Abstract
Due to lack of specification and solubility of drug molecules, patients have to take high doses of the drug to achieve the desired therapeutic effects for the treatment of diseases. To solve these problems, there are various drug carriers present in the pharmaceuticals, which can used to deliver therapeutic agents to the target site in the body. Mesoporous silica materials become known as a promising candidate that can overcome above problems and produce effects in a controllable and sustainable manner. In particular, mesoporous silica nanoparticles (MSNs) are widely used as a delivery reagent because silica possesses favorable chemical properties, thermal stability, and biocompatibility. The unique mesoporous structure of silica facilitates effective loading of drugs and their subsequent controlled release of the target site. The properties of mesoporous, including pore size, high drug loading, and porosity as well as the surface properties, can be altered depending on additives used to prepare MSNs. Active surface enables functionalization to changed surface properties and link therapeutic molecules. They are used as widely in the field of diagnosis, target drug delivery, bio-sensing, cellular uptake, etc., in the bio-medical field. This review aims to present the state of knowledge of silica containing mesoporous nanoparticles and specific application in various biomedical fields.
Keywords: Bio-distribution, evaporation-induced self-assembly, mesoporous silica nanoparticles, silica precursors, surface functionalization, target drug delivery
INTRODUCTION
The era of nanotechnology has revolutionized the drug delivery and targeting process and changed the landscape of the pharmaceutical industry. Nanoparticles have dimension below 0.1 μm or 100 nm especially in the drug delivery. The drug is dissolved, entrapped, encapsulated or attached to a nanoparticle matrix.[1] Nanoparticles size range also affects the bioavailability and bio-distribution of particles, and hence it is useful as a drug carrier.[2] Hydrophobic core is beneficial for drug loading while hydrophilic surface blocks opsonization and allow easier movement in the system. Numerous nanodevices have been reported like carbon nanotubes, quantum dots, and polymeric micelles, etc., in the field of nanotechnology. In the present scenario, mesoporous nanoparticles are emerging for their well-known drug deliver and targeting purposes. Historically, Kresge et al. have described a means of combining sol-gel chemistry with liquid-crystal-line templating to construct ordered porous molecular sieves characterized by periodic arrangements of uniformly sized mesopores (diameter between 2 nm and 50 nm) incorporated within an amorphous silica matrix.[3]
Mesoporous silica nanoparticles (MSNs) have become apparent as a promising and novel drug vehicle due to their unique mesoporous structure that preserving a level of chemical stability, surface functionality and biocompatibility ensure the controlled release, and target drug delivery of a variety of drug molecules.[3,4] Mesoporous silica materials was discovered in 1992 by the Mobile Oil Corporation have received considerable attention due to their superior textual properties such as high surface area, large pore volume, tunable pore diameter, and narrow pore size distribution.[5]
Mesoporous nanoparticles have a solid framework with porous structure and large surface area, which allows the attachment of different functional group for targeted the drug moiety to a particular site.[6] Chemically, MSNs have honeycomb-like structure and active surface. Active surface enables functionalization to modify surface properties and link therapeutic molecules.[7] Various pore geometric structures were shown in Figure 1.
Figure 1.
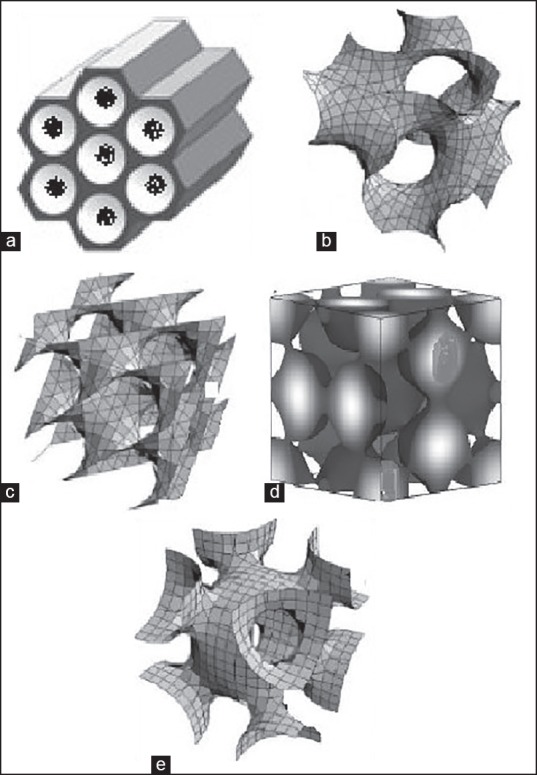
Various pore geometrics of mesoporous structure (a) 2D hexagonal p6 mm, (b) bicontinuous cubic Ia3d, (c) bicontinuous cubic pn3 m, (d) cage type pm3n, (e) cage type Im3 m
Mesoporous silica nanoparticles due to their low toxicity and high drug loading capacity, so they are used in controlled and target drug delivery system. Basically, silica is widely present in the environment in comparison to other metal oxides like titanium and iron oxides it has comparatively better biocompatibility.[8] The mesoporous form of silica has unique properties, particularly in loading of therapeutic agents at high quantities, and in the subsequent releases. Due to strong Si-O bond, silica-based mesoporous nanoparticles are more stable to external response such as degradation and mechanical stress as compared to niosomes, liposomes, and dendrimers which inhibit the need of any external stabilization in the synthesis of MSNs.[3,9] The mesoporous structure such as pore size and porosity can be tuned to the size and type of drugs. Various types of MSNs with internal structure and pore size as shown in Table 1. Another distinctive advantage of MSNs is that they have well-defined surface properties that allow easy functionalization of the silanol-containing surface to control drug loading and release.[10,11] The surface functionalization is generally needed to load proper type of drug molecules (hydrophobic/hydrophilic or positive/negative charged), specific actions can also have a natural quality or characteristics by the functionalization through chemical links with other materials such as stimuli-responsive, luminescent or capping materials, leading to smart, and multifunctional properties.[12]
Table 1.
