Abstract
Introduction
Studies suggest that both affective and cognitive processes are involved in the perception of vulnerability to cancer and that affect has an early influence in this assessment of risk. We constructed a path model based on a conceptual framework of heuristic reasoning (affect, resemblance, and availability) coupled with cognitive processes involved in developing personal models of cancer causation.
Methods
From an eligible cohort of 16 700 women in a managed care organization, we randomly selected 2524 women at high, elevated, and average risk of ovarian cancer and administered a questionnaire to test our model (response rate 76.3%). Path analysis delineated the relationships between personal and cognitive characteristics (number of relatives with cancer, age, ideas about cancer causation, perceived resemblance to an affected friend or relative, and ovarian cancer knowledge) and emotional constructs (closeness to an affected relative or friend, time spent processing the cancer experience, and cancer worry) on perceived risk of ovarian cancer.
Results
Our final model fit the data well (root mean square error of approximation (RMSEA) = 0.028, comparative fit index (CFI) = 0.99, normed fit index (NFI) = 0.98). This final model (1) demonstrated the nature and direction of relationships between cognitive characteristics and perceived risk; (2) showed that time spent processing the cancer experience was associated with cancer worry; and (3) showed that cancer worry moderately influenced perceived risk.
Discussion
Our results highlight the important role that family cancer experience has on cancer worry and shows how cancer experience translates into personal risk perceptions. This understanding informs the discordance between medical or objective risk assessment and personal risk assessment.
Introduction
The concept of risk perception has played a key role in models of health behavior, in medical and psychological research, and in strategies of informed decision-making and risk communication [1]. Despite its importance, risk perception has been described as a ‘phenomenon in search of an explanation’ [2]. A person’s perception of risk might influence decisions about whether to seek screening, undergo preventive surgery, or make behavioral changes intended to reduce risk. Yet the literature on risk perception has demonstrated that objective, probability-based, numeric risk assessments often are discordant with individuals’ perceptions of their own risk, sometimes leading to unnecessary distress, and potentially jeopardizing sound medical decision-making. Studies that have focused on genetic counseling and hereditary cancers, especially breast cancer, suggest that women overestimate their risk for cancer, irrespective of their objective risk as determined by their age and family history [3-5]. Furthermore, genetic counseling, which aims to help people understand the potential contribution of genetics to disease risk, often has only a limited effect on improving the accuracy of perceived risk [4,5] because perceived susceptibility to cancer appears to be resistant to change [6].
The lack of agreement between objective and perceived risk can be partially explained by an influence of contextual factors on risk perceptions [7], or by limitations in how perceived risk is measured [8]. More important is the growing recognition of an affective or emotional component of risk judgment in a process typically regarded as cognitive [2,9]. It has been suggested that perceived risk is not one concept but rather a construct made up of both deliberative or cognitive processing and associative or intuitive processing that might at times conflict with one another [10]. Whether emotional constructs such as worry or concern operate separately from the more cognitive aspects of risk perception or whether cognitive risk judgment and worry have a causal or reciprocal relationship bears further study [11]. More work is needed to expand our understanding of how emotional processes are integrated into risk perceptions and decision-making [12].
Judgment and decision-making theory provides guidance about how people use both rational and emotionally-based heuristics to develop judgments and facilitate decision making in the face of uncertainty or complexity [13,14]. Among the heuristics that have been used to describe how information is incorporated into an assessment of perceived cancer risk are the affect heuristic, which acknowledges the contribution of feelings in assessing a threat; the representativeness heuristic where judgment about an event is based on perceived similarity or dissimilarity to an affected person; and the availability heuristic, which poses that more salient, familiar, and imaginable events are more easily recalled and judged as probable [15,16].
A woman’s experience with cancer illness or death among relatives and friends as well as her knowledge about the hereditary nature of the cancer can evoke heuristic processing when asked to assess her personal vulnerability to cancer. The more salient a woman’s experience with cancer, in terms of the emotional intensity of the relationship with an affected relative or friend, the more likely it is that she will perceive her own risk of cancer to be high, irrespective of her objective risk. [17,18]. The intensity of a direct cancer experience may be affected by factors such as strength of the relationship, observation of negative change in the family member or friend, physical and psychological resemblance to the affected relative or friend, and the ability to talk about the experience with the affected person or with others [19].
