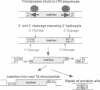
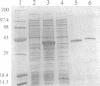
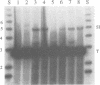
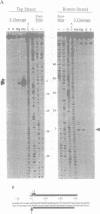
Abstract
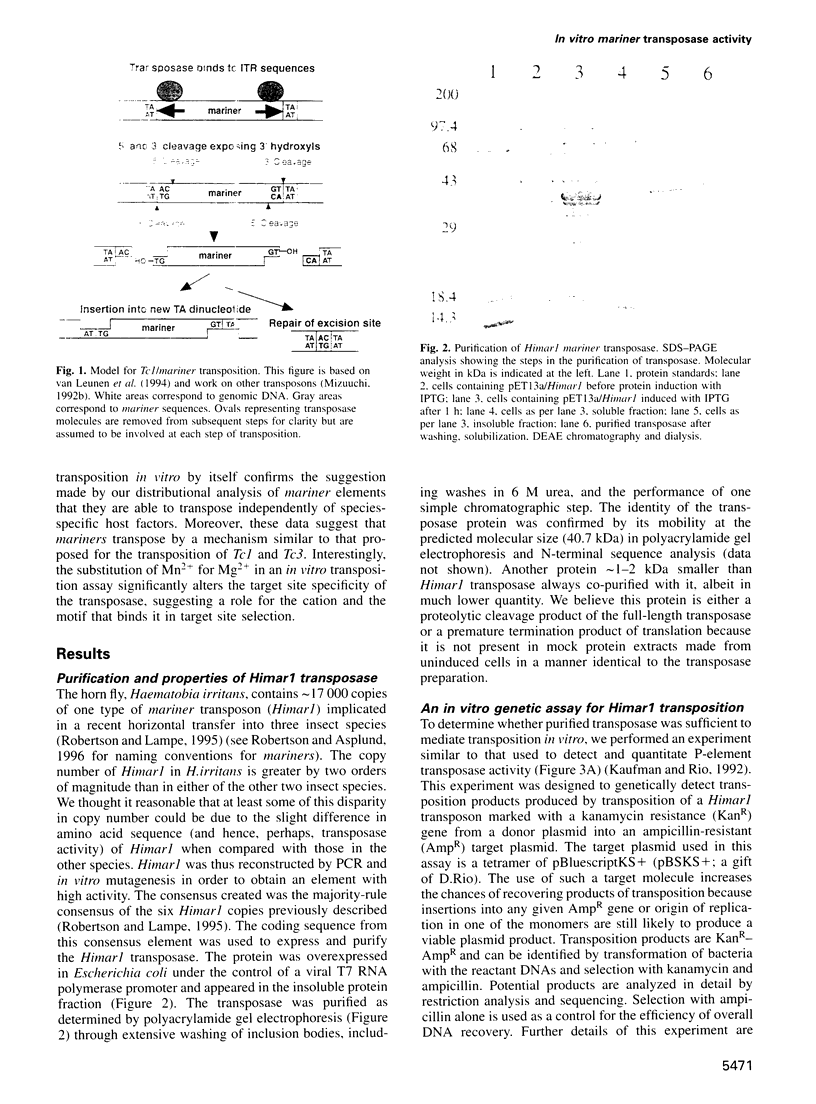
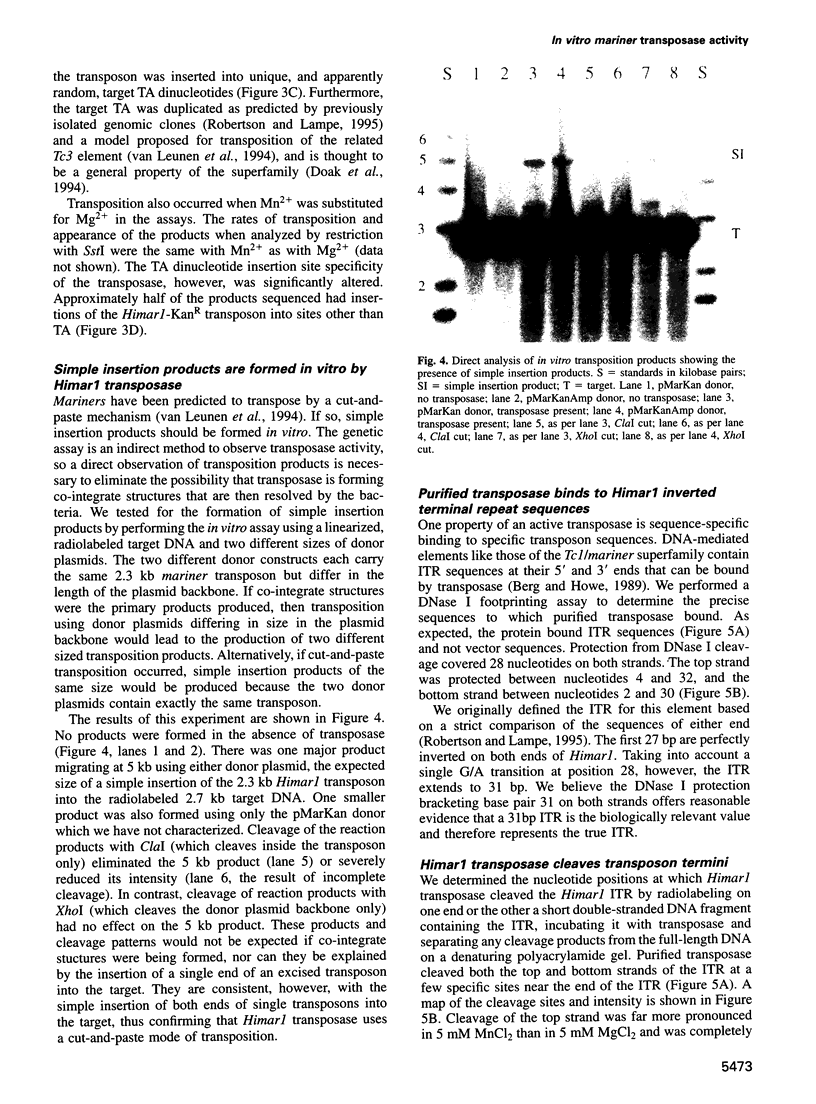
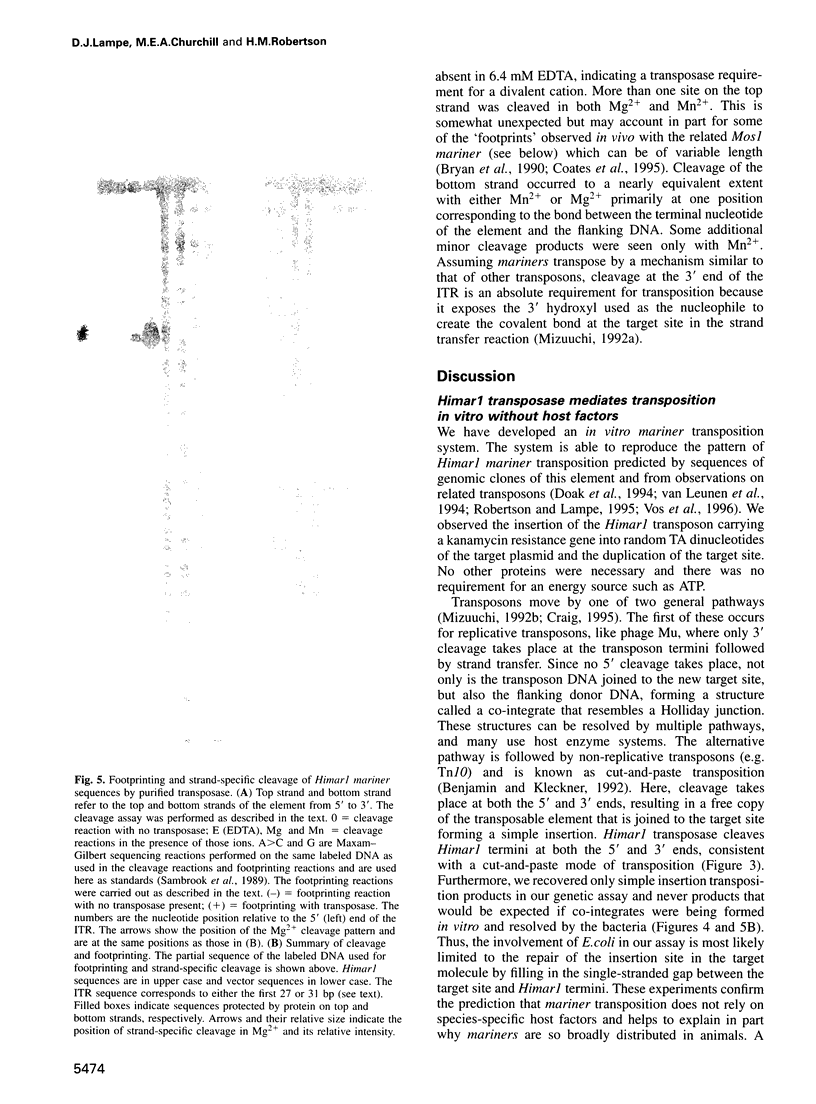
Mariners are a widespread and diverse family of animal transposons. Extremely similar mariners of the irritans subfamily are present in the genomes of three divergent insect host species, which strongly suggests that species-specific host factors are unnecessary for mobility. We tested this hypothesis by examining the activity of a purified transposase from one of these elements (Himar1) present in the horn fly, Haematobia irritans. Himar1 transposase was sufficient to reproduce transposition faithfully in an in vitro inter-plasmid transposition reaction. Further analyses showed that Himar1 transposase binds to the inverted terminal repeat sequences of its cognate transposon and mediates 5' and 3' cleavage of the element termini. Independence of species-specific host factors helps to explain why mariners have such a broad distribution and why they are capable of horizontal transfer between species.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker T. A., Luo L. Identification of residues in the Mu transposase essential for catalysis. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6654–6658. doi: 10.1073/pnas.91.14.6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T. A., Mizuuchi M., Mizuuchi K. MuB protein allosterically activates strand transfer by the transposase of phage Mu. Cell. 1991 Jun 14;65(6):1003–1013. doi: 10.1016/0092-8674(91)90552-a. [DOI] [PubMed] [Google Scholar]
- Beall E. L., Admon A., Rio D. C. A Drosophila protein homologous to the human p70 Ku autoimmune antigen interacts with the P transposable element inverted repeats. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12681–12685. doi: 10.1073/pnas.91.26.12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin H. W., Kleckner N. Excision of Tn10 from the donor site during transposition occurs by flush double-strand cleavages at the transposon termini. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4648–4652. doi: 10.1073/pnas.89.10.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchetot A., Gooding R. H. Identification of a mariner element from the tsetse fly, Glossina palpalis palpalis. Insect Mol Biol. 1995 May;4(2):89–96. doi: 10.1111/j.1365-2583.1995.tb00012.x. [DOI] [PubMed] [Google Scholar]
