys and lungs. However, its effect on inflammatory nitric oxide (NO) production and the active constituents have not yet been explored.
Objective:
In this study, we investigated the phytochemical constituents of L. cuneata and evaluated their effect on NO production using lipopolysaccharide (LPS)-stimulated BV2 cells.
Materials and Methods:
The 80% methanol extract of the aerial part of L. cuneata were used for the isolation of flavonoids. The isolated compounds were elucidated by various spectroscopic methods including nuclear magnetic resonance and mass spectrometry spectrometry. To evaluate the effect on inflammatory NO production, LPS-stimulated murine microglia BV-2 cells were used as a screening system.
Results:
Nine flavonoids were isolated from the aerial parts of L. cuneata. Among the isolated flavonoids, compounds 4, 5, 7 and 9 are reported from the genus Lespedeza for the first time. Moreover, compounds 1 and 6 showed significant inhibitory effects on NO production in LPS-stimulated BV2 cells without cell toxicity.
Conclusion:
In this study, nine flavonoids were isolated from L. cuneata. Among the compounds, only 1 and 6, which have free hydroxyl groups at both C3 and C7 showed significant inhibitory activity on NO production in LPS-stimulated BV2 cells. These results suggested L. cuneata and its flavonoid constituents as possible candidate for the treatment of various inflammatory diseases.
Keywords: BV2 microglia, flavonoids, Lespedeza cuneata, nitric oxide
INTRODUCTION
Plants belonging to the genus Lespedeza (Leguminosae) are widely distributed both in Eastern North America and in Eastern Asia, and about 40 species have been reported.[1] It is recognized for its tolerance of drought and acidity and for its ability to grow in shallow soils of low fertility. Lespedeza cuneata is now being considered as an energy crop for increasing the sustainability of agriculture and energy production in the United States.[2] In the pharmaceutical field, the aerial parts of this plant have been used to protect the function of liver, kidneys and lungs in traditional Asian medicine.[3] L. cuneata is known to contain flavonoids, pinitol, tannins and β-sitosterol.[4] Phytochemical studies have revealed that flavonoid compounds including C-glycosyl flavones (e.g. isoorientin, isovitexin, vicenin II, lucenin II, desmodin and homoadonivernith), O-glycosyl flavonols (e.g. avicularin, juglanin, trifolin, hyperin and hirsutrin) and aglycones (e.g. quercetin and kaempferol) were isolated from extracts of the aerial parts of L. cuneata.[5,6]
Flavonoids are naturally occurring polyphenolic compounds, which contain two benzene rings linked together with a heterocyclic pyran or pyrone ring. Flavonoids are normal constituents of the human diet and are known for a variety of biological activities.[7] Recently, there has been much interest in the neuroprotective effects of flavonoids, which have been shown to be effective in protecting against both age-related cognitive and motor decline in vivo.[8,9,10] This potential may reside in a number of physiological functions, including their antioxidant properties,[11] the interactions with intracellular signaling pathways, the regulation of cell survival/apoptotic genes and mitochondrial function.[12,13] These signaling cascades are also critical for the control of inflammatory processes, helping the activation of microglia in response to cytokines and the induction of inducible nitric oxide synthase and nitric oxide (NO) production.[14,15,16] Consequently, flavonoids have been suggested as the novel therapeutic agents for the reduction of the deleterious effects of inflammation and as the potential preventive drugs for degenerative disease development.[17]
Nitric oxide is a signaling molecule that plays a key role in the pathogenesis of inflammation.[18] They regulate inflammatory response and recovery from tissue damage.[19,20] Therefore, regulation of NO production in microglia could be a good target for the treatment of degenerative disorders.
In this study, we tried to identify NO production inhibitory constituents of L. cuneata employing lipopolysaccharide (LPS)-stimulated BV2 cells as a screening system. Since the structures of flavonols 1–8 were similar to each other, the effects due to hydroxylated and the position of the sugar moiety against NO production inhibitory activity was investigated.