Various types of mesoporous silica nanoparticles with their internal structure and pore diameter
The mesoporous particle could be synthesized using a simple sol-gel method or a spray drying method. Mesoporous silica has been also widely used as a coating material. MSNs density can be increased by two strategies that is, capping of the pores of MSNs with gold nanoparticles and gold plating on MSNs surface.[11]
Synthetic amorphous silica consists of nano-sized primary particles of nano or micrometer-sized aggregates and of agglomerates in the micrometer-size range. It has been used in a wide variety of industrial and consumer applications including food, cosmetics, and pharmaceutical products for many decades. Based on extensive physico-chemical, ecotoxicology, toxicology, safety, and epidemiology data, no environmental or health risks have been associated with these materials. It does not produce any toxicological effects on medicines and approved by generally recognized as safe.[13]
Nanoparticles especially which are made from a pyrolysis method for instance, can be coated with a layer of silica and produce good stability in aqueous solutions. Both applications can benefit from the facile surface chemistry of silica that allows easy coupling of targeting ligands onto the particles.[4,6] MSNs have been used in controllable drug delivery, gene transport, gene expression, biomarking, biosignal probing, imaging agent, detecting agent, drug delivery vehicles, and other important biological applications
Conventional MSNs can load a dose of therapeutic drug with 200-300 mg (maximally about 600 mg) drug/1 g silica. However, hollow MSNs also called MSNs with hollow core-mesoporous shell structure are able to achieve a super-high drug loading capacity because it provides more space to load drugs due to the hollow cores, typically >1 g drug/1 g of silica.[18]
SYNTHESIS OF MESOPOROUS SILICA NANOPARTICLES
The synthesis of MSNs occurs at a low surfactant concentration to make the structure of the ordered mesophases strongly dependent on the interaction between the growing anionic oligomers of orthosilicic acid and cationic surfactant, which changes limits of the structure of mesophases to small sizes.[3,18]
Mesoporous silica nanoparticles synthesis based on solution
The most widely used types of MSNs are mobile crystalline material (MCM-41), it consists of ordering hexagonally arrangement of cylindrical mesopores. Synthesis of MCM-41 required liquid crystal is templating of an alkyl ammonium salt that is, cetyl trimethyl ammonium bromide.[19] High concentration of amphipillic surfactant assembles into a spherical micelle in the water and hydrophilic soluble precursor like polysilicic acid or silica acid. By electrostatic and hydrogen bonding interaction, the silica precursor is concentrated at the hydrophilic interface and form an amorphous silica, which is a mold of the mesoporous product. Removal of remaining surfactant can be done by calcination and extraction method.[20]
Evaporation-induced self-assembly
This method was established in 1997. It is starting by forming a homogeneous solution of soluble silica and surfactant in ethanol, water with an initial surfactant concentration of critical micelle concentration. The Solvent evaporation process will start during dip coating for increase surfactant concentration.[21] Then driving a mixture of silica/surfactant micelles, and their further formation occur into liquid crystalline mesophases as shown in Figure 2. Film process was done by use of aerosol processing to direct the formation of mesoporous nanoparticles.[18,21] Evaporation-induced self-assembly is a non-volatile component that can be introduced into an aerosol droplet incorporated within the MSNs.
Figure 2.
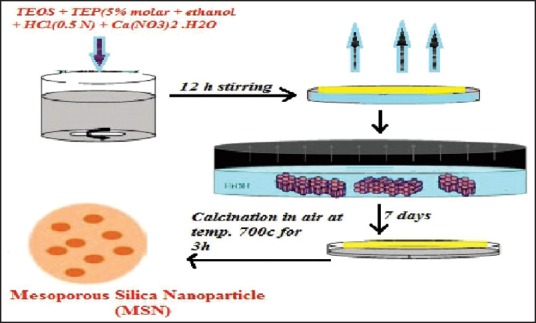
Evaporation self-induced assembly
SOL-GEL PROCESS
This process requires two-step consideration: Hydrolysis and condensation. Hydrolysis produced colloidal particles in aqueous solution, which can be stimulated at alkaline and acidic pH.[22] At neutral pH, condensation reaction takes place in which gel-like 3D network structure formed by cross-linking through silioxane bond. After drying at ambient temperature the different biomolecules embedded in a matrix structure of silica gel as shown in Figure 3. This process involves the formation of the (MCM-41) under the size range of 60-1000 nm. Several advantages of the sol-gel process like, it is a simple and cost effective process used to provide MSNs with controlled mesoporous structure and surface properties.[23] Sol-gel process is not a multistep process, so its time-saving process and required less excipients.
Figure 3.

Sol-gel process in the synthesis of mesoporous silica nanoparticles
Synthesis mechanism of mesoporous silica nanoparticles
The synthesis process of MSNs involves the replication of a surfactant liquid crystal structure and polymerization of metal oxide precursor. Removal of organic surfactant through the calcination process which form a porous structure. Various chemical constituents used in the formation MSNs were described in [Table 2].
Table 2.
Common chemical constituents used in preparation of MSNs
Silica Precursor used in mesoporous silica nanoparticles.
Organically modified precursors
They are prevented hydrolysis because an organic group attached directly to a silicon atom, which does not need oxygen bridge. It is conceded that organo-silica nanoparticles consist of better properties including large surface area, less condensed silioxane structure, and low density.[24] The limited accessibility and high cost of organic template lead to its restricted use in practical applications. Commonly used silica precursors are glycerol-derived polyol-based silanes, orthosilicic acid, sodium metasilicate, tetraethyl orthosilicate (TEOS) or tetramethoxysilane (TMOS), and tetrakis (2-hydroxyethyl) orthosilicate.[3,25]
Tetraethyl orthosilicate or TMOS was commonly used in MSNs synthesis. However, their poor water solubility requires additional organic solvent and alcohol and needs extreme conditions of pH and high temperature, which restricts their use.[26,27]
Tetrakis (2-hydroxyethyl) orthosilicate had been investigated to address the problems associated with TEOS and TMOS. It is now used in many studies as MSNs precursor because it is more biocompatible with biopolymers, more water soluble than TEOS and TMOS, and can process jellification at ambient temperature with a catalyst.[27]
Glycerol-derived polyol-based silane precursors
They are not pH dependent but very sensitive to the ionic strength of the sol. This can form optically clear monolithic MSNs. The residuals can be either removed or retained, therefore, the shrinkage during long-term storage can be minimized.[28] Orthosilicic acid was used as a silica precursor in the past but due to the extensive time consumption and requirement of freshly prepared acid, so it is not widely used anymore now a day.[29]
Sodium metasilicate
It is another precursor to sol-gel-derived silica. Formation of sodium chloride was investigated, which can cause a problem if a significant amount is generated. Later researches suggested that removing of this salt formulation by dialysis process, but it is a time and cost consuming procedure.[30] Hence, alkoxides and pure alkoxysilanes are currently widely used.
