Abstract
Introduction:
The copolymer of polyethylene glycol (PEG) and polyesters has many interesting properties, such as amphiphilicity, biocompatibility, biodegradability, and self-assembly in an aqueous environment. Diblock copolymers of PEG-polyester can form different structures such as micelles, polymersome, capsules or micro-container in an aqueous environment according to the length of their blocks.
Materials and Methods:
Herein, a series of poly (lactic acid) (PLA) and PEG diblock copolymers were synthesized through the ring-opening polymerization. The polymerization reaction and the copolymer structures were evaluated by nuclear magnetic resonance (NMR) and gel permeation chromatography (GPC). The corresponding copolymers were implemented for the formation of polymersome structures using film rehydration method. Impact of methoxy PEG chain length and hydrophobic weight fraction on particle size of polymersomes were studied, and the proper ones were selected for loading of doxorubicin (DOX) via pH gradient method.
Results and Discussion:
Results obtained from 1HNMR and GPC revealed that microwave irradiation is a simple and reliable method for the synthesis of PEG-PLA copolymers. Further analysis indicated the copolymer with relative molecular weight of PLA to PEG ratios of 3 or fEo ~ 25% produced the smallest size polymersomes. Polymersomes prepared from PEG5000 to PLA15000 were more capable in loading and sustained release of DOX than those prepared from PEG2000 to PLA6000. Conclusion: In conclusion copolymers of PEG/PLA with fOE ~25% and relatively higher molecular weight are more suitable for encapsulation and providing sustained release of DOX.
Keywords: Copolymer, doxorubicin, polyethylene glycol-poly (lactic acid), polymersome, self- assembly
INTRODUCTION
Biodegradable polyesters including poly (lactide), poly (glycolide), poly (lactide)-co-poly (glycolide) copolymers and poly (ε-caprolactone) are implemented frequently in pharmaceutical development due to their bioresorbable and biocompatible characteristics.[1,2,3,4,5]
On the other hand, polyethylene glycol (PEG) is nonbiodegradable, but biocompatible polymer which has been used for many clinical applications.[6]
Polyethylene glycol possesses notable characteristics, such as hydrophilicity and biocompatibility. Copolymerization of PEG with polyesters suggests the possibility to integrate the unique properties of PEG and biodegradable polyesters.
These copolymers consist of biodegradable hydrophobic polyester blocks and hydrophilic, biocompatible PEG segments.
The copolymer of PEG and polyesters has many interesting properties, such as amphiphilicity, biocompatibility, biodegradability, and self-assembly in an aqueous environment. Diblock copolymers of PEG-polyester can form different structures such as micelles, polymersome, capsules or micro-container in an aqueous environment according to the length of their blocks.[7]
In recent years, many pharmaceutical studies have been devoted to develop vesicular systems named polymersomes based on block copolymers as competent drug carriers, with attractive and capable characteristics.[8,9,10,11,12,13,14] Compare to liposomes, polymersomes exhibited better properties for sustained drug delivery, due to its high membrane stability and low membrane permeability that can be modulated by varying block lengths.[15]
The membrane thickness in polymersomes can be varied to a wider range (5-20 nm) in comparison with liposome (3-5 nm). Due to this property it is possible to design the desirable controlled drug delivery system by modulating the membrane thickness of polymersomes.
The bilayers stability in polymersome is an important advantage to prevent drug leakage and disintegration of the membrane encountered in lipidic vesicles.[16]
In addition, it has been proved that the release profiles and pharmacokinetics of polymersomes formulation can be controlled in vitro and in vivo.[17] As a smart drug delivery system, polymersomes with various sizes between 5 nm and 5 μm could be prepared depending on the type and chain length of copolymer. Moreover, the preparation techniques of polymersomes could influence the mean size of structures, which develop toward drug delivery systems based on polymeric vesicles.[18,19]
The outstanding polymersome characteristics such as physicochemical properties, release kinetics, and targeting chemistries of polymersome vehicle make them an ideal carrier for the encapsulation of therapeutic molecules. As demonstrated by their effective treatment of tumor models in vivo polymersomes exhibit great potency in proceeding from design and synthesis onto therapeutic application.[11]
The long-circulating properties of the 100% PEGylated biodegradable nano-sized polymersomes provide passive targeting for tumors in cancer chemotherapy by the enhanced permeability and retention effect.[20]
Successful shrinkage of the tumor after intravenously administration of doxorubicin (DOX)-paclitaxel loaded nano-polymersomes in tumor bearing nude mice suggests a great opportunity for the use of polymersomes in the effective delivery of anti-cancer drugs or therapeutic molecules.[21]
The great characteristics and exceptional properties for polymersome structures could be obtained by recent advances in block copolymer chemistries.
However, while liposomes are generally investigated for drug delivery, they are not capable in sustained release of drugs, which marks a significant inadequacy of this group of nanocarriers.