MATERIALS AND METHODS
General procedures
All organic solvents, such as hexane, chloroform (CHCl3), ethyl acetate (EtOAc), methanol (MeOH) and n-butanol (n-BuOH) used for extraction and column chromatography were of analytical grade and purchased from Duksan Chemical (Anseong, Korea). 1H nuclear magnetic resonance (1H NMR) (400 MHz) and 13C NMR (100 MHz) spectra were recorded on an Agilent 400-MR NMR spectrometer (Agilent Technologies, Santa Clara, CA) and TMS was used as an internal standard. Data processing was carried out with the MestReNova 6.0.2 program (Mestrelab research SI, www.mestrelab.com, 2009). HRESIMS spectra were obtained using an Agilent 6550 iFunnel Q-TOF liquid chromatography/mass spectrometry (LC/MS) system (Agilent Technologies, Santa Clara, CA). Preparative high-performance liquid chromatography (HPLC) was carried out using an Agilent 1260 HPLC system. Column chromatography was performed on silica gel (Kieselgel 60, 70–230 mesh and 230–400 mesh, Merck, Darmstadt, Germany) and YMC RP-18 resins (Fuji Silysia Chemical, Aichi, Japan). Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were obtained from Gibco BRL. Co. Glutamate and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carbboxylic acid (trolox), 3-(4,5-dimethylthiazol-2-yl)- 2,5- diphenyl tetrazolium bromide (MTT) and scopolamine were purchased from Sigma (USA).
Plant materials
The aerial part of L. cuneata were collected at Yongdu Mountain, Jecheon, Chungcheongbuk province, South Korea in October 2011, and authenticated by Dr. Jong Hee Park, professor of Pusan National University. A voucher specimen (YIPS-LC-140815) was deposited at the Herbarium of Pharmacy, Yonsei Institute of Pharmaceutical Sciences, Yonsei University, Incheon, Korea.
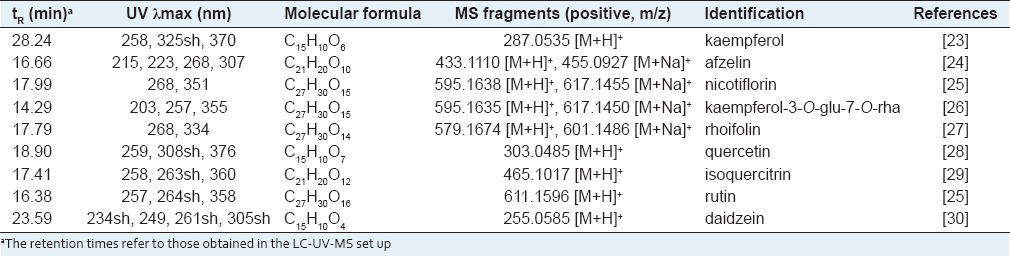
Extraction and isolation
The dried and powdered aerial part of L. cuneata (1.8 kg) were extracted with 80% MeOH four times for 3 days at room temperature. After removal of the solvent under reduced pressure in vacuo, the MeOH extract (196.74 g, yield: 10.9%) was suspended in H2O and then partitioned successively with hexane (16.15 g), CHCl3 (11.52 g) EtOAc (10.40 g) and n-BuOH (62.9 g) fraction. The n-BuOH fraction was chromatographed on a Diaion HP-20P column eluting with H2O containing increasing concentrations of MeOH (0, 40, 60, 80 and 100%) to obtain five sub-fractions Fr. A (23.72 g), Fr. B (12.55 g), Fr. C (2.12 g) Fr. D (0.32 g) and Fr. E (0.03 g). The Fr. B was chromatographed on a LH-20 column eluting with 80% MeOH to give five smaller fractions, Fr. B1 (0.8 g), Fr. B2 (0.9 g), Fr. B3 (9.24 g) Fr. B4 (0.32 g) and Fr. B5 (0.03 g). The Fr. B3 fraction was chromatographed on HPLC using J'sphere ODS H-80 (250 mm × 20 mm, 4 μm, 8 nm) column eluting with 15% aqueous acetonitrile at a flow rate of 3 mL/min to yield 2 (5.6 mg), 3 (0.7 mg), 4 (4.7 mg), 5 (3.6 mg), 7 (0.6 mg) and 8 (5.2 mg). The Fr. C was chromatographed on LH-20 column eluting with 80% MeOH to yield 1 (6.4 mg), 6 (8.1 mg) and 9 (2.0 mg). Isolated compounds were elucidated by electrospray ionization quadrupole time-of-flight mass spectrometry (ESI-Q-TOF-MS) and several NMR techniques including 1D and 2D NMR spectroscopic methods and by comparison of their data with those reported previously in the related literatures [Table 1].