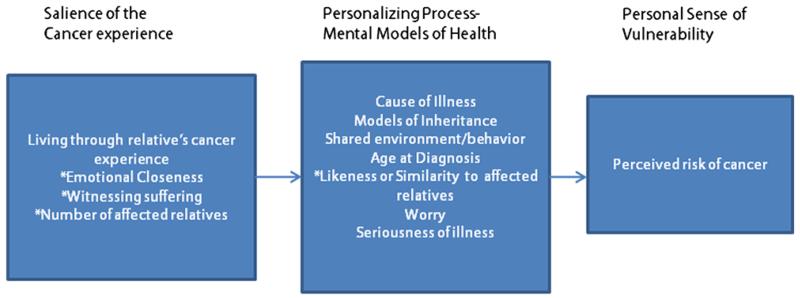
The aim of this study was to explore the cognitive and emotional antecedents of perceived risk of ovarian cancer and to elucidate the relationship between cancer worry and perceived risk of ovarian cancer. We propose that the heuristics of availability, affect (worry), and representativeness play key roles in developing personal models of cancer causation and vulnerability. Based on a conceptual framework developed by Walter and colleagues [20,21], we tested a model that included (1) the construct of salience, which is strongly influenced by personal experiences of cancer among relatives and friends; (2) knowledge of disease causation and inheritance that inform mental models of cancer; and (3) cognitive and emotional processing of the cancer experience into a perceived vulnerability to cancer (Figure 1 conceptual model).
Figure 1.
Conceptual model of perceived risk and cancer
* affect, availability and representativeness heuristics
Adapted from Walter et al., 2004; Walter and Emery 2005
The affective components of this model include living through the cancer experience of the affected person, emotional closeness to the affected person, bereavement, and anxiety and cancer worry. The cognitive component of this model includes the direct inputs into processing a mental model of cancer such as ideas about cancer causation, age, knowledge about ovarian cancer, number of relatives with cancer, and resemblance to the affected friend or relative. We believe that processing the emotional components of the cancer experience along with assessing cancer causes together influence perceived vulnerability to cancer.
Finally, because much of the research on risk perception and screening has been conducted in clinical settings with women at high risk [4,22], we sought to expand the examination of risk perception, worry, and cancer experience to women at varying risk levels in a more general population setting.
Methods
Participants
The data for this study were collected as part of a cross-sectional baseline survey on perceived risk of ovarian cancer and cancer screening among women at high, elevated, and average risk of ovarian cancer. The study was conducted among women in the Henry Ford Health System (HFHS) in Detroit, Michigan, which serves the primary and specialty health care needs of many residents in southeastern Michigan. Details on the sampling strategy and implementation of this survey have been previously published [23].
Briefly, those eligible to participate were women 30 years or older who had not been diagnosed with ovarian cancer and who reported not having had both of their ovaries removed. Participants also had to speak and understand English and respond to survey questions in English. We used a computer-assisted telephone interviewing (CATI) system to contact women, provide them with basic information about the research study, and then administer a brief eligibility screener. Immediately after administering the eligibility screening questions, the programmed CATI system randomly selected respondents for participation in a full interview. Out of an initial list of 55 887 women enrolled in the HFHS system, 20 483 were screened for eligibility (36.7%), and 16 720 were considered eligible (81.6%). Out of 3307 women who were randomly selected for a computer-assisted telephone interview, 2524 consented and completed the 35-min interview (response rate 76.3%). The survey was conducted from January 16, 2008 to December 12, 2008. Participants were provided with a $15 gift card upon completion of the interview.
We obtained approval for this study from the Institutional Review Boards of the Centers for Disease Control and Prevention and HFHS. Because we were asking respondents potentially sensitive information about their personal and family history of cancer, including previous receipt of BRCA1/BRCA2 genetic testing and the results of genetic tests, we obtained a 301(d) Certificate of Confidentiality of the Public Health Service Act for this study. We consented all respondents before conducting the survey interviews.
Measures
Our survey included original questions as well as previously-used instruments that allowed for the collection of valid and reliable data. We also adapted some existing scales to meet our specific needs. We selected variables and measures that corresponded to each of the components of our conceptual model and tested each of the measures for clarity of wording and appropriate response categories through cognitive interviewing methods.
Perceived risk of ovarian cancer
We summed three variables to create a composite measure of perceived risk of ovarian cancer. Women were asked whether their 10-year and lifetime risks of developing ovarian cancer are much higher, higher, about the same, lower, or much lower, than risk in most women their age. These perceived risk measures have been used previously in studies and have demonstrated high correlation and stability [24]. We also asked respondents if they thought their family history of cancer greatly increases, somewhat increases, has no effect on, somewhat decreases, or greatly decreases their risk of cancer. The internal consistency estimate of reliability for this scale could be considered marginally adequate given only three items (Cronbach’s alpha = 0.67).