Polymeric vesicles can encapsulate high quantity of drugs and release them at sustained rates in the desirable drug concentration, and consequently increasing the in vivo therapeutic efficacy and easing the implementation of highly toxic anti-cancer agents.[22]
In this regards, in the current study DOX was used as a model drug to investigate the best characteristic of self-assembled nano-scale PEG-poly (lactic acid) (PLA) polymeric vesicles (polymersomes) for encapsulation and controlled release of DOX.
In this report; we aimed to investigate the effect of PEG length and also hydrophilic to hydrophobic weight ratio in copolymer structure on self-assembly properties of PEG-PLA copolymers for use as a drug vehicle in chemotherapy. PEG 2000 and 5000 were used, and copolymers with ratios of PLA to PEG2000 of 1.5 and 3 and PLA to PEG5000 of 1.5, 2 and 3 were prepared.
All five copolymers were assessed for formation of polymersomes using film rehydration technique. Among these, polymers, which were able to self-assemble into polymersomes with smallest mean particle size were chosen in order to encapsulate DOX as a chemotherapeutics model drug within polymersome structures. DOX release studies were also carried out to explore the ability of these polymersomes as a carrier for sustained release of water soluble drugs.
MATERIALS AND METHODS
Methoxy-PEG with molecular weights of 2000 and 5000 Da, D,L-Lactide and Stannous octanoate (Tin(II) ethyl hexanoate) were purchased from Sigma-Aldrich (Germany). DOX hydrochloride was procured from Eurasia (New Delhi, India). Dialysis sac (cut off 3.5 kD) was purchased from Thermo Scientific (Germany). All other chemical reagents and analytical grade solvents were obtained from Merck (Germany) and were used as received.
Synthesis and purification of polyethylene glycol-poly (lactic acid) copolymers
Synthesis of PEG-PLA copolymers was carried out with five different block lengths of (PEG-PLA): 2000:3000 (copolymer 1), 2000:6000 (copolymer 2), 5000:7000 (copolymer 3), 5000:9000 (copolymer 4) and 5000:15000 (copolymer 5) by ring opening polymerization method.
The copolymers were synthesized under microwave irradiation. Briefly, for synthesize of copolymer 5 as an example, PEG5000 2.5 g were introduced into a dry round bottom flask equipped with a condenser and was placed in a Milestone Microsynth microwave (Italy). The PEG was irradiated for 10 min at 1000 watt and 120°C to increase melted PEG viscosity. Then to the dried viscous PEG, 7.5 g DL-lactide and 10 μL Sn(Oct) were added and the mixture was further irradiated and stirred at 1000 watt and 30 rpm while temperature was kept at 130°C for 25 min. In order to purify the synthesized copolymer; obtained copolymers were dissolved in an adequate amount of chloroform. Then the synthesized copolymers were precipitated by adding 10-fold cold diethyl ether. The purification process was repeated 3 times and the precipitates were freeze dried, and the products were kept at −20°C until use.
Characterization of the polyethylene glycol-poly (lactic acid) Copolymers
The 1H-nuclear magnetic resonance (1H-NMR) spectra of the PEG-PLA copolymers were recorded at room temperature using a Bruker AC 80 nuclear magnetic resonance spectrometer (Germany) in CDCl3.
The 1HNMR spectra were used to estimate Mn of copolymers from the integration ratio of resonances at 5.4 ppm originated from CH of the PLA block and CH2 of the ethylene glycol at 3.8 ppm according to the method established by Jeong et al.[23]
Molecular weights and polydispersity were determined using the Agilent gel permeation chromatography (GPC)-Add-on system and refractive index signal detector recording at 212 nm coupled to the PL gel columns and operated at temperature 25°C. The molecular weights were calibrated with polystyrene standards. Tetrahydrofuran was used as eluent (flow rate: 1 mL/min), and the sample injection volume was 10 μL.
Thermal transitions of copolymers were determined using differential scanning calorimetry (DSC) (Mettler Toledo DSC 822, Switzerland). The sample sizes were approximately 2 mg, and each sample was subjected to thermal cycles from 0 to 140°C at a rate of 10°C/min.
Self-assembly of copolymer
Blank self-assembled structures were prepared by film rehydration method. Briefly, 2 mL of the copolymer solution (10 mg/mL) in dichloromethane was transferred into a round bottom flask. The solvent was evaporated under vacuum using flask rotary evaporator at room temperature for 6 h.
The thin, dried polymer film was hydrated by addition of 2 mL distilled water at 60°C and stirred overnight under 1250 rpm continuous stirring.
The polymersome dispersion was sonicated for 30 min at 25°C ± 2°C in bath sonicator (Bandelin Sonorex Digitec, Germany) followed by extrusion 15 times through two stacked 100 nm Nucleopore polycarbonate filters using Avanti mini extruder (Avanti polar lipids) on a heat block at 60°C.