Table 1.
Identification of flavonoids from L. Cuneata
Structure elucidation of isolated compounds
Kaempferol (1)- Yellow amorphous powder; 1H-NMR (400 MHz, CD3 OD) δH: 6.17 (1H, d, J = 1.4 Hz, H-6), 6.38 (1H, s, H-8), 8.07 (1H, d, J = 8.7 Hz, H-2), 6.89 (1H, d, J = 8.7 Hz, H-3), 6.89 (1H, d, J = 8.7 Hz, H-5), 8.07 (1H, d, J = 8.7 Hz, H-6); 13C-NMR (100 MHz, CD3 OD) δC: 148.0 (C-2), 137.1 (C-3), 177.4 (C-4), 104.5 (C-4a), 162.5 (C-5), 99.2 (C-6), 165.6 (C-7), 94.4 (C-8), 160.5 (C-8a), 123.7 (C-1), 130.7 (C-2), 116.3 (C-3), 158.2 (C-4), 116.2 (C-5), 130.7 (C-6).
Afzelin (2) - Yellow amorphous powder;1H-NMR (400 MHz, DMSO-d6) δH: 0.78 (3H, d, J = 5.1 Hz, C-6’’), 5.29 (1H, s, C-1’’), 6.22 (1H, d, J = 1.6 Hz, H-6), 6.42 (1H, d, J = 1.6 Hz, H-8), 6.92 (2H, d, J = 7.3 Hz, H-3, 5), 7.76 (2H, d, J = 7.3 Hz, H-2, 6), 12.62 (1H, s, 5-OH); 13C-NMR (100 MHz, DMSO-d6) dC: 17.5 (C-6’’), 70.1 (C-2’’), 70.3 (C-3’’), 70.7 (C-5’’), 71.2 (C-4’’), 93.8 (C-8), 98.8 (C-6), 101.8 (C-1’’), 104.2 (C-10), 115.5 (C-3’,5’), 120.6 (C-1’), 130.7 (C-2’,6’) 134.2 (C-3), 156.6 (C-9), 157.3 (C-2), 160.0 (C-4’), 161.3 (C-5), 164.4 (C-7), 177.8 (C-4).
Nicotiflorin (3) - Yellow amorphous powder; 1H-NMR (400 MHz, CD3 OD) δH: 6.20 (1H, d, J = 2.0 Hz, H-6), 6.39 (1H, d, J = 2.0 Hz, H-8), 8.06 (2H, dd, J = 2.0, 6.8 Hz, H-2’, 6’), 6.89 (2H, dd, J = 2.0, 6.8 Hz, H-3’, 5’), 5.13 (1H, d, J = 7.6 Hz, H-1’’), 3.26-3.48 (5H, m, H-2’’~5’’ and H-6b’’), 3.81 (1H, d, J = 10.0 Hz, H-6a’’), 4.52 (1H, d, J = 1.6 Hz, H-1’’’), 3.64 (1H, dd, J = 1.6, 3.2 Hz, H-2’’’), 3.53 (1H, dd, J = 3.8, 9.4 Hz, H-3’’’), 3.26-3.48 (2H, m, H-4’’’, 5’’’), 1.13 (3H, d, J = 6.0 Hz, H-6’’’);13C-NMR (100 MHz, CD3 OD) δC: 158.67 (C-2), 135.69 (C-3), 179.54 (C-4), 163.11 (C-5), 100.11 (C-6), 166.13 (C-7), 95.07 (C-8), 159.55 (C-9), 105.81 (C-10), 122.89 (C-1’), 132.53 (C-2’), 116.28 (C-3’), 161.63 (C-4’), 116.28 (C-5’), 132.53 (C-6’), 104.79 (C-1’’), 75.92 (C-2’’), 78.28 (C-3’’), 71.58 (C-4’’), 77.34 (C-5’’), 68.72 (C-6’’), 102.57 (C-1’’’), 72.23 (C-2’’’), 72.44 (C-3’’’), 74.05 (C-4’’’), 69.88 (C-5’’’), 18.07 (H-6’’’).