ADVANTAGES AND DISADVANTAGES OF POROUS SILICA MATERIAL
Porous silica based materials are among the most beneficial compounds which can provide more opportunities for treatment of cancer therapy and provide a pathway toward the treatment of challenging diseases. Silica or silicon has various versatile and broad range advantages such as versatility, non-toxicity, biocompatibility, biodegradability, high surface area, pore volume, homogenous distribution of guest molecules in porous space, the ability for surface charge control, and free dispersion throughout the body.[31] The major disadvantage of porous silica nanoparticles is attributed to the surface density of silanol groups interacting with the surface of the phospholipids of the red blood cell membranes resulting in hemolysis. Another disadvantage is related to metabolic changes induced by porous silica nanoparticles leading to melanoma promotion.
Biocompatibility and bio-distribution of mesoporous silica nanoparticles
The use of the organic compound into organism requires determination of biocompatibility and bio-distribution of MSNs. On the behalf of a study conducted by Hudson et al. suggested that intraperitoneal or intravenous produce a lethal effect in mice but subcutaneous injection showed no toxicity.[23,32] On the basis of characteristics property, MSNs were targeted to different cell as describe in Figure 4. Hence, both factors depend upon high dose and large particles with consideration to surface properties, shape, and size of MSNs.
Figure 4.
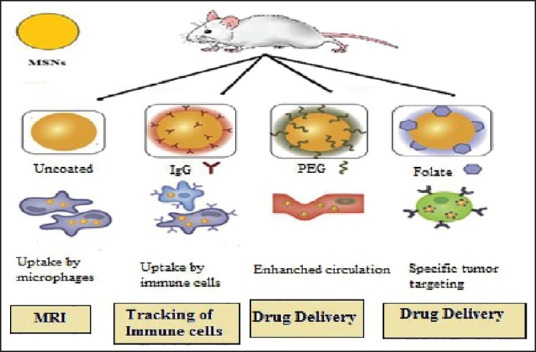
Biocompatibility and bio-distribution of mesoporous silica nanoparticles
Biocompatibility is the study of the interaction of biomaterials with the body of experimental animals to evaluate possible toxicity effects.[40]
Biocompatibility study in mice
A dose-escalating study of phosphonate-MSNs (100-130 nm) to examine biocompatibility of the material and it was shown that repeated doses of phosphonated MSNs ranging from 5 mg/kg to 50 mg/kg in mice did not result in significant toxicity as determined by body weight, histology, and hematological studies.[41] A short term treatment of five injections in 14 days and a long-term study treatment of 18 injections were carried out in mice produce no major side-effect. Different surface modification and particle size have on biocompatibility in nanoparticles, so these modifications can affect the interaction of nanoparticles in different organs and tissue.[28,30]
Bio-distribution is defined as tracking the localization of compounds of interest in the organs and tissues of experimental animals to determine the accumulation in different parts of the body. The biodistribution process using MSNs was shown in [Figure 5].
Figure 5.
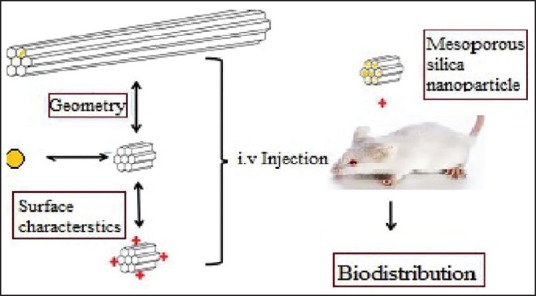
Biodistribution process of mesoporous silica nanoparticles
Bio-distribution has also been study showed that each type of porous silica (PSi)-particles tend to be removed from the body via the complement system or urine depending on the administration route. Bio-distribution of MSNs accumulated in tumors at concentrations ranging from 45 ng/mg (4 h postinjection) to 110 ng/mg (24 h postinjection) before decreasing to 65 ng/mg (48 h postinjection).[42] The highest accumulation of nanoparticles was found to be in the kidney and lungs. The other organs, including liver, heart, intestine, and spleen had a low accumulation in 48 h postinjection ranging from 3 ng/mg to 10 ng/mg. Targeting of the MSNs of cancer cells by attaching folate to their surface resulted in an increase in tumor accumulation. Experimental study showed that positively charged nanoparticles are excreted from the liver into the gastrointestinal tract and excreted from the animal in the feces while negatively charged MSNs are sequestered within the liver.[43] Therefore, the charge on the surface of the nanoparticles may play an important role in their bio-distribution. Another property that affects the bio-distribution of material used is the shape of the nanoparticles. Huang et al. showed that MSNs with a short rod-shape accumulate in the liver and long rod-shaped nanoparticles accumulate in the spleen.[42] The excretion of MSNs through the urine and feces has been reported by various groups. This study using phosphonate-MSNs up to 94% of the silica was excreted from the mice 4 days after injection with 73% being detected in the urine and 21% in the feces
Functionalization of mesoporous silica nanoparticles
Co-condensation process allows direct functionalization of the silica surface during the synthesis of the material, which was shown to be preferential for the internal surface of the mesopores. Typically, organosilanes (R-TMS or R-TES) where R is an organic functional group, while TMS is trimethoxysilane and TES is triethoxysilane is added into the reaction solution together with TEOS or immediately after the addition of TEOS which affords incorporation of organosilanes in the final material.[43]
Postsynthesis strategies for functionalization
Postsynthetic grafting strategy
In general, postsynthesis grafting is useful for functionalizing mesoporous silicates with active molecules that are incompatible with the chemistry of the initial sol. It is often desirable to functionalize accessible silica surfaces, such as the surface of nanoparticles or areas near pore orifices.[44] Postsynthesis grafting involves the same types of silane linkers that are commonly utilized in the bifunctional strategy.