Characterization of polymersomes
The measurement of polymersome size and size distribution was carried out by dynamic light scattering (DLS). Briefly, 20 μL of polymersome suspensions (10 mg/mL) was diluted in 980 μL of deionized 18 MΩ water and then analyzed with zetasizer (NANO-ZS, Malvern, UK) at a scattering angle of 90 equipped with a 4 mW He-Ne laser operating at 633 nm using back-scattering detection. All measurements were performed in triplicate at 25°C.
Before ultrasonication and extrusion procedure, the self-assembled blank structures were observed by light microscope (Olympus/CHK, Taiwan) at various magnification levels (×40 or ×100).
Transmission electron microscopy was also performed to indicate the spherical and homogeneity of polymersomes shapes. Dispersion (5 μL) of blank polymersomes after performing ultrasonication and extrusion procedure was placed on a carbon-coated copper grid (300 mesh), and the vesicles were fixed using 2% osmium tetroxide. At next stage, the grid was washed with deionized water and held to dry at room temperature for 3 h. The grid was then mounted on the transmission electron microscope (LEO/Zeiss 910, Germany), and images were captured, at different magnifications (×24,000, ×30,000).
Doxorubicin loading
Doxorubicin loaded polymersomes were prepared by pH gradient method.[24] For this purpose the thin, dried polymer film of copolymers 2 and 5, were hydrated using 2 mL aqueous ammonium sulfate 250 mM at 60°C under 1250 rpm continuous stirring overnight.
The polymersome dispersion was sonicated for 30 min at 25°C ± 2°C in bath sonicator (Bandelin Sonorex Digitec, Germany), followed by extrusion 15 times through two stacked 100 nm Nucleopore polycarbonate filters using Avanti mini extruder (Avanti polar lipids) on a heat block at 60°C.
The sonicated polymersomes were kept undisturbed for 24 h in the refrigerator at 4°C.
To establish a transmembrane pH gradient, dialysis against 10% sucrose solution was carried out. Briefly, the dialysis tubing (cut off 3.5 kD) was filled with 2 mL ammonium sulfate polymersome suspension. Then the sac was immersed in a flask containing 200 mL of 10% sucrose solution. The contents of the flask were stirred at 400 rpm, and the flask was closed with the parafilm. The dialysis was carried out for 48 h to develop a transmembrane pH gradient.
After pH gradient establishment, volumes equivalent to 20 mg of polymersomes (10 mg/mL) were incubated with the 400 μL of DOX stock solution (10 mg/mL DOX which was prepared in 10% sucrose solution) for 10 h at 60°C ± 2°C in bath oil. At the final stage, ultrafiltration (MWCO 30 kD) was used to remove free DOX.
In order to determine the encapsulation efficiency (EE) of DOX in polymersomes, 20 μL of DOX loaded polymersomes was mixed with 980 μL of dimethylsulfoxide in order to break the structure of nano-vesicles. The solution was then analyzed by spectrophotometry measurements at A = 480 nm using ultraviolet (UV) –visible spectrophotometer (UV-160A Shimadzu, Japan).
Encapsulation efficiency (EE %) was then calculated using Equation 1:
Encapsulation efficiency (EE %) = (Wt/Wi×100) (1)
Where Wt is a total amount of drug in the polymersomes and Wi is the total quantity of drug added initially to perform loading procedure.
Release study
The in vitro release of DOX from PEG-PLA polymersome formulations was assessed up to 5 days (150 h) under physiological condition (pH 7.4, 37°C).
One milliliter of each formulation (PolyDOX-5 containing 1.96 mg DOX/mL and PolyDOX-2 containing 1.006 mg DOX/mL) or DOX suspension with equivalent concentration of DOX (1.96 mg DOX/mL or 1.006 mg DOX/mL) were introduced into a dialysis bag (MWCO 3.5 kD) and then immersed into 100 mL of PBS (0.1 M, pH 7.4) medium in a shaker incubator set at 90 rpm and 37°C.
Samples (1 mL) were withdrawn at definite times and were replaced by addition of 1 mL of the fresh release medium. Samples were analyzed in 96-well plate by the spectrophotometry measurement at A = 480 nm using Synergy H4 Hybrid Multi-Mode Microplate Reader (Biotek, Model: H4MLFPTAD). For each time point, the absorbance of 3 well (volume: 200 μL) was read, and the reported results are the mean of three determinations.
RESULTS AND DISCUSSIONS
Synthesis of polyethylene glycol-poly (lactic acid)
The five sets of PEG-PLA copolymers were successfully synthesized by ring opening polymerization method.
The 1HNMR spectrum of the copolymer-5 as an example is shown in Figure 1 which is representative for all synthesized copolymers. The signal at 3.8 ppm is attributed to the methyl group at the end of the PEG block in the copolymer chains. Its integral serves as an internal standard to calculate the number average chain length of the PEG. The signal at 5.4 ppm is related to the CH of lactide at PLA block and with its integral the number average chain length of the PLA can be estimated. The results of these analyzes for all copolymers are summarized in Table 1.
Figure 1.