Cancer worry
We adapted the Breast Cancer Worry Scale [25] to assess participants’ levels of concern about ovarian cancer and the extent to which it affects daily functioning. Using the responses of never, rarely, sometimes, a lot, and all the time, participants reported how often they have thought about their chances of developing ovarian cancer, how often thoughts of getting ovarian cancer have affected their mood, and how often these thoughts have affected their ability to perform daily activities. This scale has been used in studies of ovarian cancer screening [26]. Responses were summed across the three variables. The internal consistency estimate of reliability for the worry measure in this survey was adequate (Cronbach’s alpha = 0.76).
Family history of cancer
Participants were asked about breast and ovarian cancers in first-degree and second-degree relatives on both the mother’s and father’s side of the family. Participants were also asked whether any blood relatives had cancer other than breast or ovarian cancer and the type of cancer. Self-reports of cancer in family members, particularly first-degree relatives, have been found to be accurate and reliable [27]. We created two variables for analysis: (1) a count of first-degree and second-degree relatives with ovarian cancer and (2) a count of all relatives with any cancers.
Anxiety
Anxiety plays an important role in information processing [28]. Our survey included The State-Trait Anxiety inventory [29]. This is a well-validated scale that measures underlying (trait) and situational (state) anxiety. Participants were asked to respond to a series of statements about being generally or currently calm, tense, upset, relaxed, or worried using a four-point Likert-type scale (1 = not at all, 2 = somewhat, 3 = moderately, 4 = very much). For this study sample, the Cronbach’s alpha values for state and trait anxiety were 0.78 and 0.79, respectively, demonstrating adequate internal consistency for both subscales.
Cancer experience
We used the Connection to the Experience of Cancer Scale to measure the experience of cancer among friends or relatives [19]. Participants were asked to identify the relative or friend with cancer to whom they felt the closest and whether that person survived cancer. Participants were asked about how much time was spent with the friend or relative, how much negative change they observed in that person, how often they spoke with the friend or relative about their cancer, how much they resemble the friend or relative physically or in terms of personality, how often they think about the cancer experience and how much the experience has affected the participant. For each item, response options include three levels: not close or never, somewhat, or sometimes, and very or a lot. The three factors extracted from this scale were closeness/time spent (i.e., how close and how much time was spent with the affected person before and during their illness), resemblance (i.e., physical and personality resemblance to affected person) and time processing (i.e., time spent talking with the affected person about cancer, the effect of the cancer experience on how the participant thinks about her own health, and the time spent talking with friends or relatives about cancer experience). Psychometric data on the scale from this study sample have been reported previously [19].
Bereavement
A significant component of the cancer experience is bereavement. Witnessing the difficult course of a disease and death of a close relative from cancer may be particularly salient in the development of a woman’s sense of vulnerability to cancer and her worry about cancer. Women who have had a close relative die of cancer are more worried or distressed about cancer than those whose relatives survived [30] and breast cancer death in the family has been shown to be a strong predictor of medical decision-making behaviors [31]. Our survey participants were asked if the relative or friend with cancer that they identified was still living (yes/no). This question was not specific to cause of death although the majority were cancer deaths.
Ovarian cancer knowledge
Women’s general knowledge about ovarian cancer can influence the cognitive assessment of their perceived risk. To assess knowledge, participants were asked whether seven factors increase, decrease, or have no effect on a woman’s chances of getting ovarian cancer. These included: being hit in the abdomen; having one or more close relatives with ovarian cancer; giving birth; having breast, colorectal or endometrial cancer; getting older; having many sexual partners; and taking oral contraceptives. Participants were also asked whether they believed that women with ovarian cancer never experience symptoms and whether ovarian cancer causes more deaths than breast cancer. Correct answers were summed across the items for a total knowledge score.
Cancer cause
To operationalize how women have formulated their own cognitive models of inheritance, they were asked to give an open-ended response to what they think might increase their own chances of getting cancer. Answers were dichotomized into family history/genetic causes versus all other causes.
Seriousness of cancer
The perceived seriousness of an illness might influence a women’s sense of vulnerability to that illness. Women were asked how much they agreed or disagreed with the statement, ‘Getting ovarian cancer would be a serious problem.’ Response options included strongly agree, agree, disagree, or strongly disagree.
Demographics
Several studies have shown that older women report lower levels of perceived risk of cancer than younger women, so age was included as a potential determinant of perceived risk [32,33]. We excluded women younger than 30 years of age because both ovarian cancer risk and the likelihood of experiencing cancer among family members and friends were lower for women 29 years of age and younger.
