In Brief
This article provides an overview of the development of insulins, oral agents, and noninsulin injectable agents used in the management of hyperglycemia in patients with diabetes. It also briefly reviews the pharmacological impact and salient side effects of these medications.
The management of diabetes has changed dramatically during the past several thousand years. The option preferred by “experts” of the pharaoh of Egypt 3,500 years ago was a mixture of “water from the bird pond,” elderberry, fibers from the asit plant, milk, beer, cucumber flower, and green dates.1 Although our therapeutic options today are significantly more effective, they will likely be considered arcane by our successors 100 years from now if the current trajectory in treatment development continues. Clearly, however, the current pharmacological armamentarium used to manage diabetes has resulted in a dramatic reduction in morbidity and mortality. This article provides a brief overview of the development history and effectiveness of various agents used in the pharmacological management of diabetes.
Insulin
Before the 1920s, there were no effective pharmacological agents for the management of diabetes. Because of this, type 1 diabetes was a fatal malady. This changed dramatically with Frederick Banting’s work.
Dr. Banting served as a surgeon in World War I. Captain Banting initially spent some time in hospitals in England, but later was sent to the front as a battalion medical officer, where he was wounded by shrapnel. He received a Military Cross for his courage in action.2 After returning from the war, Dr. Banting opened an office outside of Toronto, Canada. After seeing only one patient in the first month of his practice (a patient seeking a prescription for ethanol), Banting embarked upon a career in academics.
One of his first teaching assignments involved carbohydrate metabolism. This led to his interest in diabetes and his erroneous assumption that one needed to surgically ligate the pancreatic duct and then wait 6–8 weeks before extracting anything that might be useful from the endocrine portion of the gland. Over time, and without the ligation step, he was able to extract a substance from canine pancreas glands that had an impact on hyperglycemia in other diabetic animals.
Banting and his student, Charles Best, continued working on various extraction processes. By December 1921, they were using a process that combined equal parts of ground-up beef pancreas and slightly acidic alcohol. The solution was filtered, washed twice with toluene, and filter sterilized. This test solution was given to dogs to determine potency.
Leonard Thompson was the first patient to receive insulin. He was a 14-year-old boy who weighed 65 lb, was pale, smelled of acetone, was losing hair, had a distended abdomen, and was later described as looking like the victim of a concentration camp. On 11 January 1922, a young house officer, Ed Jeffery, injected 7.5 cc of Banting and Best’s extract (described as a thick brown muck) into each buttock of the patient. A sterile abscess developed at the site of one of the injections, but the patient’s blood glucose dropped.
After that injection, the push to perfect the extraction process and commercialize insulin was on. Banting’s team entered into an agreement with Eli Lilly and Company, and, by July 1922, the first bottles of Lilly’s Iletin (insulin) arrived in Banting’s office. Insulin was commercially available in the United States by 1923.
The next major advancement in insulin was its crystallization in 1926.3 The technique of insulin crystallization led to improved soluble (regular) insulin purity and also opened the door to insulin formulation modifications with different time-action profiles. There was a great need for an extended-action insulin. With the availability of only rapid-acting insulin, patients required multiple daily injections and had to be awakened at night for injections. Children not awaked for nighttime injections were at risk for a significant reduction in growth, or diabetic dwarfism syndrome. Children with diabetic dwarfism syndrome, which was also known as Mauriac’s syndrome, suffered from stunted growth, hepatomegaly, and delayed puberty.4 In 1936, the first commercially available, extended-action insulin, PZI (protamine zinc insulin), was released. This formulation was composed of an amorphous combination of protamine, zinc, and insulin. PZI continues to be used today in the management of cats with diabetes.3
The next major development in insulin formulation came in 1946, when the Nordisk Insulin Laboratory in Denmark released the second extended-action insulin, NPH (neutral protamine Hagedorn).3 This insulin contained ∼ 10% of the protamine found in PZI along with zinc insulin crystals. This insulin was shorter acting than PZI and could be combined with regular insulin.
In 1956, the lente series of insulin was introduced: ultralente, lente, and semilente. These formulations were synthesized by altering the content of the excess zinc. Ultralente is a microcrystalline formulation that is long acting. Semilente is more amorphous than ultralente and has a time-action profile that is slightly slower in onset than regular insulin. Lente is composed of a 70:30 mixture of ultralente and semilente and is intermediate acting.