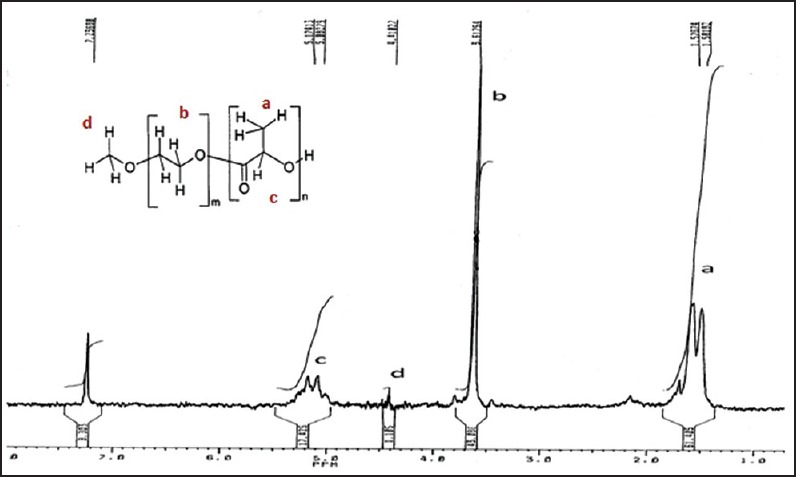
1H nuclear magnetic resonance of polyethylene glycol5000-PLA15000 (copolymer 5)
Table 1.
Copolymer characteristics determined by HNMR and GPC
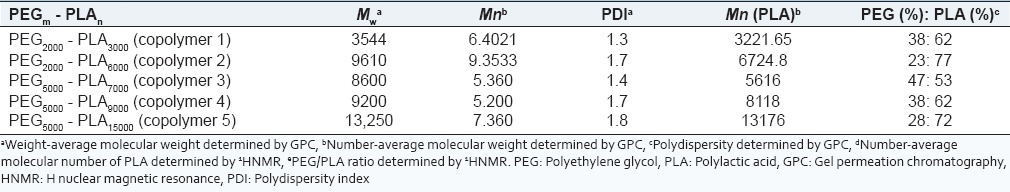
Furthermore, Table 1 shows the molecular weight data resulting from GPC analysis of the polymers after synthesis. The polydispersity of the copolymers indicate a narrow molecular weight distribution (<2) for all copolymers. These results suggest that microwave irradiation is a reliable method for the synthesis of PEG-PLA copolymers.
The DSC diagrams of copolymer 2 and 5 are demonstrated in Figure 2 which shows transition temperatures at about 40°C and 50°C for copolymers 2 and 5, respectively. The transition temperature of copolymers was found to be affected by copolymer molecular weight so that transition temperature shifted to higher temperatures with increasing molecular weight of the copolymer. According to the Flory-Fox theory [Equation 2], glass transition temperatures relates to the number average molecular weight and free volume in the polymer structure.[25]
Figure 2.
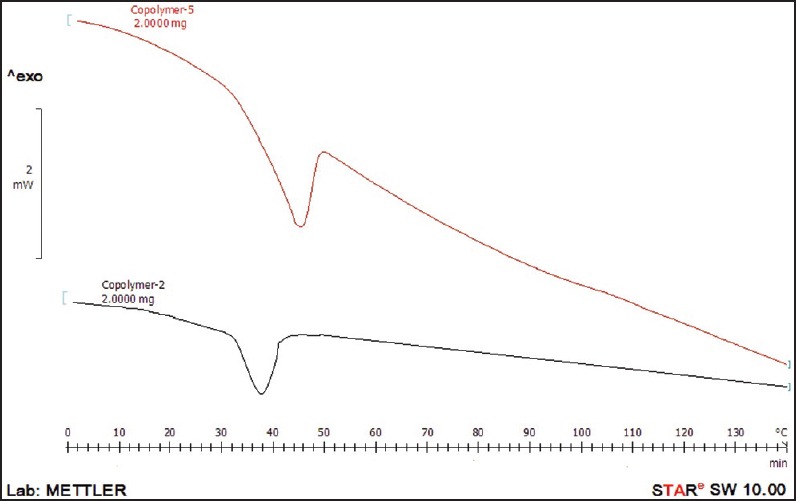
Differential scanning calorimetry thermogram of copolymer 5 and copolymer 2

In mentioned equation, Tg,∞ is the maximum glass transition temperature that can achieve at a theoretical infinite molecular weight and K is an empirical parameter that is related to the free volume present in the polymer sample.
In this regard, copolymer 5 with longer chain lengths (higher molecular weight) has fewer chain ends per total units and less free volume than copolymer 2 consisting of short chain.
In other words, chain ends can be viewed as an impurity when considering the packing of chains and more impurity results in a lower Tg.
Polyethylene glycol-poly (lactic acid) polymersomes
Self-assembly of the copolymers with various ratios of hydrophobic to hydrophilic block in the polymeric backbone was evaluated. PEG is completely soluble in an aqueous medium. In contrast to PEG, aliphatic polyesters produce solid particles and aggregate in an aqueous environment.