Statistical analysis
Means, standard deviations, and intercorrelations were computed for all study variables. In addition, all variables were checked for normality and presence of outliers.
Path model analysis
Following our conceptual model [20,21], we used path analysis to quantify direct and indirect pathways through which the salience of the cancer experience (time spent with affected person, bereavement, and observation of change in the affected person’s quality of life) leads to the emotional processing of the experience (time spent thinking/speaking about the cancer experience), which, in turn, are hypothesized to influence a personal sense of vulnerability (perceived risk). Other variables directly influencing perceived risk were ovarian cancer knowledge, the number of relatives with ovarian cancer, age of the respondent, the number of relatives with any cancer, and belief in a genetic cause of cancer in the affected person. Model fit was assessed with several fit indices: most importantly, the CFI, the RMSEA, and the NFI. RMSEA values at or above 0.10 indicate a poorly fitting model with values between 0.05 and 0.09, indicating an average fit and values below 0.05 indicating a very good fit. Well-fitting models have a CFI value approaching 1.0 (>0.90) and a NFI value >0.90 [34].
Analyses were carried out with EQS Version 6. The default estimation method typically used in path analyses is maximum likelihood—a method that assumes normality of variables. As several of our variables violated this assumption, we used a robust maximum likelihood estimation procedure [35].
Results
Of the 2524 women who completed the CATI interview, 250 did not have a close relative or friend with cancer and 101 chose not to reveal whether they could identify such a person. We deleted 162 additional observations with missing or do not know values on the variables of interest, leaving a total of 2011 observations for our analysis. Participants ranged in age from 30 to 77 years with an average age of 55 years. The majority were-non-Hispanic white (68%), had some college education (63%), were married (67%), and reported a relatively high annual income (61% with income at least $50 000).
Table 1 displays the means, standard deviations, and Pearson correlation coefficients among the 14 variables used in the path model. Most variables did not exhibit extreme skewness (>2) or extreme kurtosis (.7) Cancer worry and number of ovarian cancer relatives had the highest skewness values of 2.1 and 1.7, respectively. The seriousness variable was excluded from further analyses because 96% of respondents endorsed the seriousness of ovarian cancer. Perceived risk was most strongly correlated with number of relatives with cancer and cancer worry. Closeness/time spent was positively correlated with time spent processing the cancer experience and state anxiety was correlated with trait anxiety. Bereavement (the close person was alive) was negatively associated with observing a negative change in quality of life.
Table 1.
Correlations among variables used in the model along with their means and standard deviations (n = 2011)
|
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| Perceived risk | — | |||||||||||||
| # of ovarian cancer relatives |
0.29** | — | ||||||||||||
| # relatives with any cancer |
0.23** | 0.21** | — | |||||||||||
| Age | −0.17** | −0.04 | 0.05* | — | ||||||||||
| Time spent (general closeness) |
0.04* | 0.11** | 0.14** | −0.02 | — | |||||||||
| Time processing | 0.14** | 0.08** | 0.10** | −0.02 | 0.35** | — | ||||||||
| Resemblance | 0.13** | 0.11** | 0.15** | −0.06** | 0.31** | 0.22** | — | |||||||
| Bereavement | 0.01 | −0.12 ** | −0.02 | −0.08** | −0.09** | −0.08** | −0.10** | — | ||||||
| Cancer knowledge | 0.13** | 0.03 | 0.13** | −0.23** | −0.03 | 0.01 | 0.05* | 0.04 | — | |||||
| Cancer cause | 0.19** | 0.27** | 0.27** | −0.12** | 0.05* | 0.10** | 0.06** | 0.02 | 0.17** | — | ||||
| Cancer worry | 0.31** | 0.17** | 0.03 | −0.03 | 0.02 | 0.19** | 0.10** | −0.04 | 0.01 | 0.03 | — | |||
| State anxiety | 0.12** | 0.03 | 0.03 | −0.04 | −0.05* | 0.06* | 0.04 | −0.04 | 0.02 | 0.01 | 0.19** | — | ||
| Trait anxiety | 0.19** | 0.03 | 0.06** | −0.23** | −0.06* | 0.05* | 0.03 | −0.01 | 0.07** | 0.03 | 0.13** | 0.53** | — | |
| Change QoL (negative change) |
0.04 | 0.08** | 0.05* | 0.01 | 0.12** | 0.26** | 0.04 | −0.46** | 0.04* | 0.01 | 0.06** | 0.08** | 0.09** | — |
| Mean | 8.98 | 0.20 | 2.52 | 55.14 | 7.77 | 8.27 | 3.90 | 0.43 | 3.95 | 0.25 | 3.86 | 8.63 | 10.37 | 2.13 |
| s.d. | 2.46 | 0.41 | 1.95 | 10.72 | 1.40 | 1.83 | 1.16 | 0.50 | 1.69 | 0.43 | 1.49 | 3.14 | 3.33 | 0.79 |
QoL. quality of life.