Backfilling strategy
Backfilling is a simple strategy for introducing active molecules into the empty pores of mesostructured silica. Empty mesopores are filled by exposing a material to a solution or vapor containing the active molecules and allowing them to diffuse into it.[45] Backfilling does not result in chemically modified materials but is less widely useful.
Surface modification
Various characteristics of MSNs like the Surface area, zeta-potential, hydrophilicity, functional groups on surface, affinity, and selectivity that influence the bioavailability of the particles. By changing these characteristic properties of the particle modified more effective drug carriers.[46]
Target specificity
Mesoporous silica nanoparticles have ability to reach the correct target tissue mainly tumor on the basis of surface modification. Selectivity of nanoparticles improved by conjugation various target molecules via covalent bonding. Widely used targeting agents are organic molecules that already exist in the system as they usually are nontoxic, biodegradable and stable in the wide range of pH.[45]
Polyethylene glycol
Polyethylene glycol (PEG) is also nontoxic and causes no immunological response improving further its usefulness in surface modifications of nanoparticles. A surface modification of nanoparticles is PEGylation, in which the particles are coated with PEG chains. PEG can be attached to the particles with either covalent bonds, adsorption or by entrapping PEG moieties within the surface.[47,48]
APPLICATIONS OF MESOPOROUS SILICA NANOPARTICLES
MSNS shows various applications in the different field as shown in Figure 6.
Figure 6.
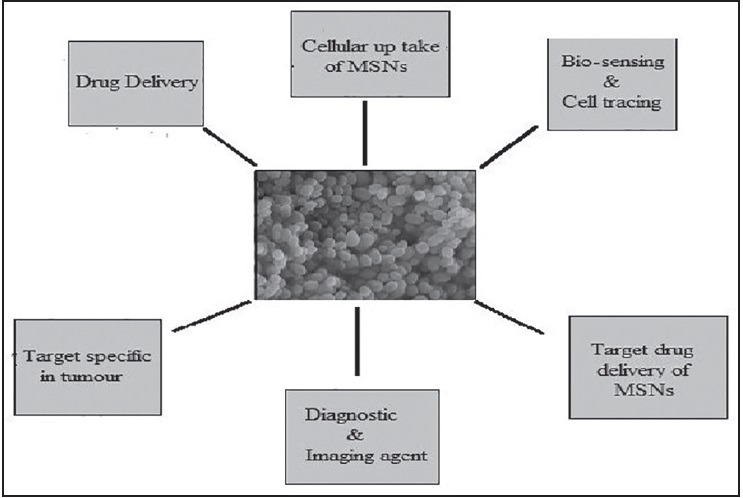
Applications of mesoporous silica nanoparticles in various bio-medical fields
Drug delivery
Porous silica based materials for drug delivery applications emerged in the late 1990's with reports of silica nanopore membranes; one major advantage of the PSi materials is that they can be tailored for continuous or triggered drug release depending on the application.[30] The paracellular delivery of insulin across an intestinal Caco-2 cell monolayer using micro fabricated PSi particles was one of the first examples of drug delivery using this material. Because of high surface area, the selectivity of the adsorption substance and low toxicity MSNs were widely used in adsorption of toxic molecules and drug delivery system.[49,50] In general, people use activated carbon to remove toxic chemicals however it showed poor removal ability
Conventional MSNs can load a dose of therapeutic drug with 200-300 mg (maximally about 600 mg) drug/1 g silica. For example, conventional glucose-responsive insulin delivery systems suffer from the decrease of insulin release with repeated cycles[18,51] [Table 3].
Table 3.
List of various types of drugs delivered through mesoporous silica nanoparticles
Cellular uptake of mesoporous silica nanoparticles
Silica based material nanoparticles uptake by cell produce no detectable toxic effects.[58] The mechanism of cellular uptake appears to be mediated by an active endocytosis pathway as MSNs endocytosis is inhibited by a decrease in temperature to 4°C that is incubation with metabolic inhibitors. Functionalization of the external surface of MSNs with groups in which cells express specific receptors like folic acid, which notably enhances the uptake efficiency of the material by cells and functionalization of the particles with groups that alter their zeta-potentials affects not only the efficiency of their internalization, but also the uptake mechanism.[56,59]
Bio-sensing and cell tracing
Mesoporous silica nanoparticles used as bio-sensing element due to their size and versatile chemistry of the structure. The unique surface properties and the small particle sizes of these nanoparticle-based sensor systems used for the detection of analysts within individual cells both in vivo and in vitro.[60] Nanoparticles do not suffer from fluorescent, self-quenching, and other diffusion-related problems. Therefore, the ability to functionalize the surface of nanoparticles with large quantities of cell-recognition or other site-directing compounds produces as an ideal agent for cell tracing.[42,49]
Target specific in tumor
The application of porous silica materials for cancer therapy has been emerging as a new interesting field of interdisciplinary research among chemistry, medicine, material science, biology, and pharmacology.[22,34] Selective targeting strategies employ ligands that specifically bind to receptors on the cell surface of interest to promote nanocarrier binding and internalization. This strategy requires that receptors are highly overexpressed by cancer cells relative to normal cells.[53] The effective drug treatment by MSNs in the cancerous cell as shown in Figure 7. The outstanding advantages of porous nanomaterials is the ability of surface functionalization with targeting moieties is the most exciting way to deliver the drug to a cancerous cell.
Figure 7.