Increasing hydrophilicity of polyesters via the copolymerization with PEG forces these copolymers toward the formation of polymersomes or micelles structures.
Previously, the effect of different volume of blocks on linear diblock copolymers was verified and reported.[8,26,27]
It was shown previously that, in linear amphiphilic copolymers, the hydrophilic volume fraction (fEO) <25% creates aggregates and solid particles, meanwhile values between 25% and 40% gave polymersomes; fEO in the range of 40-50% resulted in hollow tubules, and micelles were formed when fEO was >50%.[27]
In current study, the influence of hydrophilic volume fraction of PEG-PLA copolymers in the range of 25-40% on the size and polydispersity of resultant polymersome was investigated.
The sizes and polydispersity of blank polymersomes formed from PEG-PLA copolymers via film rehydration method were measured by DLS [Table 2].
Table 2.
Particle size and size distributions of blank polymersomes created by film hydration method
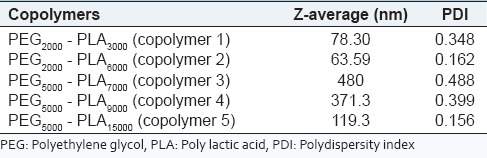
It should be mentioned that the film rehydration method used to prepare polymersomes were reproducible.
Obtained results showed that the smallest particle size with narrow size distribution was belonged to methoxy PEG (mPEG)2000-PLA6000 and mPEG5000-PLA15000 copolymers. The mPEG5000-PLA15000 and mPEG2000-PLA6000 were more efficient in developing nano-sized polymersomes.
As a result, hydrophilic volume fraction of ~25% of polymer backbone or PLA to PEG ratio of 3 was the best ratio of segments in diblock PEG-PLA copolymer for creation of nano-polymersome structures.
Transmission electron microscopy images of nano-polymersomes related to copolymer 2 and 5 demonstrate the spherical shape of self-assembled vesicles [Figure 3].
Figure 3.
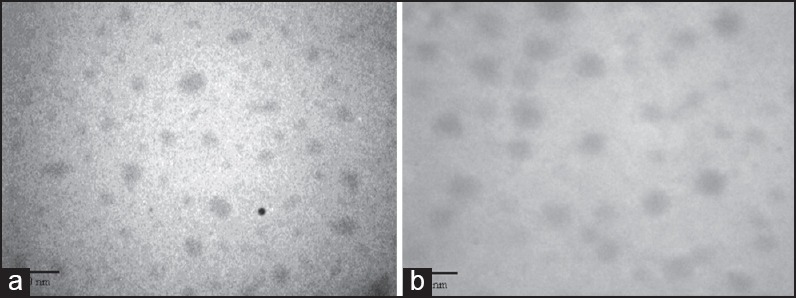
Transmission electron microscopy image of blank polymersomes of (a) copolymer 2, (b) copolymer 5. The images were recorded at magnifications of ×24,000
Doxorubicin loaded polymersomes
Characterization and release profiles
Doxorubicin loading was performed by pH gradient method. The EE of DOX was about 98.085% for copolymer 5 and 50.3% for copolymer 2 [Table 3].
Table 3.
Characteristics of DOX-loaded PEG-PLA polymersomes

The hydrodynamic radius of DOX loaded polymersome was determined to be about 115 nm for polymersomes of copolymer 5 (PolyDOX-5) and 67 nm for polymersomes of copolymer 2 (PolyDOX-2) by DLS with a narrow size distribution of 0.156 and 0.162 respectively [Figure 4].
Figure 4.
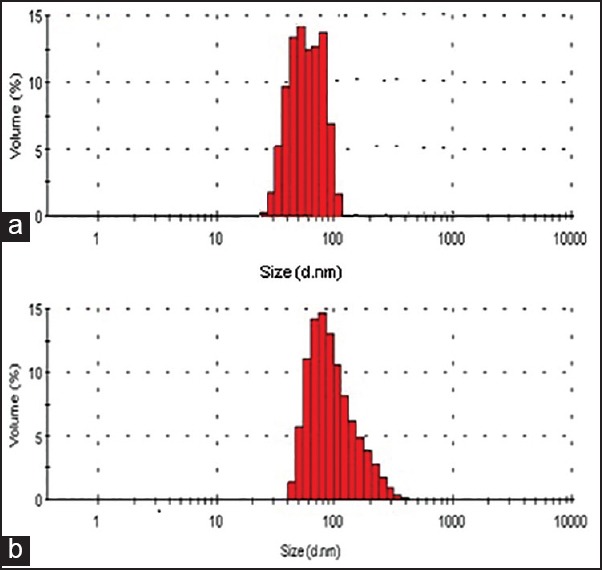
Particle size and size distributions of doxorubicin (DOX) loaded polymersomes of (a) polyethylene glycol (PEG)2000-poly (lactic acid) (PLA)6000 (PolyDOX-2) and (b) PEG5000-PLA15000 (PolyDOX-5)
Obtained results indicated that compared to PolyDOX-2, PolyDOX-5 encapsulated DOX more efficiently. This may be due to larger interior aqueous compartment for remote loading of DOX after creating of pH gradient.