Kaempferol 3-O-glucosyl-7-O-rhamnoside (4). Yellow amorphous powder;1H NMR (400 MHz, CD3 OD) δH: 8.08 (2H, d, J = 8.7 Hz, H-2’, 6’), 6.91 (2H, d, J = 8.7 Hz, H-3’, 5’), 6.23 (1H, d, J = 1.5 Hz, H-6), 6.41 (1H, d, J = 1.5 Hz, H-8), 5.14 (1H, d, J = 7.2 Hz, H-1’’), 3.24-3.98 (6H, H-2’’ -H-6’’), 4.53 (1H, d, J = 5.8 Hz, H-1’’’), 3.24-3.98 (4H, H-2’’’ -H-5’’’), 1.14 (3H, d, J = 6.0 Hz, H-6’’’);13C NMR (100 MHz, CD3 OD) δC: 156.6 (C-2), 133.5 (C-3), 177.5 (C-4), 161.3 (C-5), 99.8 (C-6), 164.2 (C-7), 93.8 (C-8), 156.9 (C-9), 104.2 (C-10), 121.1 (C-1’), 130.9 (C-2’, C-6’), 115.2 (C-3’, C-5’), 159.9 (C-4’), 101.6 (C-1’’), 74.4 (C-2’’), 76.7 (C-3’’), 70.9 (C-4’’), 76.0 (C-5’’), 67.1 (C-6’’), 100.8 (C-1’’’), 70.5 (C-2’’’), 70.2 (C-3’’’), 72.1 (C-4’’’), 68.3 (C-5’’’), 17.7 (C -’’’).
Rhoifolin (5) - White amorphous powder; 1H NMR (400 MHz, CD3 OD) δH: 1.32 (3H, d, J = 6.0 Hz, 6’’’–CH3), 5.18 (1H, d, J = 7.5 Hz, H-100), 5.28 (1H, d, J = 1.8 Hz, H-1’’’), 6.44 (1H, d, J = 2.1 Hz, H-6), 6.64 (1H, s, H-3), 6.76 (1H, d, J = 2.1 Hz, H-8), 6.91 (1H, d, J = 9.0 Hz, H-30, 50), 7.86 (1H, dd, J = 9.0 Hz, H-2’, 6’); 13C-NMR (100 MHz, CD3 OD) δC: 164.4 (C-2), 104.1 (C-3), 184.0 (C-4), 159.0 (C-5), 99.8 (C-6), 166.7 (C-7), 95.9 (C-8), 162.9 (C-9), 107.1 (C-10), 123.0 (C-1’), 129.6 (C-2’), 117.1 (C-3’), 162.9 (C-4’), 117.1 (C-3’), 129.6 (C-6’), 99.8 (C-1’’), 79.1 (C-2’’), 78.3 (C-3’’), 72.2 (C-4’’), 79.1 (C-5’’), 62.4 (C-6’’), 101.0 (C-1’’’), 71.4 (C-2’’’), 72.2 (C-3’’’), 74.0 (C-4’’’), 70.0 (C-5’’’), 18.3 (C-6’’’).
Quercetin (6) - Yellow amorphous powder; 1H-NMR (400 MHz, DMSO-d6) δH: 6.17 (s, 1H, H-6), 6.404 (s, 1H, H-8), 7.54 (d, 1H, H-2’), 6.88 (d, 1H, H-3’), 7.66 (s, 1H, H-6’). 13C-NMR (100 MHz, DMSO-d6) δC: 147.20 (C-2), 136.14 (C-3), 176.24 (C-4), 161.18 (C-5), 98.59 (C-6), 164.35 (C-7), 93.74 (C-8), 156.60 (C-9), 103.40 (C-10), 122.41 (C-1’), 115.42 (C-2’), 145.47 (C -’), 148.10 (C-4’), 115.94 (C-5’), 120.40 (C-6’).