All insulin preparations available before 1983 were derived from animal sources (primarily beef and pork). This changed in 1983, when the first recombinant medication, human insulin, was approved.5 One of the primary problems at the time of the release of human insulin was the pharmacokinetic/pharmacodynamic profiles of the available insulins. The search for a “flat” basal insulin and a rapid-acting insulin that more closely approximated physiological insulin secretory patterns accelerated after the release of human insulin.
In 1996, the first rapid-acting human insulin analog, lispro, was approved.5 This was followed in the past 15 years with a succession of additional insulin analogs, including the rapid-acting insulins aspart and glulisine and the long-acting basal analogs glargine and detemir. The U.S. Food and Drug Administration (FDA) declined to approve degludec, an ultra-long-acting insulin (duration of 42 hours), in 2013. However this compound is available in Europe and will probably be resubmitted for approval in the United States.6
In addition to the formulation changes described above, myriad advancements in the area of insulin delivery devices and routes of administration have been made or are being worked on, including better syringes, pulmonary insulin, insulin pumps, and closed-loop insulin delivery systems. Insulin is widely used today in patients with type 1 or type 2 diabetes and is arguably the most effective and predictable (in most, but not all cases) of all of the current antihyperglycemic agents.
Biguanides
French lilac, or goat’s rue (Galega officinalis), was used as a folk remedy for diabetes in Southern and Eastern Europe during medieval times.7 In the early 20th century, the antihyperglycemic moiety in this plant, guanidine, was isolated. Frank et al.8 synthesized a guanidine compound called Synthalin in Germany and used it to treat diabetes during the 1920s.3 Homologs of guanidine (e.g., Synthalin) were used for a short period but were hepatotoxic, and the use of these compounds all but ended with the discovery and proliferation of insulin. However, in later years, there was a resurgence in interest in the biguanides. In the 1960s and 1970s, phenformin was widely studied in the United States, while metformin was studied in France and buformin was studied in Germany.9 Although phenformin and buformin were used clinically, their relationship to lactic acidosis led to their withdrawal from the market in most countries.
Metformin was introduced in 1959 as an antihyperglycemic agent but was not approved in the United States until the 1990s. Today, metformin is the only clinically significant biguanide and is the most widely used antihyperglycemic agent in the world. Its primary mechanism of action is its ability to reduce hepatic glucose production, but it also reduces glucose via a mild increase in insulin-stimulated glucose uptake.7 This medication is generally well tolerated and is typically associated with a significant reduction in A1C levels (∼ 1.5%).7
Sulfonylureas
The history of the sulfonylureas (SUs) began in 1937 with the observation of the hypoglycemic activity of synthetic sulfur compounds.10 Five years later, Marcel Janbon and his colleagues were treating patients with the antibiotic para-amino-sulfonamide-isopropyl-thiodiazole for typhoid and observed hypoglycemia.11 In 1946, Auguste Loubatieres confirmed that aryl SU compounds stimulated release of insulin and therefore required some pancreatic β-cell function to elicit an effect.3,10
In the 1950s, the first SU, tolbutamide, was marketed in Germany.11 This was followed by the introduction of the other first-generation agents: chlorpropamide, acetohexamide, and tolazamide. The next advancement in SU therapy in the United States did not occur until the release of the more potent second-generation agents glipizide and glyburide in 1984. These agents had been in use in Europe for several years before this.11 The next SU agent, glimepiride, which is sometimes referred to as a third-generation agent, was released in 1995.