Correlation is significant at the 0.01 level.
Correlation is significant at the 0.05 level.
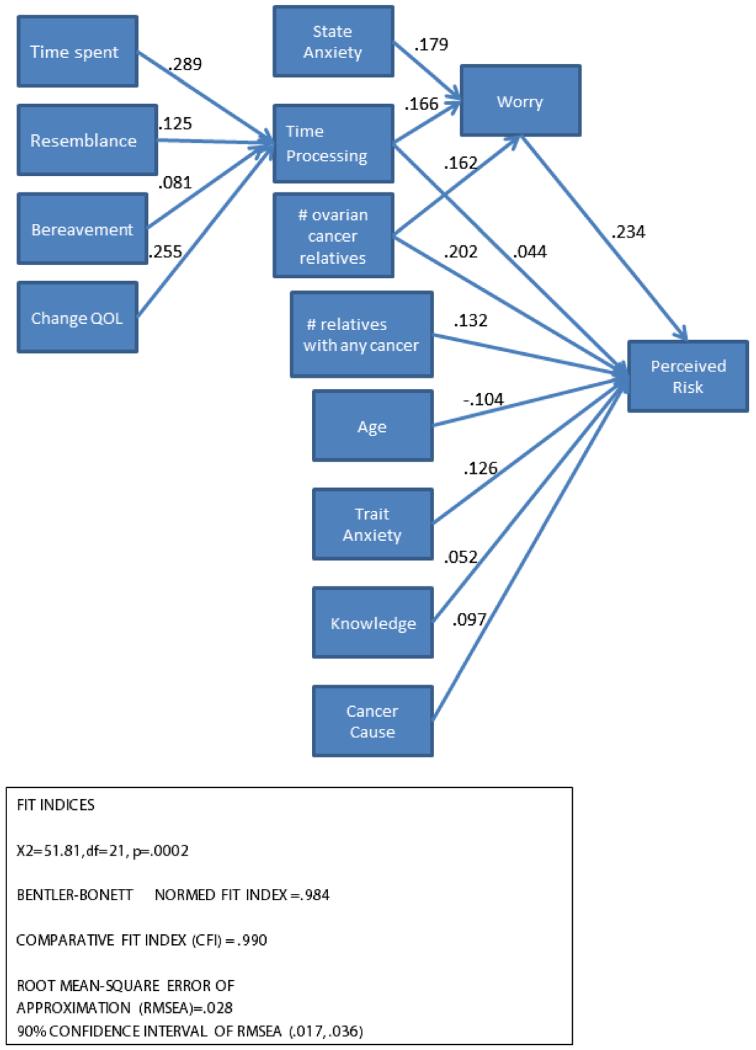
Our initial path model in which time spent processing the cancer experience, worry, and the other covariates led directly to perceived risk achieved an average fit (RMSEA = 0.06 and CFI = 0.94). We conducted an exploratory investigation that separated the emotional from the cognitive aspects of perceived risk by creating a path from the closeness/time spent through time processing to worry and then to perceived risk. We believe this modification more closely aligns with hypotheses about the dual nature of judgments such as perceived risk, in addition to suggesting how emotional processes are integrated into risk perceptions. In this model, we classified resemblance as part of the cancer experience that predicted time spent processing. This path model was a very good fit to the data (CFI = 0.99; RMSEA = 0.028 [0.017, 0.036]; NFI = 0.98), and all path coefficients were statistically significant (Figure 2). Overall, the model accounted for 23% of the variability (r-squared) in perceived risk. Because of the satisfactory model fit and the conceptual interpretability of the model, we elected not to make any further modifications.
Figure 2.
Path analytic model of time processing, worry, and perceived risk for 2011 study participants showing standardized path coefficients
Although the path coefficients for resemblance and bereavement were statistically significant, the strongest predictors of time processing were time spent with the affected person and observation of negative change in that person. Higher scores on time processing the cancer experience, state anxiety, and the number of ovarian cancer relatives were all positively associated with worry. Although statistically significant, the path coefficient for time processing to perceived risk was small. Worry, in turn, strongly predicted perceived risk as did the number of relatives with ovarian cancer. The cognitive components of perceived risk (i.e., the number of relatives with cancer, a higher cancer knowledge score, and a belief in the genetic causes of cancer) positively predicted perceived risk. As would be expected from the literature, age was negatively associated with perceived risk.