Isoquercitrin (7) - Yellow amorphous powder; 1H-NMR (400 MHz, CD3OD) δH: 6.19 (1H, brs, H-6), 6.37 (1H, brs, H-8), 7.70 (1H, s, H-2’), 6.88 (1H, d, J = 8.3 Hz, H-5’), 7.59 (1H, d, J = 8.3 Hz, H-6’), 5.24 (1H, d, J = 7.5 Hz, H-1’’), 3.37-3.50 (3H, m, H-2’’ -4’’), 3.19-3.23 (1H, m, H-5’’), 3.71 (1H, dd, J = 1.8, 12.0 Hz, H-6a’’), 3.57 (1H, dd, J = 5.1, 12.0 Hz, H-6b’’); 13C-NMR (100 MHz, CD3OD) ξC: 158.79 (C-2), 135.48 (C-3), 179.25 (C-4), 162.86 (C-5), 99.94 (C-6), 166.25 (C-7), 94.72 (C-8), 158.33 (C-9), 105.50 (C-10), 122.96 (C-1’), 115.90 (C-2’), 145.77 (C-3’), 149.72 (C-4’), 117.43 (C-5’), 123.07 (C-6’), 104.26 (C-1’’), 75.69 (C-2’’), 78.35 (C-3’’), 71.18 (C-4’’), 78.08 (C-5’’), 62.53 (C-6’’).
Rutin (8) - Yellow amorphous powder; 1H NMR (400 MHz, DMSO-d6) δH: 1.00 (3H, d, J = 6.0 Hz, H-5’’’), 3.04-3.72 (10H, m, sugar H), 4.39 (1H, s, H-1’’’), 5.34 (1H, d, J = 7.5 Hz, Glc H-1), 6.20 (1H, d J = 1.2 Hz, H-6), 6.39 (1H, d, J = 1.2 Hz, H-8), 6.84 (1H, d, J = 8.5 Hz, H-5′), 7.53 (1H, d, J = 2.5 Hz, H-2’), 7.55 (1H, dd, J = 2.5, 8.5 Hz, H-6’), 12.59 (1H, s, 5-OH); 13C NMR (100 MHz, DMSO-d6) δC: 156.6 (C-2), 133.3 (C-3), 177.3 (C-4), 161.2 (C-5), 98.7 (C-6), 164.2 (C-7), 93.6 (C-8), 156.4 (C-9), 104.1 (C-10), 121.6 (C-1’), 115.3 (C-2’), 144.7 (C-3’), 148.4 (C-4’), 116.3 (C-5’), 121.2 (C-6’), 101.2 (C-1’’), 74.1 (C-2’’), 76.5 (C-3’’), 70.6 (C-4’’), 75.9 (C-5’’), 67.0 (C-6’’), 100.7 (C-1’’), 70.4 (C-2’’’), 70.0(C-3’’’), 71.9 (C-4’’’), 68.2 (C-5’’’), 17.7 (C-6’’’).
Daidzein (9) - White amorphous powder;1H NMR (400 MHz, DMSO-d6) δH: 6.79 (2H, d, J = 8.4 Hz, H-3’, 5’), 6.84 (1H, d, J = 2.1 Hz, H-8), 6.92 (1H, dd, J = 8.8, 2.1 Hz, H-6), 7.37 (2H, d, J = 8.4 Hz, H-2’, 6’), 7.95 (1H, d, J = 8.8 Hz, H-5), 8.27 (1H, s, H-2), 9.52 (1H, s, H-4’), 10.75 (1H, s, H-7); 13C NMR (100 MHz, DMSO-d6) δC: 102.2 (C-8), 115.1 (C-3’, 5’), 115.2 (C-6), 116.8 (C-4a), 122.7 (C-1’), 123.6 (C-3), 127.4 (C-5), 130.2 (C-2’, 6’), 152.9 (C-2), 157.3 (C-4’), 157.6 (C-8a), 162.7 (C-7), 174.8 (C-4).
Measurements of nitric oxide in lipopolysaccharide-stimulated BV2 microglia cells
BV-2 cells were maintained in DMEM supplemented with 5% FBS and 1% penicillin-streptomycin. To measure NO production, BV-2 cells were dispensed in wells of a 96-well plate (2 at 104 cells/well). After 24 h, the cells were pretreated with compounds for 30 min and stimulated with 100 ng/mL LPS for 24 h. Nitrite, a soluble oxidation product of NO, was measured in the culture media using the Griess reaction. The supernatant was harvested and mixed with an equal volume of Griess reagent (1% sulphanilamide, 0.1% N-1-naphthylethylenediamine dihydrochloride in 5% phosphoric acid). After 10 min, the absorbance at 540 nm was measured using an Emax microplate reader (molecular devices). Sodium nitrite was used as a standard to calculate the nitrite concentration. Cell viability was measured using a MTT assay. NG-Monomethyl-L-arginine (L-NMMA), a well-known NO synthase inhibitor, was tested as a positive control.