Present multi-drug delivery systems of mesoporous silica nanoparticles in targeted delivery in the treatment of tumor cells
Targeted MSNs therapies are used to block the growth and spread of cancer by interfering directly with specific molecules involved in tumor growth and progression or indirectly by stimulating the immune system to recognize and destroy cancer cells.[61] For example, the delivery of many toxic antitumor drugs requires “zero release” before reaching the targeted cells or tissues. Efficient delivery of doxorubicin (DOX) using MSNs coated with a PEG copolymer using 50 nm MSNs, when coated with the copolymer they can reach a size of 110 nm.[16,62] Cancerous therapy was done through injected of 120 mg/kg of nanoparticles on a weekly basis for 3 weeks to a KB-31 xenograft model. The DOX-loaded nanoparticles demonstrated 85% tumor inhibition compared with 70% inhibition by the free drug.[63] Therefore, there is an important need for using porous nanoparticles as nanovaccines to treat cancer
Diagnostic and imaging agent
In recent years, multifunctional nanomaterials are used for simultaneous imaging and therapy. The field has expanded as rapidly as “theranostic” has been coined to describe platforms that serve dual roles in the field of diagnostic and therapeutic agents.[64,65] Ideally, these nanomaterials will be suitable for long-term quantitative imaging at low doses and safely cleared from the body after imaging is complete. Silica-based imaging nanoprobes are most commonly used for optical, magnetic resonance imaging (MRI) or a combination of both modalities.[66] Silica nanoparticles are an excellent carrier for facile loading of a wide variety of imaging and therapeutic moieties, making them promising candidates for theranostic applications
Targeted drug delivery of mesoporous silica nanoparticles
Specific targeting is highly attractive as an approach to spontaneously distinguish the site of disease diagnosis and as a result, this technique reduces drug administration dosage and diminishes toxic side-effects of drugs during circulation.[67] Both passive strategies and active surface decoration methods have been applied to the fabrication of novel MSNs based drug delivery systems for targeted release.[68]
Passive strategies
Passive routes passive accumulation of MSNs in tumor tissue can be realized by the enhanced permeability and retention (EPR) effect, a theory first postulated by Matsumura and Maeda.[69] They hypothesized that the differential localization of macromolecules, as well as particles of certain sizes, is attributed to the tumor microenvironment, the relative slow elimination rate, and poor lymphatic drainage. Effectiveness of the EPR effect can be mediated by the particle size, surface charge or hydrophobicity.[70,71]
Active surface decoration
Surface decoration with targeting ligands efforts have been made to functionalize the surfaces of MSNs with cancer-specific targeting ligands for an enhanced MSNs uptake by cancer cells compared to non-cancerous cells. One such ligand is folic acid,[72] as folate receptors are known to be overexpressed in several types of human cancer including ovarian, endometrial, colorectal, breast, and lung.[73] Besides folic acid, other small cell nutrient molecules such as mannose and 100 were also shown to selectively improve the uptake of MSNs by breast cancer cells.
PATENTED CITATION OF MESOPOROUS SILICA NANOPARTICLES
The various types of patent in the field of MSN were shown in [Table 4].
Table 4.
Patented citations
Future prospective
Mesoporous silica nanoparticles involve the identification of precise targets (cells and receptors) related to specific clinical conditions, and it is a choice of the appropriate nanocarriers to achieve the required responses at target site while minimizing the side effects of respective target molecule. Thus, in vivo potential of nanotechnology toward targeted imaging and drug delivery to be realized, that MSNs have to get smarter effects on a wide range of treatment in various diseases. MSNs are targeted contrast agents that improve the resolution of cancer cells to the single cell level. It is act as good diagnostic agents if iron oxide nanocrystals where incorporated into the mesoporous particles for magnetic manipulation and MRI. To further develop mesoporous silica for oral administration, the mechanisms behind the effects on weight and fat reduction needs to be investigated fully. Therefore, it is essential that fundamental research be carried out to address these issues if the successful efficient application of these technologies is going to be achieved.
CONCLUSIONS
In conclusion, this review describes in detail the recent progress of synthesizing and functionalizing MSNs and using these nanomaterials as targeted release and biological cell delivery vehicles. On the other side, the synthesis methods to functional MSNs with a core-shell structure are able to produce an acceptable amount of nanoparticles. Hence, they can be used as a unique essential drug carrier to overcome unwanted side effects. A versatile approach for the selective functionalization of MSNs has been developed. Hence, they have a wonderful approach for the application in the different field like drug delivery, diagnostic and imaging agent, and target drug delivery in the treatment in cancer. It is important to underline that one of the most valuable characteristics of silica nanoparticles is the possibility to use them to merge different materials, to combine various functionalities, that is to say to act as a basis to obtain multifunctional medicine dedicated nanoplatforms enabling multimodal imaging and simultaneous diagnosis and therapy. MSNPs are promising nanocarriers to efficiently transport and site-specifically deliver highly toxic drugs, like chemotherapeutic agents for cancer treatment. MSNs are efficiently transported site specific drug delivery of highly toxic drug such chemotherapeutic agents for effective cancer treatment. They have stimuli responsive drug releases so, it enhancing and minimizing the side effects of anti-cancer drugs in cancer therapy.
Footnotes
Source of Support: Nil.
Conflicts of Interest: None declared.