SUs are widely used, generally safe, inexpensive, and relatively predictable. Their primary use-limiting side effect is hypoglycemia, although they are also associated with weight gain. Generally, an A1C reduction of 1–2% can be expected in a responsive patient with type 2 diabetes.7
Thiazolidinediones
Thiazolidinediones (TZDs), which are also known simply as “glitazones,” were initially introduced to the U.S. market in 1996. These agents are peroxisome proliferator–activated receptor-γ activators whose mechanisms of action are enhancement of skeletal muscle insulin sensitivity and reduction in hepatic glucose production.12 These agents do not increase the risk of hypoglycemia and have a more durable effect than metformin or SUs.12
Troglitazone was the first agent in this category to be approved by the FDA.13 As troglitazone use increased, idiosyncratic hepatic failure began to be reported. By March 2000, the FDA had received reports of 63 hepatic failure cases resulting in death in patients treated with troglitazone, and shortly thereafter, the drug was removed from the market.14
Two other TZDs, pioglitazone and rosiglitazone, which are currently on the market, have each been linked to nonhypoglycemic issues. Both agents have been linked to fluid retention and must be used with caution in patients with congestive heart failure. Pioglitazone has been shown to potentially have a modest beneficial impact on cardiovascular disease but has also been associated with a possible increase in the incidence of bladder cancer.12 Until recently, rosiglitazone was not widely available because of concerns that it was associated with an increased risk of myocardial infarction (MI). The FDA, which had previously placed restrictions on rosiglitazone, began to ease those restrictions in November 2013. Their change in position was based on the findings of the large Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycemia in Diabetes (RECORD) study, which concluded that people treated with rosiglitazone did not have an elevated risk of MI compared to patients taking other antihyperglycemic medications.15
TZDs are typically associated with an A1C decrease of 0.5–1% in most patients.7 There are no significant differences in A1C lowering between pioglitazone and rosiglitazone.
α-Glucosidase Inhibitors
α-Glucosidase inhibitors (AGIs) exert a local effect on the brush border of the small intestine, inhibiting α-glucosidase enzymes, which are responsible for the breakdown of oligosaccharides, trisaccharides, and disaccharides. These enzymes include maltase, isomaltase, gluocoamylase, and sucrase. Inhibition of these enzyme systems effectively reduces the rate of absorption of carbohydrates but does not alter the absolute absorption. The result is reduced postprandial glucose levels, with a modest effect on fasting glucose.7 The reduction of A1C observed with AGIs is typically 0.5–1.0%.
The first drug in this category to reach the market was acarbose, which was approved by the FDA in 1995. A second AGI, miglitol, was approved in 1996. These drugs are available but not widely used, probably because of their modest impact on A1C, their need for multiple daily doses, and their gastrointestinal (GI) side effects.7,12
Meglitinides
The meglitinides (also called “glinides”) have a mechanism of action similar to that of the SUs but are structurally unrelated to SUs. This class of medication lowers blood glucose levels by stimulating insulin release from the pancreas.7 As with the SUs, glinide-induced insulin stimulation is dependent on functioning pancreatic β-cells. However, the effect of these drugs is glucose dependent and diminishes at low glucose concentrations. The glinides bind to receptors in the pancreas, but the configuration of their binding is different from that observed with SUs. The glinides have a more rapid onset and a shorter duration of action than the second-generation SUs, which necessitates multiple daily dosing.
Glinides can cause hypoglycemia, but they do so at a rate lower than that observed with the SUs. A1C reduction from glinides is generally between 1 and 1.5%.7,11 The first agent in this class, repaglinide, was approved by the FDA in 1997, and a second agent, nateglinide, was approved in 2000.11
Glucagon-Like Peptide-1 Receptor Agonists
The idea of an “incretin effect” was long known and based on experimental data demonstrating a greater insulin response with oral glucose administration versus intravenous glucose administration. The generalities of the incretin-insulin pathway were worked out by the 1980s. Two key studies evaluated the impact of native glucagon-like peptide-1 (GLP-1) in normal subjects and in patients with type 2 diabetes.16,17 Both of these trials demonstrated a significant increase in insulin response and in the reversal of hyperglycemia in patients with type 2 diabetes who were hyperglycemic and received native GLP-1.