In this regards, Yang et al. demonstrated that longer PLA chain length and higher molecular weight of polymers produced particles with larger diameter and this increased drug loading efficiencies.[28] In this study, PolyDOX-5 with larger particle size and higher molecular weight of the copolymer encapsulated higher concentration of DOX within polymersomes.
Figure 5a and b illustrates DOX release profiles in phosphate buffer (pH 7.4) within 5 days.
Figure 5.
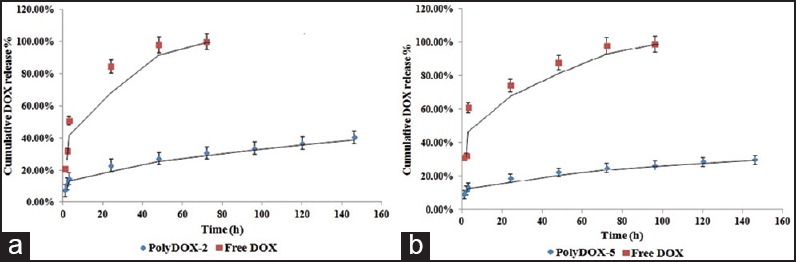
Doxorubicin (DOX) release profile of (a) PolyDOX-2 (DOX loaded polyethylene glycol [PEG]2000-poly [lactic acid] [PLA]6000 polymersomes) and (b) PolyDOX-5 (DOX loaded PEG5000-PLA15000 polymersomes) at pH = 7.4 and 37°C up to 5 days
Polymersome formulations of DOX (PolyDOX-2, PolyDOX-5) exhibited an initial burst release followed by a sustained, continuous release.
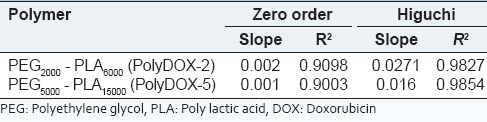
The release kinetics of DOX showed the best fit for Higuchi model for both formulations [Table 4].
Table 4.
The release kinetic of DOX from PEG-PLA polymersomes
Time for 18-24% DOX release was around 24 h for both formulations, and DOX gradually released during 5 days in a sustained and controlled manner. This type of release pattern for DOX could be advantageous in cancer chemotherapy. The initial burst release (18-24% over 24 h) provides a primary dose, and the remaining dose is released in a sustained manner.
It is important to gain a sustained release of therapeutic DOX concentration in clinical chemotherapy because continuous low-dose administration of DOX is more capable in inducing apoptosis than a single high-dose exposure of DOX.[29]
By the way, the behavior of the nanoparticles in in vivo might be different due to the presence of hydrolytic enzymes, which could contribute to the degradation of polymeric bilayer and results in faster drug release.
The in vitro release profile of the DOX from polymersomes was fitted to Higuchi model, and the results demonstrated that diffusion of drug through the vesicles was the main mechanism controlling the rate of drug release.
The higher burst release and release rate of DOX in PolyDOX-2 formulation (copolymer 2) is thought to be due to the smaller size of particles and higher permeability of vesicle membrane due to thin bilayer of polymersomes (copolymer 2).
It has been shown that polymer molecular weight can also affect polymer degradation and drug release rates so that an increase in molecular weight could decrease diffusivity and drug release rate.[30,31,32] It is well-known that the main release kinetics for many drugs is diffusion through water-filled pores in vesicles. In this system, polymer degradation produces soluble monomers or oligomers that can diffuse out of the particle leaving behind water filled pores. These small monomers or oligomers are generated more rapidly due to faster degradation of low molecular weight polymers.[33,34,35]
On the other hand, the release profile of PolyDOX-2 versus PolyDOX-5 formulation indicated that polymersome size will affect the rate of drug release. As the size decreased for PolyDOX-2, the surface area to volume ratio of the particle increased. Thus, for a given rate of drug diffusion through the polymersome, the rate of drug leakage or burst release will increase with decreasing particle size. In addition, water permeation into smaller particles may be faster due to the small particle diameter.
Moreover, because of lower Mw of PEG in PolyDOX-2 formulation, deformation of nano-polymersomes could occur more easily due to the small molecular chain and low flexibility of nano-structures.[7]
CONCLUSIONS
Altogether, the particle size of PEG-PLA polymersomes can be changed by choosing PEG chain length and PEG to PLA ratio using film rehydration technique as an excellent reproducible preparation method. Here we demonstrated that the fOE ~25% was the best hydrophilic volume fraction in diblock copolymers for formation of smallest nano-sized polymersome structures. In this regard, two different formulations with different molecular weight of copolymers were formed and evaluated for control release of DOX.