Statistical analysis
Data were evaluated for statistical significance by ANOVA test using a computerized statistical package. The data were considered to be statistically significant if the P ≤ 0.05.
RESULTS AND DISCUSSION
Nine compounds were isolated and their structures were identified as kaempferol (1), afzelin (2), nicotiflorin (3), kaempferol-3-O-β-glucopyranosyl-7-O-α-rhamnoside (4), rhoifolin (5), quercetin (6), isoquercitrin (7), rutin (8) and daidzein (9) [Figure 1]. Among them, seven flavonoids (2–5 and 7–9) were isolated from L. cuneata for the first time. Moreover, compounds 4, 5, 7 and 9 are reported from the genus Lespedeza for the first time. The present phytochemical investigation has further enriched our knowledge about the chemistry of L. cuneata and has identified compounds 2–5 and 7–9 could be potential chemotaxonomic markers for the species.
Figure 1.
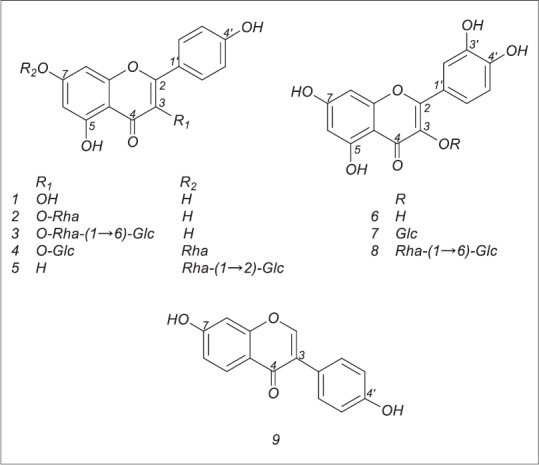
Chemical structures of compounds 1–9
Many flavonoids have been intensively studied on their inhibitory effects on inflammatory NO production.[21] Among the studied flavonoids, the bioactivity of various flavonoids is related to the number of hydroxyl groups on the flavonoid B-ring and the presence of the sugar moiety.[22] In the light of this, it appears that interactions might be structure-dependent, meaning that different flavonoids are likely to express different cellular outcomes. Thus, the effect of the isolated flavonoids on NO production in LPS-stimulated BV2 cells were evaluated to estimate the structure-dependency.
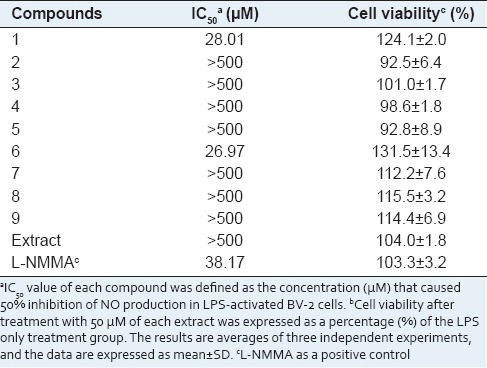
Nitric oxide inhibitory activities of the isolated compounds (1–9) and L. cuneata extract were evaluated by examining the inhibition of NO production in LPS-activated microglia BV-2 cells [Table 2]. Among the tested compounds, 1 and 6 significantly inhibited NO production with the IC50 values of 28.01 and 26.97 μM, respectively, which displayed more potent activity than L-NMMA, a well-known NOs inhibitor. None of the isolates (1–9) showed cytotoxicity at the concentrations up to 50 mM. The result showed that compounds with the sugar moiety (2–5 and 7–8) did not show the inhibition of NO production compared to the aglycones (1 and 6). As results, we suggested that the presence of a hydroxyl group attached at C-3 on the C-ring may be the functional group responsible for the NO inhibitory properties of flavonoids.
Table 2.