GLP-1 and its analogs reduce glucose levels via a glucose-linked enhancement of insulin secretion. The short half-life (1–2 minutes) of native GLP-1 (because of its rapid degradation by the enzyme dipeptidyl peptidase-4 [DPP-4; discussed below]) led to the search for GLP-1 analogs and DPP-4 inhibitors. One analog, exendin-4, was isolated from the salivary gland venom of the Gila monster (Heloderma suspectum).7
Exenatide, a synthetically produced form of exendin-4, was the first GLP-1 receptor agonist to become available for clinical use in 2005.18 A second GLP-1 receptor agonist, liraglutide, was approved in 2010. In 2012, a long-acting (once-weekly) form of exenatide was approved. A new drug application (NDA) for dulaglutide, another once-weekly GLP-1 agonist, was submitted to the FDA in October 2013.19
Other agents in this class are currently under development, including lixisenatide and albiglutide. The NDA for lixisenatide was submitted to the FDA but later rescinded in 2013. It is expected that the NDA for lixisenatide will be resubmitted in 2015.20
GLP-1 receptor agonists, which are all administered subcutaneously, are generally associated with 0.5–1% reductions in A1C levels.7,11 Weight loss is one advantage of treatment with incretin-based agents. However, these compounds can cause significant GI side effects, particularly early in therapy, and concerns about associations between GLP-1 receptor agonists and pancreatitis are ongoing.
DPP-4 Inhibitors
As noted above, with the elucidation of the incretin-insulin pathway, researchers became interested in the development of DPP-4 inhibitors, agents that could be taken orally and would prolong the circulating half-life of endogenous incretins. The first of these agents to become available in the United States was sitagliptin, which was approved in 2006.21 This was followed by the release of saxagliptin and linagliptin. Alogliptin was approved by the FDA in 2013. Vildagliptin has been approved for use in Europe but is not available in the United States.
These compounds are associated with an A1C reduction of ∼ 0.8%.10 They are weight neutral and do not tend to cause hypoglycemia.7 However, pancreatitis has been reported in patients treated with DPP-4 inhibitors.11
Amylin Agonists
The endogenous neuroendocrine hormone amylin was discovered in 1987. Amylin is co-secreted with insulin by the β-cells in equimolar amounts.7 Patients with type 2 diabetes have reduced amounts of amylin, whereas patients with type 1 diabetes have essentially no amylin.11 The only amylin analog currently on the market is pramlintide, which was approved by the FDA in 2005. Its physiological effect includes weight loss, delayed gastric emptying, and a reduction in both postprandial glucose and glucagon. The primary side effect is nausea.
Pramlintide has a modest effect on A1C reduction of ∼ 0.5%. This compound is usually reserved for use in patients with type 1 diabetes treated with intensive insulin therapy.11 It reduces postprandial glucose excursions via the mechanisms mentioned above.
Bromocriptine
Bromocriptine is a dopamine agonist that was approved for use in the United States as an antihyperglycemic medication in 2009.12 Its mechanism is not certain but may be related to its dopaminergic activity in the brain and the subsequent inhibition of sympathetic tone.11 Its impact on glycemia is modest, with A1C reductions of up to 0.7%.11
Colesevelam
Colesevelam is an interesting compound that has a dual effect of lowering LDL cholesterol and reducing blood glucose levels. This drug was specifically developed for its ability to bind bile acids, effectively removing them from circulation and resulting in reductions in LDL cholesterol. The mechanism of action of the glucose lowering observed with this compound is not known. The drug was approved by the FDA for use in patients with type 2 diabetes in 2008.11
Colesevelam is typically associated with an A1C reduction of ∼ 0.5% and LDL cholesterol reduction of 13%.7 Its side effects are similar to those encountered with AGIs and are primarily gastrointestinal. Also, it should be noted that colesevelam may cause a slight increase in triglycerides.
Sodium Glucose Co-Transporter 2 Inhibitors
The sodium glucose co-transporter 2 (SGLT-2) inhibitors are a novel group of compounds that antagonize a high-capacity, low-affinity glucose transporter found primarily in the kidney.22 This transporter is responsible for ∼ 90% of glucose reabsorption in the kidney. When this transporter is antagonized, excess glucose in the renal tubules is not reabsorbed, and glucose is excreted in the urine. This results in a net loss of glucose and a reduction in hyperglycemia.
A recent meta-analysis of placebo-controlled studies evaluating SGLT-2 inhibitors reported A1C reductions of 0.5–0.6% in patients treated with these agents.23 In addition to reducing hyperglycemia, SGLT-2 inhibitors have also been associated with slight reductions in weight and BMI.