REFERENCES
- 1.Swami A, Jinjun S, Suresh G, Votruba RA, Nagesh K, Farokhzad CO. Nanoparticles for targeted and temporally controlled drug delivery. In: Svenson S, Prud’homme RK, editors. Multifunctional Nanoparticles for Drug Delivery, Applications: Imaging, Targeting, and Delivery, Nanostructure Science Technology. Bostan, USA: Springer; 2012. pp. 9–25. [Google Scholar]
- 2.Mohanraj VJ, Chen Y. Nanoparticle: A review. Trop J Pharm Res. 2006;5:561–73. [Google Scholar]
- 3.Sooyeon K, Singh RK, Wojciech C. Silica-based mesoporous nanoparticles for controlled drug delivery. J Tissue Eng. 2013;4:1–35. doi: 10.1177/2041731413503357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brannon-Peppas L. Recent advances on the use of biodegradable microparticles and nanoparticles in controlled drug delivery. Int J Pharm. 1995;116:1–9. [Google Scholar]
- 5.Kresge CT, Leonowicz ME, Roth WJ. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature. 1992;359:710–2. [Google Scholar]
- 6.Shchipunov YA, Burtseva YV, Karpenko TY, Shevchenko NM, Zvyagintseva TN, Starikayasss M, et al. Highly efficient immobilization of endo-1, 3-beta-d-glucanases (laminarinases) from marine mollusks in novel hybrid polysaccharide-silica nanocomposites with regulated composition. J Mol Catal. 2006;40:16–23. [Google Scholar]
- 7.Klichko Y, Liong M, Choi E, Angelos S, Nel AE, Stoddart E, et al. Mesostructured silica for optical functionality, nanomachines, and drug delivery. J Am Ceram Soc. 2009;92:S2–10. doi: 10.1111/j.1551-2916.2008.02722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tourne-Peteilh C, Begu S, Lerner DA, Galarneau A, Lafont U, Devoisselle JM. Sol-gel one-pot synthesis in soft conditions of mesoporous silica materials ready for drug delivery system. J Solgel Sci Technol. 2012;61:455–62. [Google Scholar]
- 9.Liong M, Lu J, Kovochich M, Xia T, Ruehm SG, Nel AE, et al. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano. 2008;2:889–96. doi: 10.1021/nn800072t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hongmin C, Zipeng Z, Jin X. Label-free luminescent mesoporous silica nanoparticles for imaging and drug delivery. Theranostic. 2013;3:650–7. doi: 10.7150/thno.6668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie J, Lee S, Chen X. Nanoparticle-based theranostic agents. Adv Drug Deliv Rev. 2012;62:1064–79. doi: 10.1016/j.addr.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin X, Xie J, Niu G, Zhang F, Gao H, Yang M, et al. Chimeric ferritin nanocages for multiple function loading and multimodal imaging. Nano Lett. 2011;11:814–9. doi: 10.1021/nl104141g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fruijtier-Pölloth C. The toxicological mode of action and the safety of synthetic amorphous silica-a nanostructured material. Toxicology. 2012;294:61–79. doi: 10.1016/j.tox.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Vivero-Escoto JL, Luis J. Surface functionalized mesoporous silica nanoparticles for intracellular drug delivery. Diss Abstr Int. 2009;71:621–38. [Google Scholar]
- 15.Selvam P, Bhatia SK, Sonwane CG. Recent advances in processing and characterization of periodic mesoporous MCM-41 silicate molecular sieves. Ind Eng Chem Res. 2001;40:3237–61. [Google Scholar]
- 16.Gary-Bobo M, Hocine O, Brevet D, Maynadier M, Raehm L, Richeter S, et al. Cancer therapy improvement with mesoporous silica nanoparticles combining targeting, drug delivery and PDT. Int J Pharm. 2012;423:509–15. doi: 10.1016/j.ijpharm.2011.11.045. [DOI] [PubMed] [Google Scholar]
- 17.Yiu HH, Wright PA. Enzymes supported on ordered mesoporous solids: A special case of an inorganic-organic hybrid. Mater Chem. 2005;15:3690–700. [Google Scholar]
- 18.Trewyn BG, Slowing II, Giri S, Chen HT, Lin VS. Synthesis and functionalization of a mesoporous silica nanoparticle based on the sol-gel process and applications in controlled release. Acc Chem Res. 2007;40:846–53. doi: 10.1021/ar600032u. [DOI] [PubMed] [Google Scholar]
- 19.Radin S, Ducheyne P, Kamplain T, Tan BH. Silica sol-gel for the controlled release of antibiotics. I. Synthesis, characterization, and in vitro release. J Biomed Mater Res. 2001;57:313–20. doi: 10.1002/1097-4636(200111)57:2<313::aid-jbm1173>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 20.Rambabu A. Novel synthesis, structure and functions of mesoporous silica materials. Uppsala: Acta Universitatis Upsaliensis; 2010. pp. 13–24. [Google Scholar]
- 21.Chen CY, Burkett SL, Li HX Davis ME. Ordered mesoporous and macro porous inorganic film and membrane. Microporous Mater. 1993;2:27–34. [Google Scholar]
- 22.Firouzi A, Kumar D, Bull LM, Besier T, Sieger P, Huo Q, et al. Cooperative organization of inorganic-surfactant and biomimetic assemblies. Science. 1995;267:1138–43. doi: 10.1126/science.7855591. [DOI] [PubMed] [Google Scholar]
- 23.Shahbazi MA, Herranz B, Santos HA. Nanostructured porous Si-based nanoparticles for targeted drug delivery. Biomatter. 2012;2:296–312. doi: 10.4161/biom.22347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yanes RE, Tamanoi F. Development of mesoporous silica nanomaterials as a vehicle for anticancer drug delivery. Ther Deliv. 2012;3:389–404. doi: 10.4155/tde.12.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu J, Liong M, Zink JI, Tamanoi F. Mesoporous silica nanoparticles as a delivery system for hydrophobic anticancer drugs. Small. 2007;3:1341–6. doi: 10.1002/smll.200700005. [DOI] [PubMed] [Google Scholar]
- 26.Bagwe RP, Hilliard LR, Tan W. Surface modification of silica nanoparticles to reduce aggregation and nonspecific binding. Langmuir. 2006;22:4357–62. doi: 10.1021/la052797j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hudson SP, Padera RF, Langer R, Kohane DS. The biocompatibility of mesoporous silicates. Biomaterials. 2008;29:4045–55. doi: 10.1016/j.biomaterials.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu J, Liong M, Li Z, Zink JI, Tamanoi F. Biocompatibility, biodistribution, and drug-delivery efficiency of mesoporous silica nanoparticles for cancer therapy in animals. Small. 2010;6:1794–805. doi: 10.1002/smll.201000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Souris JS, Lee CH, Cheng SH, Chen CT, Yang CS, Ho JA, et al. Surface charge-mediated rapid hepatobiliary excretion of mesoporous silica nanoparticles. Biomaterials. 2010;31:5564–74. doi: 10.1016/j.biomaterials.2010.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang X, Li L, Liu T, Hao N, Liu H, Chen D, et al. The shape effect of mesoporous silica nanoparticles on biodistribution, clearance, and biocompatibility in vivo. ACS Nano. 2011;5:5390–9. doi: 10.1021/nn200365a. [DOI] [PubMed] [Google Scholar]