Polyethylene glycol-PLA copolymers produced nano-polymersomes with different sizes and different diffusion rates for DOX, resulting in differences in DOX release profile. By increasing PEG and PLA chain lengths and consequently polymer molecular weight in PolyDOX-5 formulation drug release rate and diffusivity decreased, and this system released DOX in sustained and controlled manner.
ACKNOWLEDGMENTS
The authors are grateful for the financial support provided by the Iran National Science Foundation (No. 9000719) and the Mashhad University of Medical Sciences (No. 901051, No. 87797).
Footnotes
Source of Support: Nil.
Conflicts of Interest: None declared.
REFERENCES
- 1.Piao LH, Deng MX, Chen XS, Jiang LS, Jing XB. Ring-opening polymerization of epsilon-caprolactone and L-lactide using organic amino calcium catalyst. Polymer. 2003;44:2331. [Google Scholar]
- 2.Sodergard A, Stolt M. Properties of lactic acid based polymers and their correlation with composition. Prog Polym Sci. 2002;27:1123–63. [Google Scholar]
- 3.Vainionpaa S, Rokkanen P, Tormala P. Surgical applications of biodegradable polymers in human tissue. Prog Polym Sci. 1989;14:679–716. [Google Scholar]
- 4.DellaErba R, Groeninckx G, Maglio G, Malinconico M, Migliozzi A. Immiscible polymer blends of semicrystalline biocompatible components: Thermal properties and phase morphology analysis of PLLA/PCL blends. Polymer. 2001;42:7831. [Google Scholar]
- 5.Rusa CC, Tonelli AE. Polymer/polymer inclusion compounds as a novel approach to obtaining a PLLA/PCL intimately compatible blend. Macromolecules. 2000;33:5321. [Google Scholar]
- 6.Zhong ZY, Zhang J, Gan ZH, Jing XB. A novel rare earth coordination catalyst for polymerization of biodegradable aliphatic lactones and lactides. Polym Int. 1998;45:60. [Google Scholar]
- 7.Xiao RZ, Zeng ZW, Zhou GL, Wang JJ, Li FZ, Wang AM. Recent advances in PEG-PLA block copolymer nanoparticles. Int J Nanomedicine. 2010;5:1057–65. doi: 10.2147/IJN.S14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Discher DE, Eisenberg A. Polymer vesicles. Science. 2002;297:967–73. doi: 10.1126/science.1074972. [DOI] [PubMed] [Google Scholar]
- 9.Levine DH, Ghoroghchian PP, Freudenberg J, Zhang G, Therien MJ, Greene MI, et al. Polymersomes: A new multi-functional tool for cancer diagnosis and therapy. Methods. 2008;46:25–32. doi: 10.1016/j.ymeth.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Upadhyay KK, Agrawal HG, Upadhyay C, Schatz C, Le Meins JF, Misra A, et al. Role of block copolymer nanoconstructs in cancer therapy. Crit Rev Ther Drug Carrier Syst. 2009;26:157–205. doi: 10.1615/critrevtherdrugcarriersyst.v26.i2.20. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed F, Pakunlu RI, Srinivas G, Brannan A, Bates F, Klein ML, et al. Shrinkage of a rapidly growing tumor by drug-loaded polymersomes: PH-triggered release through copolymer degradation. Mol Pharm. 2006;3:340–50. doi: 10.1021/mp050103u. [DOI] [PubMed] [Google Scholar]
- 12.Upadhyay KK, Le Meins JF, Misra A, Voisin P, Bouchaud V, Ibarboure E, et al. Biomimetic doxorubicin loaded polymersomes from hyaluronan-block-poly(gamma-benzyl glutamate) copolymers. Biomacromolecules. 2009;10:2802–8. doi: 10.1021/bm9006419. [DOI] [PubMed] [Google Scholar]
- 13.Kim Y, Tewari M, Pajerowski JD, Cai S, Sen S, Williams JH, et al. Polymersome delivery of siRNA and antisense oligonucleotides. J Control Release. 2009;134:132–40. doi: 10.1016/j.jconrel.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schatz C, Louguet S, Le Meins JF, Lecommandoux S. Polysaccharide-block-polypeptide copolymer vesicles: Towards synthetic viral capsids. Angew Chem Int Ed Engl. 2009;48:2572–5. doi: 10.1002/anie.200805895. [DOI] [PubMed] [Google Scholar]
- 15.Christian DA, Cai S, Bowen DM, Kim Y, Pajerowski JD, Discher DE. Polymersome carriers: From self-assembly to siRNA and protein therapeutics. Eur J Pharm Biopharm. 2009;71:463–74. doi: 10.1016/j.ejpb.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Discher BM, Bermudez H, Hammer DA, Discher DE. Cross-linked polymersome membranes: Vesicles with broadly adjustable properties. J Phys Chem B. 2002;106:2848–54. [Google Scholar]
- 17.Antonietti M, Forster S. Vesicles and liposomes: A self-assembly principle beyond lipids. Adv Mater. 2003;15:1323–33. [Google Scholar]