- Bryan G., Garza D., Hartl D. Insertion and excision of the transposable element mariner in Drosophila. Genetics. 1990 May;125(1):103–114. doi: 10.1093/genetics/125.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates C. J., Turney C. L., Frommer M., O'Brochta D. A., Warren W. D., Atkinson P. W. The transposable element mariner can excise in non-drosophilid insects. Mol Gen Genet. 1995 Nov 15;249(2):246–252. doi: 10.1007/BF00290372. [DOI] [PubMed] [Google Scholar]
- Colloms S. D., van Luenen H. G., Plasterk R. H. DNA binding activities of the Caenorhabditis elegans Tc3 transposase. Nucleic Acids Res. 1994 Dec 25;22(25):5548–5554. doi: 10.1093/nar/22.25.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig N. L. Unity in transposition reactions. Science. 1995 Oct 13;270(5234):253–254. doi: 10.1126/science.270.5234.253. [DOI] [PubMed] [Google Scholar]
- Deng W. P., Nickoloff J. A. Site-directed mutagenesis of virtually any plasmid by eliminating a unique site. Anal Biochem. 1992 Jan;200(1):81–88. doi: 10.1016/0003-2697(92)90280-k. [DOI] [PubMed] [Google Scholar]
- Doak T. G., Doerder F. P., Jahn C. L., Herrick G. A proposed superfamily of transposase genes: transposon-like elements in ciliated protozoa and a common "D35E" motif. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):942–946. doi: 10.1073/pnas.91.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide D., Anderson P. Insertion and excision of Caenorhabditis elegans transposable element Tc1. Mol Cell Biol. 1988 Feb;8(2):737–746. doi: 10.1128/mcb.8.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Fernàndez J., Bayascas-Ramírez J. R., Marfany G., Muñoz-Mármol A. M., Casali A., Baguñ J., Saló E. High copy number of highly similar mariner-like transposons in planarian (Platyhelminthe): evidence for a trans-phyla horizontal transfer. Mol Biol Evol. 1995 May;12(3):421–431. doi: 10.1093/oxfordjournals.molbev.a040217. [DOI] [PubMed] [Google Scholar]
- Grindley N. D., Leschziner A. E. DNA transposition: from a black box to a color monitor. Cell. 1995 Dec 29;83(7):1063–1066. doi: 10.1016/0092-8674(95)90132-9. [DOI] [PubMed] [Google Scholar]