- 31.Fan J, Fang G, Wang X, Zeng F, Xiang Y, Wu S. Targeted anticancer prodrug with mesoporous silica nanoparticles as vehicles. Nanotechnology. 2011;22:455102. doi: 10.1088/0957-4484/22/45/455102. [DOI] [PubMed] [Google Scholar]
- 32.Vivero-Escoto JL, Slowing II, Trewyn BG, Lin VS. Mesoporous silica nanoparticles for intracellular controlled drug delivery. Small. 2010;6:1952–67. doi: 10.1002/smll.200901789. [DOI] [PubMed] [Google Scholar]
- 33.Sakai-Kato K, Hasegawa T, Takaoka A, Kato M, Toyo'oka T, Utsunomiya-Tate N, et al. Controlled structure and properties of silicate nanoparticle networks for incorporation of biosystem components. Nanotechnology. 2011;22:205702. doi: 10.1088/0957-4484/22/20/205702. [DOI] [PubMed] [Google Scholar]
- 34.Shchipunov YA, Ruiz-Hitzky E, Ariga K, Lvov YM. Bio-inorganic Hybrid Nanomaterials: Strategies, Syntheses, Characterization and Applications. Weinheim: Wiley-VCH Verlag GmbH & Co., KGaA; 2008. Entrapment of biopolymers into sol-gel derived silica nanocomposites; pp. 75–111. [Google Scholar]
- 35.Cademartiri R, Anany H, Gross I, Bhayani R, Griffiths M, Brook MA. Immobilization of bacteriophages on modified silica particles. Biomaterials. 2010;31:1904–10. doi: 10.1016/j.biomaterials.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 36.Shchipunov YA, Karpenko TY, Krekoten AV. Hybrid organic-inorganic nanocomposites fabricated with a novel biocompatible precursor using sol-gel processing. Compos Interface. 2005;11:587–607. [Google Scholar]
- 37.Colby MW, Osaka A, Mackenzie JD. Effects of temperature on formation of silica-gel. J Non Cryst Solids. 1986;82:37–41. [Google Scholar]
- 38.Shchipunov YA, Karpenko TY, Krekoten AV, Postnova IV. Gelling of otherwise nongelable polysaccharides. J Colloid Interface Sci. 2005;287:373–8. doi: 10.1016/j.jcis.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Wang S. Ordered mesoporous materials for drug delivery. Microporous Mesoporous. 2009;117:1–9. [Google Scholar]
- 40.Lu J, Li Z, Zink JI, Tamanoi F. In vivo tumor suppression efficacy of mesoporous silica nanoparticles-based drug-delivery system: Enhanced efficacy by folate modification. Nanomedicine. 2012;8:212–20. doi: 10.1016/j.nano.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knezevic N. Functionalized Mesoporous Silica Nanoparticles for Stimuli-Responsive and Targeted Drug Delivery. Ames, Iowa: Digit Repository, Lowa State University; 2009. pp. 1–96. [Google Scholar]
- 42.Portin L. Layer by Layer Assembly of the Polyelectrolyte on Mesoporous Silicon. Finland: Biosciences, University of Eastern Finland; 2012. pp. 1–59. [Google Scholar]
- 43.Kennedy LC, Bickford LR, Lewinski NA, Coughlin AJ, Hu Y, Day ES, et al. A new era for cancer treatment: Gold-nanoparticle-mediated thermal therapies. Small. 2011;7:169–83. doi: 10.1002/smll.201000134. [DOI] [PubMed] [Google Scholar]
- 44.He Q, Zhang Z, Gao F, Li Y, Shi J. In vivo biodistribution and urinary excretion of mesoporous silica nanoparticles: Effects of particle size and PEGylation. Small. 2011;7:271–80. doi: 10.1002/smll.201001459. [DOI] [PubMed] [Google Scholar]
- 45.Rytkonen J, Miettinen R, Kaasalainen M, Lehto V, Salonen J, Narvanen A. Functionalization of mesoporous silicon nanoparticles for targeting and bioimaging purposes. J Nanomater. 2012 doi:10.1155/2012/896562. [Google Scholar]
- 46.Owens DE, 3rd, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 47.Bimbo LM. Biocampatability and Biofunctionalization of Mesoporous Silicon Particles. Finland: Divi of Pharmaceutical Technology, University of Helsiniki; 2012. [Google Scholar]
- 48.Slowing II, Trewyn BG, Giri S, Lin Victor SY. Mesoporous silica nanoparticles for drug delivery and bio-sensing applications. Adv Funct Mater. 2007;17:1225–36. [Google Scholar]
- 49.Bergman L, Kankaanpaa L, Tiitta S, Duchanoy A, Li L, Heino J, et al. Intracellular degradation of multilabeled poly (ethylene imine) - Mesoporous silica-silica nanoparticles: Implications for drug release. Mol Pharm. 2013;10:1795–803. doi: 10.1021/mp3005879. [DOI] [PubMed] [Google Scholar]
- 50.Yu T, Malugin A, Ghandehari H. Impact of silica nanoparticle design on cellular toxicity and haemolytic activity. ACS Nano. 2011;5:5717–28. doi: 10.1021/nn2013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee S, Kim MS, Lee D, Kwon TK, Khang D, Yun HS, et al. The comparative immunotoxicity of mesoporous silica nanoparticles and colloidal silica nanoparticles in mice. Int J Nanomedicine. 2013;8:147–58. doi: 10.2147/IJN.S39534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma X, Zhao Y, Ng KW, Zhao Y. Integrated hollow mesoporous silica nanoparticles for target drug/siRNA co-delivery. Eur J. 2013;19:15593–603. doi: 10.1002/chem.201302736. [DOI] [PubMed] [Google Scholar]
- 53.Zhang J, Yuan ZF, Wang Y, Chen WH, Luo GF, Cheng SX, et al. Multifunctional envelope-type mesoporous silica nanoparticles for tumor-triggered targeting drug delivery. J Am Chem Soc. 2013;135:5068–73. doi: 10.1021/ja312004m. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Q, Liu F, Nguyen KT, Ma X, Wang X, King, et al. Multifunctional mesoporous silica nanoparticles for cancer-targeted and controlled drug delivery. Adv Funct Mater. 2012;22:5144–56. [Google Scholar]
- 55.Mamaeva V, Rosenholm JM, Bate-Eya LT, Bergman L, Peuhu E, Duchanoy A, et al. Mesoporous silica nanoparticles as drug delivery systems for targeted inhibition of Notch signaling in cancer. Mol Ther. 2011;19:1538–46. doi: 10.1038/mt.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Halamova D, Zelenak V. NSAID naproxen in mesoporous matrix MCM-41: Drug uptake and release properties. Incl Phenom Macro. 2012;72:15–23. [Google Scholar]
- 57.Lin Y, Yu VS, Lai C, Jeftinija S, Jeftinija DM. 2012. US12411869.