- 18.Hernandez JR, Checot F, Gnanou Y, Lecommandoux S. Toward ‘smart’ nano-objects by self-assembly of block copolymers in solution. Prog Polym Sci. 2005;30:691–724. [Google Scholar]
- 19.Hearnden V, Lomas H, Macneil S, Thornhill M, Murdoch C, Lewis A, et al. Diffusion studies of nanometer polymersomes across tissue engineered human oral mucosa. Pharm Res. 2009;26:1718–28. doi: 10.1007/s11095-009-9882-6. [DOI] [PubMed] [Google Scholar]
- 20.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J Control Release. 2000;65:271–84. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed F, Pakunlu RI, Brannan A, Bates F, Minko T, Discher DE. Biodegradable polymersomes loaded with both paclitaxel and doxorubicin permeate and shrink tumors, inducing apoptosis in proportion to accumulated drug. J Control Release. 2006;116:150–8. doi: 10.1016/j.jconrel.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Svenson S, Prudhomme RK, editors. Multifunctional Nanoparticles for Drug Delivery Applications: Imaging, Targeting, and Delivery, Nanostructure Science and Technology. New York: Springer Science + Business Media, LLC; 2012. pp. 9–29. [Google Scholar]
- 23.Jeong B, Choi YK, Bae YH, Zentner G, Kim SW. New biodegradable polymers for injectable drug delivery systems. J Control Release. 1999;62:109–14. doi: 10.1016/s0168-3659(99)00061-9. [DOI] [PubMed] [Google Scholar]
- 24.Bolotin EM, Grant GJ, Barenholz Y, Turndorf H, Piskoun B. Liposomal Bupivacaine compositions prepared using an ammonium sulfate gradient. U.S. Patent, No.6162462. [Google Scholar]
- 25.Fox TG, Flory PJ. Second-order transition temperatures and related properties of polystyrene. J Appl Phys. 1950;21:581–91. [Google Scholar]
- 26.Derakhshandeh K, Erfan M, Dadashzadeh S. Encapsulation of 9-nitrocamptothecin, a novel anticancer drug, in biodegradable nanoparticles: Factorial design, characterization and release kinetics. Eur J Pharm Biopharm. 2007;66:34–41. doi: 10.1016/j.ejpb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Discher BM, Won YY, Ege DS, Lee JC, Bates FS, Discher DE, et al. Polymersomes: Tough vesicles made from diblock copolymers. Science. 1999;284:1143–6. doi: 10.1126/science.284.5417.1143. [DOI] [PubMed] [Google Scholar]
- 28.Yang ZL, Li XR, Yang KW, Liu Y. Amphotericin B-loaded poly(ethylene glycol)-poly(lactide) micelles: Preparation, freeze-drying, and in vitro release. J Biomed Mater Res A. 2008;85:539–46. doi: 10.1002/jbm.a.31504. [DOI] [PubMed] [Google Scholar]
- 29.Mattsson W, Borgström S, Landberg T. An effective low-dose adriamycin regimen as secondary chemotherapy for metastatic breast cancer patients. Cancer. 1980;46:433–7. doi: 10.1002/1097-0142(19800801)46:3<433::aid-cncr2820460302>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed F, Discher DE. Self-porating polymersomes of PEG-PLA and PEG-PCL: Hydrolysis-triggered controlled release vesicles. J Control Release. 2004;96:37–53. doi: 10.1016/j.jconrel.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 31.Alonso MJ, Gupta RK, Min C, Siber GR, Langer R. Biodegradable microspheres as controlled-release tetanus toxoid delivery systems. Vaccine. 1994;12:299–306. doi: 10.1016/0264-410x(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 32.Le Corre P, Le Guevello P, Gajan V, Chevanne F, Le Verge R. Preparation and characterization of bupivacaine-loaded polylactide and poly(lactide-glycolide) microspheres. Int J Pharm. 1994;107:41–9. [Google Scholar]
- 33.Liggins RT, Burt HM. Paclitaxel loaded poly(L-lactic acid) microspheres: Properties of microspheres made with low molecular weight polymers. Int J Pharm. 2001;222:19–33. doi: 10.1016/s0378-5173(01)00690-1. [DOI] [PubMed] [Google Scholar]
- 34.Blanco D, Alonso MJ. Protein encapsulation and release from poly(lactide-co-glycolide) microspheres: Effect of the protein and polymer properties and of the co-encapsulation of surfactants. Eur J Pharm Biopharm. 1998;45:285–94. doi: 10.1016/s0939-6411(98)00011-3. [DOI] [PubMed] [Google Scholar]
- 35.Mehta RC, Thanoo BC, Deluca PP. Peptide containing microspheres from low molecular weight and hydrophilic poly(D,L-lactide-co-glycolide) J Control Release. 1996;41:249–57. [Google Scholar]