- Holton T. A., Graham M. W. A simple and efficient method for direct cloning of PCR products using ddT-tailed vectors. Nucleic Acids Res. 1991 Mar 11;19(5):1156–1156. doi: 10.1093/nar/19.5.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M., Berg P. Altering the specificity of restriction endonuclease: effect of replacing Mg2+ with Mn2+. Biochemistry. 1978 Jan 10;17(1):131–138. doi: 10.1021/bi00594a019. [DOI] [PubMed] [Google Scholar]
- Jacobson J. W., Medhora M. M., Hartl D. L. Molecular structure of a somatically unstable transposable element in Drosophila. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8684–8688. doi: 10.1073/pnas.83.22.8684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyaprakash A., Hoy M. A. Complete sequence of a mariner transposable element from the predatory mite Metaseiulus occidentalis isolated by an inverse PCR approach. Insect Mol Biol. 1995 Feb;4(1):31–39. doi: 10.1111/j.1365-2583.1995.tb00005.x. [DOI] [PubMed] [Google Scholar]
- Kaufman P. D., Doll R. F., Rio D. C. Drosophila P element transposase recognizes internal P element DNA sequences. Cell. 1989 Oct 20;59(2):359–371. doi: 10.1016/0092-8674(89)90297-3. [DOI] [PubMed] [Google Scholar]
- Kaufman P. D., Rio D. C. Germline transformation of Drosophila melanogaster by purified P element transposase. Nucleic Acids Res. 1991 Nov 25;19(22):6336–6336. doi: 10.1093/nar/19.22.6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman P. D., Rio D. C. P element transposition in vitro proceeds by a cut-and-paste mechanism and uses GTP as a cofactor. Cell. 1992 Apr 3;69(1):27–39. doi: 10.1016/0092-8674(92)90116-t. [DOI] [PubMed] [Google Scholar]
- Kulkosky J., Jones K. S., Katz R. A., Mack J. P., Skalka A. M. Residues critical for retroviral integrative recombination in a region that is highly conserved among retroviral/retrotransposon integrases and bacterial insertion sequence transposases. Mol Cell Biol. 1992 May;12(5):2331–2338. doi: 10.1128/mcb.12.5.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohe A. R., Moriyama E. N., Lidholm D. A., Hartl D. L. Horizontal transmission, vertical inactivation, and stochastic loss of mariner-like transposable elements. Mol Biol Evol. 1995 Jan;12(1):62–72. doi: 10.1093/oxfordjournals.molbev.a040191. [DOI] [PubMed] [Google Scholar]
- Medhora M., Maruyama K., Hartl D. L. Molecular and functional analysis of the mariner mutator element Mos1 in Drosophila. Genetics. 1991 Jun;128(2):311–318. doi: 10.1093/genetics/128.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuuchi K. Polynucleotidyl transfer reactions in transpositional DNA recombination. J Biol Chem. 1992 Oct 25;267(30):21273–21276. [PubMed] [Google Scholar]