- 58.Vallet-Regi M, Izquierdo-Barba I, Colilla M. Structure and functionalization of mesoporous bioceramics for bone tissue regeneration and local drug delivery. Philos Trans R Soc A. 2012;370:1400–21. doi: 10.1098/rsta.2011.0258. [DOI] [PubMed] [Google Scholar]
- 59.Balas F, Manzano M, Horcajada P, Vallet-Regí M. Confinement and controlled release of bisphosphonates on ordered mesoporous silica-based materials. J Am Chem Soc. 2006;128:8116–7. doi: 10.1021/ja062286z. [DOI] [PubMed] [Google Scholar]
- 60.Du H, Hamilton PD, Reilly MA, d'Avignon A, Biswas P, Ravi N. A facile synthesis of highly water-soluble, core-shell organo-silica nanoparticles with controllable size via sol-gel process. J Colloid Interface Sci. 2009;340:202–8. doi: 10.1016/j.jcis.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 61.Colilla M, Izquierdo-Barba I, Vallet-Regi M. Phosphorus-containing SBA-15 materials as bisphosphonate carriers for osteoporosis treatment. Microporous Mesoporous Mater. 2010;135:51–9. [Google Scholar]
- 62.Lai CY, Trewyn BG, Jeftinija DM, Jeftinija K, Xu S, Jeftinija S, et al. A mesoporous silica nanosphere-based carrier system with chemically removable CdS nanoparticle caps for stimuli-responsive controlled release of neurotransmitters and drug molecules. J Am Chem Soc. 2003;125:4451–9. doi: 10.1021/ja028650l. [DOI] [PubMed] [Google Scholar]
- 63.Vivero-Escoto JL, Huxford-Phillips RC, Lin W. Silica-based nanoprobes for biomedical imaging and theranostic applications. Chem Soc Rev. 2012;41:2673–85. doi: 10.1039/c2cs15229k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Janib SM, Moses AS, MacKay JA. Imaging and drug delivery using theranostic nanoparticles. Adv Drug Deliv Rev. 2010;62:1052–63. doi: 10.1016/j.addr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu X, Wu M, Zhao JX. Recent development of silica nanoparticles as delivery vectors for cancer imaging and therapy. Nanomedicine. 2014;10:297–312. doi: 10.1016/j.nano.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang LS, Wu LC, Lu SY, Chang LL, Teng IT, Yang CM. Bio functionalized phospholipid-capped mesoporous silica nanoshuttles for targeted drug delivery: Improved water suspensibility and decreased nonspecific protein binding. ACS Nano. 2010;4:4371–9. doi: 10.1021/nn901376h. [DOI] [PubMed] [Google Scholar]
- 67.Arap W, Pasquilini R, Montalti M, Petrizza L, Prodi L, Rampazzo E, et al. Luminescent silica nanoparticles for cancer diagnosis. Curr Med Chem. 2013;20:2195–211. doi: 10.2174/0929867311320170005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Siefker J, Karande P, Coppens MO. Packaging biological cargoes in mesoporous materials: Opportunities for drug delivery. Expert Opin Drug Deliv. 2014;11:1781–93. doi: 10.1517/17425247.2014.938636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–92. [PubMed] [Google Scholar]
- 70.Kapse-Mistry S, Govender T, Srivastava R, Yergeri M. Nanodrug delivery in reversing multidrug resistance in cancer cells. Front Pharmacol. 2014;5:159. doi: 10.3389/fphar.2014.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tarn D, Ashley CE, Xue M, Carnes EC, Zink JI, Brinker CJ. Mesoporous silica nanoparticle nanocarriers: Biofunctionality and biocompatibility. Acc Chem Res. 2013;46:792–801. doi: 10.1021/ar3000986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baeza A, Colilla M, Vallet-Regí M. Advances in mesoporous silica nanoparticles for targeted stimuli-responsive drug delivery. Expert Opin Drug Deliv. 2015;12:319–37. doi: 10.1517/17425247.2014.953051. [DOI] [PubMed] [Google Scholar]
- 73.Singh D, McMillan JM, Liu XM, Vishwasrao HM, Kabanov AV, Sokolsky-Papkov M, et al. Formulation design facilitates magnetic nanoparticle delivery to diseased cells and tissues. Nanomedicine (Lond) 2014;9:469–85. doi: 10.2217/nnm.14.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dower WJ, Barrett RW, Gallop MA, Needels MC. Needels MC. 1997 US08146886. [Google Scholar]
- 75.Chandler VS, Jerrold FR, Chandler MB. 1991. US8540814.
- 76.Schultz S, Schultz DA, Smith DR, Mock JJ, Silva TJ. 2001. US09027048.
- 77.Foundation Inc CR, Ow H, Wiesne UB. 2004. PCT/US2003/037793.
- 78.Zink JI, Nel AE, Tian X, Zhaoxia J, Huan M, et al. 2012. US13428830.
- 79.Chao KJ, Yang CM. 2003. US10059995.
- 80.Lin V, Trewyn SY, Brian G, Whitman HS, Chad M. 2006. US10945545.
- 81.Martens J, Den GV, Humbeeck JV, Aerts C, Mellaerts R. 2007. US11575014.