Discussion
Our results describe how the emotional experience of cancer as lived through an affected person can exert a strong influence on a person’s cancer worry and subsequently on their sense of vulnerability to cancer. We framed our analysis on a conceptual model that incorporated the cancer experience and processing of that experience as a guide to understanding how perceived risk is constructed, and modified our initial path model to attempt to discriminate between the emotional and cognitive antecedents. The direct effects of worry and the number of relatives with ovarian cancer on perceived risk were the strongest predictors in the final model.
Perceived risk is elicited as a response to questions about numeric risk or comparative risk and it is important to recognize that a woman’s response to a risk perception question will incorporate her cancer experiences. The model presented here suggests that it is important and reasonable to think of the emotional component of perceived risk as an ‘affectively charged evaluative reaction’ to a question that incorporates both a person’s experience and cognitive understanding [36]. Our model is also consistent with the concept that affect and cognition are inextricably linked, and that focusing solely on cognitive and objective information might not be successful in risk communication. A recent intervention study of tailored breast cancer risk communication found that after receiving such information, women not only misreported their risk scores, but also tended to believe that the risk scores did not adequately capture their cancer family history background. This occurred despite the heavy reliance of family history in the construction of risk scores [37]. If risk scores are typically calculated by number and type of relative and age at diagnosis, clearly something about the experience of cancer in the family will not be captured. It is also important to recognize that the concepts of risk, chance, and likelihood are challenging for both lay people and professionals. A recent study of individuals in a low-income community found that 49% and 63% of respondents who were asked about their ‘perceived chance’ and ‘perceived likelihood’ of developing colorectal cancer chose ‘don’t know’ when explicitly given that choice [38].
Perspectives from the field of judgment and decision making [14] and from models that describe how both cognitive and emotional processes are involved in risk construction and in regulation of health threats [21] guided the selection of measures and instruments in the current study. We developed measures that attempted to capture the emotional components of perceived risk—specifically those related to women’s experience of cancer among friends or relatives—which allowed us to examine the connection between emotional processes and perceived risk. We were able to conduct this survey in a large, racially-diverse managed care population, which is a strength because much of the research on genetic risk and counseling has been conducted among women attending specialized health clinics or recruited from family members of women at high risk who are likely to differ from women in community settings with respect to perceived risk and family history of cancer [39]. Our population provided a reasonable compromise between a general population-based survey and a clinic-based survey.
We note several limitations. Family history was self-reported. Although reporting of first-degree relatives is generally considered reliable, some studies have described an under-reporting of ovarian cancer in relatives, especially second-degree relatives [27]. Another potential limitation is the modest reliability of some of the measures in this study because of fewer response options. The three response categories for the questions in the Connection to the Experience of Cancer Scale was motivated by a preference for fewer categories in cognitive testing and to reduce response burden. Our data were cross-sectional in nature, which limited our ability to make inferences about causal attributes. Finally, although our sample was racially diverse, it represented one health plan in one area of the country and may not be generalizable to vastly different populations.
Elucidating the relationship between cancer experiences among relatives and friends and cancer worry adds to our knowledge about how individuals perceive their vulnerability to cancer. Developing the methods to effectively measure, solicit, and use this information can help us construct more effective risk communications. Furthermore, explicitly recognizing the importance of the cancer experience among relatives or friends can help identify women who might be especially prone to distress regarding cancer and who might benefit from additional counseling. Finally, with rapid growth in genetic and molecular medicine, information on personal susceptibility to a host of diseases is increasingly available to individuals who want to avoid or reduce potential health problems.
Acknowledgements
We thank Susan Zaro (ICF International) for project oversight. Funding support was provided by the Centers for Disease Control and Prevention (Contract No. 200-2002-00574, Task order 0015).