Inhibitory effect on NO production of L. cuneata extract and compounds 1-9 in LPS-activated BV-2 cells
CONCLUSION
From the aerial part of L. cuneata, nine flavonoids were isolated by chromatographic methods. The isolated compounds were elucidated by ESI-Q-TOF-MS and several NMR techniques. Among the isolates, compounds 1 and 6 exhibited significant NO inhibitory activities in LPS-stimulated microglial BV-2 cells. Compared to the other inactive compounds, these two active compounds have two free hydroxyl groups at both C3 and C7 positions. These results suggest the possible contribution of these hydroxyl groups to the NO inhibitory activity of flavonoids. Taken together, compounds 1 and 6 might be promising candidates for the treatment of various inflammatory diseases.
Footnotes
Source of Support: This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2011-0025129).
Conflict of Interest: None declared.
REFERENCES
- 1.Han JE, Chung KH, Nemoto T, Choi BH. Phylogenetic analysis of eastern Asian and eastern North American disjunct Lespedeza (Fabaceae) inferred from nuclear ribosomal ITS and plastid region sequences. Bot J Linn Soc. 2010;164:221–35. [Google Scholar]
- 2.Lau CS, Carrier DJ, Howard LR, Lay JO, Jr, Archambault JA, Clausen EC. Extraction of antioxidant compounds from energy crops. Appl Biochem Biotechnol. 2004;113-116:569–83. doi: 10.1385/abab:114:1-3:569. [DOI] [PubMed] [Google Scholar]
- 3.Kwon DJ, Kim JK, Ham YH, Bae YS. Flavone glycosides from the aerial parts of Lespedeza cuneata G. Don. J Korean Soc Appl Biol Chem. 2007;50:344–7. [Google Scholar]
- 4.Matsuura S, Iinuma M, Ito E, Takami H, Kagei K. G Don., translator. Studies on the constituents of the useful plants. VIII. The constituents of Lespedeza cuneata. Yakugaku Zasshi. 1978;98:1542–4. doi: 10.1248/yakushi1947.98.11_1542. [DOI] [PubMed] [Google Scholar]
- 5.Kwon DJ, Bae YS. Flavonoids from the aerial parts of Lespedeza cuneata. Biochem Syst Ecol. 2009;37:46–8. [Google Scholar]
- 6.Matsuzaki K, Wang YQ, Takahashi K, Okuyama T. Flavonoid glycosides of Lespedeza species. Shoyakugaku Zasshi. 1990;44:251–3. [Google Scholar]
- 7.Hämäläinen M, Nieminen R, Vuorela P, Heinonen M, Moilanen E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators Inflamm 2007. 2007 doi: 10.1155/2007/45673. 45673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, McEwen JJ, et al. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J Neurosci. 1999;19:8114–21. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vauzour D, Vafeiadou K, Rodriguez-Mateos A, Rendeiro C, Spencer JP. The neuroprotective potential of flavonoids: A multiplicity of effects. Genes Nutr. 2008;3:115–26. doi: 10.1007/s12263-008-0091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams CM, El Mohsen MA, Vauzour D, Rendeiro C, Butler LT, Ellis JA, et al. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radic Biol Med. 2008;45:295–305. doi: 10.1016/j.freeradbiomed.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Bastianetto S, Zheng WH, Quirion R. Neuroprotective abilities of resveratrol and other red wine constituents against nitric oxide-related toxicity in cultured hippocampal neurons. Br J Pharmacol. 2000;131:711–20. doi: 10.1038/sj.bjp.0703626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spencer JP. The impact of flavonoids on memory: Physiological and molecular considerations. Chem Soc Rev. 2009;38:1152–61. doi: 10.1039/b800422f. [DOI] [PubMed] [Google Scholar]
- 13.Williams RJ, Spencer JP, Rice-Evans C. Flavonoids: Antioxidants or signalling molecules. Free Radic Biol Med. 2004;36:838–49. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Bhat NR, Zhang P, Lee JC, Hogan EL. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J Neurosci. 1998;18:1633–41. doi: 10.1523/JNEUROSCI.18-05-01633.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaminska B, Gozdz A, Zawadzka M, Ellert-Miklaszewska A, Lipko M. MAPK signal transduction underlying brain inflammation and gliosis as therapeutic target. Anat Rec (Hoboken) 2009;292:1902–13. doi: 10.1002/ar.21047. [DOI] [PubMed] [Google Scholar]