The primary side effect of SGLT-2 inhibition is an increase in urinary or genital infections. These infections are much more common than in placebo-treated patients (about four times as many) but are usually mild.23 Canagliflozin was the first SGLT-2 inhibitor to be approved by the FDA, in March 2013.24 Dapagliflozin was approved in the United States in early 2014. Empagliflozin and other SGLT-2 inhibitors are under development.
Conclusion
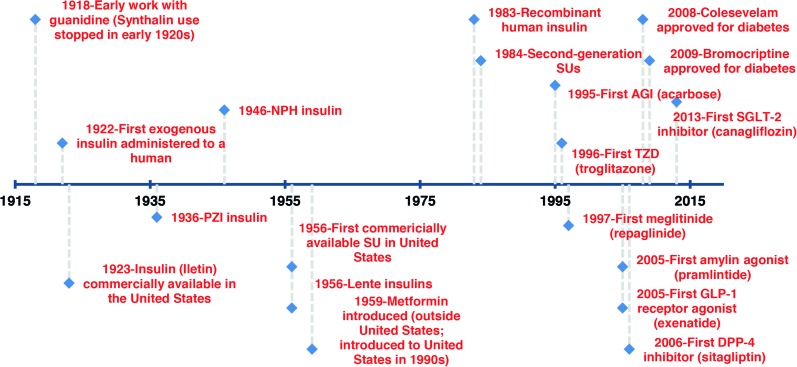
There are now 11 different categories of medications directed at the management of hyperglycemia in patients with diabetes. These compounds have been developed during the past 90 years (Figure 1), and among these categories, myriad subtypes exist. Additionally, the potential permutations of various combinations of these agents is staggering and can be bewildering to the clinicians trying to design the optimum therapy regimen for a given patient.
Figure 1.
History of diabetes medications.
We continue to struggle, as we should, with questions of efficacy and the potential detrimental effects of antihyperglycemic medications. At the same time, we should be mindful of the fact that the outlook for patients with diabetes today is much better than what they would have encountered in the 1920s or even in the 1970s. A recent poster presented at the European Association for the Study of Diabetes 2013 meeting reported that the life expectancy of people with type 1 diabetes (aged 20–24 years) is about 11–14 years less than that of individuals without diabetes.25 This is in stark contrast to data presented in 1975 that reported a 27-year difference in life expectancy between patients with type 1 diabetes and those without diabetes.26 Clearly, we are headed in the right direction, with the goal of having no gap between the two populations in terms of life expectancy or even the outright prevention of all types of diabetes. In the interim, advances in pharmacotherapy have made, and future advances will continue to make, a positive difference in the lives of our patients.
References
- 1.Sanders LJ: From Thebes to Toronto and the 21st century: an incredible journey. Diabetes Spectrum 15:56–60, 2002 [Google Scholar]
- 2.Bliss M: The Discovery of Insulin. Chicago, University of Chicago Press, 1982 [Google Scholar]
- 3.Galloway JA: Diabetes Mellitus. 9th ed Indianapolis, Ind., Eli Lilly and Company, 1988 [Google Scholar]
- 4.Daneman D, Drash AL, Lobes LA, Becker DJ, Baker LM, Travis LB: Progressive retinopathy with improved control in diabetic dwarfism (Mauriac’s syndrome). Diabetes Care 4:360–365, 1981 [DOI] [PubMed] [Google Scholar]
- 5.Defeat Diabetes, Foundation : History of diabetes in timeline. Available from http://www.defeatdiabetes.org/about_diabetes/text.asp?id=Diabetes_Timeline. Accessed 4 December 2013
- 6.Medscape : FDA rejects Novo Nordisk’s insulin degludec [article online]. 11 February 2013. Available from http://www.medscape.com/viewarticle/779077. Accessed 2 January 2014
- 7.White JR: Overview of the medications used to treat type 2 diabetes. In Medications for the Treatment of Diabetes. White JR, Campbell RK, Eds. Alexandria. Va., American Diabetes Association, 2008, p. 5–15 [Google Scholar]
- 8.Frank E, Nothnamm M, Wagner A: Über synthetische dargestellte Korper mit Insulinartiger Wirkung auf den normallen und diabetisched Organismus. Klin Wchnschr 5:2011, 1926 [Google Scholar]
- 9.Alberti KGMM, Zimmet P, Defronzo RA: International Textbook of Diabetes Mellitus. 2nd ed. New York, John Wiley & Sons, 1997 [Google Scholar]