Footnotes
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Miller SM, Bowen DJ, Campbell MK, et al. Current research promises and challenges in behavioral oncology: Report from the American society of preventive oncology annual meeting, 2002. Cancer Epidemiol Biomarkers Prev. 2004;13:171–180. doi: 10.1158/1055-9965.epi-463-2. [DOI] [PubMed] [Google Scholar]
- 2.Sjöberg L. Factors in risk perception. Risk Anal. 2000;20:1–11. [PubMed] [Google Scholar]
- 3.Hopwood P. Breast cancer risk perception: what do we know and understand? Breast Cancer Res. 2000;2:387–391. doi: 10.1186/bcr83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sivell S, Elwyn G, Gaff CL, et al. How risk is perceived, constructed and interpreted by clients in clinical genetics, and the effects on decision making: Systematic review. J Genet Couns. 2008;17:30–63. doi: 10.1007/s10897-007-9132-1. [DOI] [PubMed] [Google Scholar]
- 5.Braithwaite D, Emery J, Walter F, Prevost AT, Sutton S. Psychological impact of genetic counseling for familial cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2004;96:122–133. doi: 10.1093/jnci/djh017. [DOI] [PubMed] [Google Scholar]
- 6.McQueen A, Swank PR, Bastian LA, Vernon SW. Predictors of perceived susceptibility of breast cancer and changes over time: a mixed modeling approach. Health Psychol. 2008;27:68–77. doi: 10.1037/0278-6133.27.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leventhal H, Kelly K, Leventhal EA. Population risk, actual risk, perceived risk, and cancer control: a discussion. J Natl Cancer Inst. 1999;25:81–85. doi: 10.1093/oxfordjournals.jncimonographs.a024214. Monographs. [DOI] [PubMed] [Google Scholar]
- 8.Woloshin S, Schwartz LM, Black WC, Welch HG. Women’s perceptions of breast cancer risk: how you ask matters. Med Decis Making. 1999;19:221–229. doi: 10.1177/0272989X9901900301. [DOI] [PubMed] [Google Scholar]
- 9.Slovic P, Finucane ML, Peters E, et al. Risk as analysis and risk as feelings: some thoughts about affect, reason, risk, and rationality. Risk Anal. 2004;24:311–322. doi: 10.1111/j.0272-4332.2004.00433.x. [DOI] [PubMed] [Google Scholar]
- 10.Windschitl PD, Martin R, Flugstad AR. Context and the interpretation of likelihood information: the role of intergroup comparisons on perceived vulnerability. J Pers Soc Psychol. 2002;82:742–755. doi: 10.1037//0022-3514.82.5.742. [DOI] [PubMed] [Google Scholar]
- 11.Loewenstein GF, Weber EU, Hsee CK, Welch N. Risk as feelings. Psychol Bull. 2001;127:267–286. doi: 10.1037/0033-2909.127.2.267. [DOI] [PubMed] [Google Scholar]
- 12.Marteau TM, Weinman J. Self-regulation and the behavioural response to DNA risk information: a theoretical analysis and framework for future research. Soc Sci Med. 2006;62:1360–1368. doi: 10.1016/j.socscimed.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Kahneman D, Slovic P, Tversky A. Judgment Under Uncertainty: Heuristics and Biases. Cambridge University Press; Cambridge: 1982. [DOI] [PubMed] [Google Scholar]
- 14.Peters E, McCaul KD, Stefanek M, Nelson W. A heuristics approach to understanding cancer risk perception: contributions from judgment and decision-making research. Ann Behav Med. 2006;31:45–52. doi: 10.1207/s15324796abm3101_8. [DOI] [PubMed] [Google Scholar]
- 15.Katapodi MC, Facione NC, Humphreys JC, et al. Perceived breast cancer risk: heuristic reasoning and search for a dominance structure. Soc Sci Med. 2005;60:421–432. doi: 10.1016/j.socscimed.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Rees G, Fry A, Cull A. A family history of breast cancer: women’s experiences from a theoretical perspective. Soc Sci Med. 2001;52:1433–1440. doi: 10.1016/s0277-9536(00)00248-3. [DOI] [PubMed] [Google Scholar]
- 17.Absetz P, Aro AR, Sutton SR. Factors associated with breast cancer risk perception and psychological distress in a representative sample of middle-aged Finnish women. Anxiety Stress Coping. 2002;15:61–73. [Google Scholar]
- 18.Zakowski SG, Valdimarsdottir HB, Bovbjerg DH, et al. Predictors of intrusive thoughts and avoidance in women with family histories of breast cancer. Ann Behav Med. 1997;19:362–369. doi: 10.1007/BF02895155. [DOI] [PubMed] [Google Scholar]