- Mizuuchi K. Transpositional recombination: mechanistic insights from studies of mu and other elements. Annu Rev Biochem. 1992;61:1011–1051. doi: 10.1146/annurev.bi.61.070192.005051. [DOI] [PubMed] [Google Scholar]
- Plasterk R. H. The origin of footprints of the Tc1 transposon of Caenorhabditis elegans. EMBO J. 1991 Jul;10(7):1919–1925. doi: 10.1002/j.1460-2075.1991.tb07718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pridmore R. D. New and versatile cloning vectors with kanamycin-resistance marker. Gene. 1987;56(2-3):309–312. doi: 10.1016/0378-1119(87)90149-1. [DOI] [PubMed] [Google Scholar]
- Robertson H. M., Lampe D. J. Recent horizontal transfer of a mariner transposable element among and between Diptera and Neuroptera. Mol Biol Evol. 1995 Sep;12(5):850–862. doi: 10.1093/oxfordjournals.molbev.a040262. [DOI] [PubMed] [Google Scholar]
- Robertson H. M., MacLeod E. G. Five major subfamilies of mariner transposable elements in insects, including the Mediterranean fruit fly, and related arthropods. Insect Mol Biol. 1993;2(3):125–139. doi: 10.1111/j.1365-2583.1993.tb00132.x. [DOI] [PubMed] [Google Scholar]
- Robertson H. M. The mariner transposable element is widespread in insects. Nature. 1993 Mar 18;362(6417):241–245. doi: 10.1038/362241a0. [DOI] [PubMed] [Google Scholar]
- Vos J. C., De Baere I., Plasterk R. H. Transposase is the only nematode protein required for in vitro transposition of Tc1. Genes Dev. 1996 Mar 15;10(6):755–761. doi: 10.1101/gad.10.6.755. [DOI] [PubMed] [Google Scholar]
- Vos J. C., Plasterk R. H. Tc1 transposase of Caenorhabditis elegans is an endonuclease with a bipartite DNA binding domain. EMBO J. 1994 Dec 15;13(24):6125–6132. doi: 10.1002/j.1460-2075.1994.tb06959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos J. C., van Luenen H. G., Plasterk R. H. Characterization of the Caenorhabditis elegans Tc1 transposase in vivo and in vitro. Genes Dev. 1993 Jul;7(7A):1244–1253. doi: 10.1101/gad.7.7a.1244. [DOI] [PubMed] [Google Scholar]
- van Luenen H. G., Colloms S. D., Plasterk R. H. The mechanism of transposition of Tc3 in C. elegans. Cell. 1994 Oct 21;79(2):293–301. doi: 10.1016/0092-8674(94)90198-8. [DOI] [PubMed] [Google Scholar]