- 16.Wen J, Ribeiro R, Zhang Y. Specific PKC isoforms regulate LPS-stimulated iNOS induction in murine microglial cells. J Neuroinflammation. 2011;8:38. doi: 10.1186/1742-2094-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spencer JP, Vafeiadou K, Williams RJ, Vauzour D. Neuroinflammation: Modulation by flavonoids and mechanisms of action. Mol Aspects Med. 2012;33:83–97. doi: 10.1016/j.mam.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 18.Sharma JN, Al-Omran A, Parvathy SS. Role of nitric oxide in inflammatory diseases. Inflammopharmacology. 2007;15:252–9. doi: 10.1007/s10787-007-0013-x. [DOI] [PubMed] [Google Scholar]
- 19.Dawson TM, Dawson VL, Snyder SH. Neurol. A novel neuronal messenger molecule in brain: The free radical, nitric oxide; pp. 297–311. [DOI] [PubMed] [Google Scholar]
- 20.Wyman T, Rohrer D, Kirigiti P, Nichols H, Pilcher K, Nilaver G, et al. Promoter-activated expression of nerve growth factor for treatment of neurodegenerative diseases. Gene Ther. 1999;6:1648–60. doi: 10.1038/sj.gt.3300989. [DOI] [PubMed] [Google Scholar]
- 21.Levites Y, Weinreb O, Maor G, Youdim MB, Mandel S. Green tea polyphenol (-)-epigallocatechin-3-gallate prevents N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopaminergic neurodegeneration. J Neurochem. 2001;78:1073–82. doi: 10.1046/j.1471-4159.2001.00490.x. [DOI] [PubMed] [Google Scholar]
- 22.Li FQ, Wang T, Pei Z, Liu B, Hong JS. Inhibition of microglial activation by the herbal flavonoid baicalein attenuates inflammation-mediated degeneration of dopaminergic neurons. J Neural Transm. 2005;112:331–47. doi: 10.1007/s00702-004-0213-0. [DOI] [PubMed] [Google Scholar]
- 23.Adebayo AH, Tan NH, Akindahunsi AA, Zeng GZ, Zhang YM. Anticancer and antiradical scavenging activity of Ageratum conyzoides L. (Asteraceae) Pharmacogn Mag. 2010;6:62–6. doi: 10.4103/0973-1296.59968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SK, Kim HJ, Choi SE, Park KH, Choi HK, Lee MW. Anti-oxidative and inhibitory activities on nitric oxide (NO) and prostaglandin E2 (COX-2) production of flavonoids from seeds of Prunus tomentosa Thunberg. Arch Pharm Res. 2008;31:424–8. doi: 10.1007/s12272-001-1174-9. [DOI] [PubMed] [Google Scholar]
- 25.Park SY, Lee SY, Kang SS. Chemical constituents of Lathyrus davidii. Nat Prod Sci. 2008;14:281–8. [Google Scholar]
- 26.Iwashina T, Matsumoto S, Nishida M, Nakaike T. New and rare flavonol glycosides from asplenium trichomanes-ramosum as stable chemotaxonomic markers. Biochem Syst Ecol. 1995;23:283–90. [Google Scholar]
- 27.Lee EJ, Kim JS, Kim HP, Lee JH, Kang SS. Phenolic constituents from the flower buds of Lonicera japonica and their 5-lipoxygenase inhibitory activities. Food Chem. 2010;120:134–9. [Google Scholar]
- 28.Dhasan PB, Jegadeesan M, Kavimani S. Cucurbitacins isolated from the fruits of Momordica cymbalaria Hook f. Pharmacogn Mag. 2008;4:96. doi: 10.4103/0974-8490.60575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He D, Huang Y, Ayupbek A, Gu D, Yang Y, Aisa HA, et al. Separation and purification of flavonoids from black currant leaves by high-speed countercurrent chromatography and preparative HPLC. J Liq Chromatogr Relat Technol. 2010;33:615–28. doi: 10.1080/10826071003608447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coward L, Barnes NC, Setchell KD, Barnes S. Genistein, daidzein, and their. beta.-glycoside conjugates: Antitumor isoflavones in soybean foods from American and Asian diets. J Agric Food Chem. 1993;41:1961–7. [Google Scholar]