- 19.Hawkins NA, McCarty F, Peipins LA, Rodriguez JL. Measuring the degree of closeness to the cancer experience: development and initial validation of the connection to the experience of cancer scale (CONNECs) Patient Educ Couns. 2012;89:292–299. doi: 10.1016/j.pec.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walter FM, Emery J. ‘Coming down the line’—patients’ understanding of their family history of common chronic disease. Ann Fam Med. 2005;3:405–414. doi: 10.1370/afm.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walter FM, Emery J, Braithwaite D, Marteau TM. Lay understanding of familial risk of common chronic diseases: a systematic review and synthesis of qualitative research. Ann Fam Med. 2004;2:583–594. doi: 10.1370/afm.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heshka JT, Palleschi C, Howley H, Wilson B, Wells PS. A systematic review of perceived risks, psychological and behavioral impacts of genetic testing. Genet Med. 2008;10:19–32. doi: 10.1097/GIM.0b013e31815f524f. [DOI] [PubMed] [Google Scholar]
- 23.Leadbetter S, Hawkins NA, Scholl LE, et al. Recruiting women for a study on perceived risk of cancer: influence of survey topic salience and early versus late response. Prev Chronic Dis. 2013;10:E75. doi: 10.5888/pcd10.120293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gurmankin Levy A, Shea J, Williams SV, Quistberg A, Armstrong K. Measuring perceptions of breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15:1893–1898. doi: 10.1158/1055-9965.EPI-05-0482. [DOI] [PubMed] [Google Scholar]
- 25.Lerman C, Schwartz M. Adherence and psychological adjustment among women at high risk for breast cancer. Breast Cancer Res Treat. 1993;28:145–155. doi: 10.1007/BF00666427. [DOI] [PubMed] [Google Scholar]
- 26.Andersen MR, Peacock S, Nelson J, et al. Worry about ovarian cancer risk and use of ovarian cancer screening by women at risk for ovarian cancer. Gynecol Oncol. 2002;85:3–8. doi: 10.1006/gyno.2001.6556. [DOI] [PubMed] [Google Scholar]
- 27.Murff HJ, Spigel DR, Syngal S. Does this patient have a family history of cancer? An evidence-based analysis of the accuracy of family cancer history. JAMA. 2004;292:1480–1489. doi: 10.1001/jama.292.12.1480. [DOI] [PubMed] [Google Scholar]
- 28.Cameron LD, Jago L. Emotion regulation interventions: a common-sense model approach. Br J Health Psychol. 2008;13:215–221. doi: 10.1348/135910708X288800. [DOI] [PubMed] [Google Scholar]
- 29.Spielberger CD. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press, Inc.; Palo Alto: 1983. [Google Scholar]
- 30.Hopwood P, Shenton A, Lalloo F, Evans DG, Howell A. Risk perception and cancer worry: An exploratory study of the impact of genetic risk counselling in women with a family history of breast cancer. J Med Genet. 2001;38:139–142. doi: 10.1136/jmg.38.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tracy KA, Quillin JM, Wilson DB, et al. The impact of family history of breast cancer and cancer death on women’s mammography practices and beliefs. Genet Med. 2008;10:621–625. doi: 10.1097/gim.0b013e31817c0355. [DOI] [PubMed] [Google Scholar]
- 32.Rubinstein WS, O’Neill SM, Rothrock N, et al. Components of family history associated with women’s disease perceptions for cancer: a report from the family healthware impact trial. Genet Med. 2011;13:52–62. doi: 10.1097/GIM.0b013e3181fbe485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang C, O’Neill SM, Rothrock N, et al. Comparison of risk perceptions and beliefs across common chronic diseases. Prev Med. 2009;48:197–202. doi: 10.1016/j.ypmed.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu LT, Bentler PM. Cutoff criteria for fit indices in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Model. 1999;6:1–55. [Google Scholar]
- 35.Santorra A, Bentler P. Corrections to test statistics and standard errors in covariance structure analysis. Proceedings of the Business and Economic Statistics Section of the American Statistical Association. 1994:308–313. [Google Scholar]
- 36.Russell JA, Barrett LF. Core affect, prototypical emotional episodes, and other things called emotion: dissecting the elephant. J Pers Soc Psychol. 1999;76:805–819. doi: 10.1037//0022-3514.76.5.805. [DOI] [PubMed] [Google Scholar]
- 37.Scherer LD, Ubel PA, McClure J, et al. Belief in numbers: when and why women disbelieve tailored breast cancer risk statistics. Patient Educ Couns. 2013;92:253–259. doi: 10.1016/j.pec.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waters EA, Hay JL, Orom H, Kiviniemi MT, Drake BF. “Don’t know” responses to risk perception measures: implications for underserved populations. Med Decis Making. 2013;33:271–281. doi: 10.1177/0272989X12464435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katapodi MC, Lee KA, Facione NC, et al. Predictors of perceived breast cancer risk and the relation between perceived risk and breast cancer screening: a meta-analytic review. Prev Med. 2004;38:388–402. doi: 10.1016/j.ypmed.2003.11.012. [DOI] [PubMed] [Google Scholar]