- 10.Levine R: Sulfonylureas: background and development of the field. Diabetes Care 7 (Suppl. 1):3–7, 1984 [PubMed] [Google Scholar]
- 11.Quianzon CCL, Cheikh I: History of current non-insulin medications for diabetes mellitus. J Community Hosp Intern Med Perspect. Published online 15 October 2012. (doi: 10.3402/jchimp.v2i3.19081) [DOI] [PMC free article] [PubMed]
- 12.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Farrannini E, Nauck M, Peters A, Tsapas A, Wender R, Mathhews DR: Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 35:1364–1379, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kendall DM: Thiazolidinediones: the case for early use. Diabetes Care 29:154–157, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Lumpkin MM: Troglitazone: presentation to advisory committee. Published 19 May 2000. Available from www.fda.gov/ohrms/dockets/ac/00/slides/3615s1a.PPT. Accessed 4 December 2013
- 15.Nature World News : FDA eases restrictions on Glaxo diabetes drug Avandia. Published 26 November 2013. Available from http://www.natureworldnews.com/articles/5075/20131126/fda-eases-restrictions-glaxos-drug.htm. Accessed 4 December 2013
- 16.Gutniak M, Orskov C, Holst JJ, Ahrén B, Efendic S: Antidiabetogenic effect of glucagon-like peptide-1 (7-37) in diabetic and non-diabetic patients with diabetes mellitus. N Engl J Med 326:1316–1322, 1992 [DOI] [PubMed] [Google Scholar]
- 17.Nathan DM, Schreiber E, Fogel H, Mojsov S, Habener JF: Insulinotropic action of glucagon-like peptide-1 (7-37) in diabetic and non-diabetic patients with diabetes mellitus. Diabetes Care 15:270–276, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Aroda VR, Ratner R: The safety and tolerability of GLP-1 receptor agonist in the treatment of type 2 diabetes: a review. Diabetes Metab Res Rev 27:528–542, 2011 [DOI] [PubMed] [Google Scholar]
- 19. diatribe: Eli Lilly submits GLP-1 dulaglutide to FDA and unveils injection device [article online]. Available from http://diatribe.us/issues/59/new-now-next/5. Accessed 4 December 2013.
- 20.Stuart C: Sanofi pull lixisenatide’s FDA application [article online]. Cardiovascular Business. Available from http://www.cardiovascularbusiness.com/topics/prevention/sanofi-pulls-lixisenatide%E2%80%99s-fda-application. Accessed 4 December 2013 [Google Scholar]
- 21.Neumiller JJ: Differential chemistry (structure), mechanism of action, and pharmacology of GLP-1 receptor agonist and DPP-4 inhibitors. J Am Pharm Assoc 49 (Suppl. 1):S14–S29, 2009 [DOI] [PubMed] [Google Scholar]
- 22.White J: Apple trees to sodium glucose co-transporter inhibitors: a review of SGLT-2 inhibition. Clinical Diabetes 28:5–10, 2010 [Google Scholar]
- 23.Monami M, Nardini C, Mannucci E: Efficacy and safety of sodium glucose co-transport-2 inhibitors in type 2 diabetes: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. Electronically published ahead of print on 29 December 2013 (doi: 0.1111/dom.12244) [DOI] [PubMed]
- 24. Forbes.com: FDA approves first SGLT2 inhibitor for diabetes [article online]. 29 March 2013. Available from http://www.forbes.com/sites/larryhusten/2013/03/29/fda-approves-first-sglt2-inhibitor-for-diabetes. Accessed 3 January 2014.
- 25.Nainggolan L: Life expectancy greatly improved in type 1 diabetes [article online]. 25 September 2013. Available from http://www.medscape.com/viewarticle/811610. Accessed 4 December 2013
- 26.Goodkin G: Mortality factors in diabetes: a 20 year mortality study [Abstract]. J Occup Med 17:716–721, 1975 [PubMed] [Google Scholar]