Abstract
Botulinum neurotoxins (BoNTs) are produced by various species of clostridia and are potent neurotoxins which cause the disease botulism, by cleaving proteins needed for successful nerve transmission. There are currently seven confirmed serotypes of BoNTs, labeled A-G, and toxin-producing clostridia typically only produce one serotype of BoNT. There are a few strains (bivalent strains) which are known to produce more than one serotype of BoNT, producing either both BoNT/A and /B, BoNT/A and /F, or BoNT/B and /F; designated as Ab, Ba, Af, or Bf. Recently, it was reported that Clostridium botulinum strain Af84 has three neurotoxin gene clusters: bont/A2, bont/F4, and bont/F5. This was the first report of a clostridial organism containing more than two neurotoxin gene clusters. Using a mass spectrometry based proteomics approach, we report here that all three neurotoxins, BoNT/A2, /F4, and /F5, are produced by C. botulinum Af84. Label free MSE quantification of the three toxins indicated that toxin composition is 88% BoNT/A2, 1% BoNT/F4, and 11% BoNT/F5. The enzymatic activity of all three neurotoxins was assessed by examining the enzymatic activity of the neurotoxins upon peptide substrates which mimic the toxins’ natural targets and monitoring cleavage of the substrates by mass spectrometry. We determined that all three neurotoxins are enzymatically active. This is the first report of three enzymatically active neurotoxins produced in a single strain of Clostridium botulinum.
Introduction
Botulinum neurotoxins (BoNTs) are toxic proteins which are produced by Clostridium botulinum and some related clostridia. Intoxication with BoNT causes the disease known as botulism, which can be fatal if untreated. BoNT consists of a heavy chain which binds to receptors of neurons and a light chain which has enzymatic activity, joined by a disulfide bond. The light chain is a highly-specific protease, cleaving proteins necessary for nerve impulse transmission. BoNTs are currently grouped into seven confirmed serotypes, A-G, based on their response to antisera. Additionally, the different serotypes of toxins possess differential enzymatic activity. BoNT/A, /C, and /E cleave SNAP-25 (synaptosomal-associated protein)1–6, and BoNT/B, /D, /F and /G cleave synaptobrevin-2 (also known as VAMP-2, vesicle-associated membrane protein)7–12. BoNT/A, /B, /C, /D, /E, and /F are also known to exhibit genetic and amino acid variance within each serotype, and this variance is designated as subtype, with the addition of a number following the letter, i.e. subtype BoNT/A2. All subtypes within a serotype have been shown to cleave their protein target in the same location, with the exception of BoNT/F57.
Our laboratory previously reported on our ability to detect BoNT via its enzymatic activity upon a peptide substrate13. In this procedure, BoNT is mixed with a peptide substrate which is a shortened version of the toxin’s natural target, SNAP-25 or synaptobrevin-2. BoNT cleaves the peptide substrate in a serotype-specific location, so that each serotype can be identified by its unique enzymatic cleavage pattern. BoNT/A cleaves SNAP-25 between 196Q and 197R1,2,4,5, and BoNT/C cleaves SNAP-25 at the adjacent residue, between 197R and 198A3,6. BoNT/E also cleaves SNAP-25, but cleaves it between 180R and 181I5. BoNT/B, /D, /F, and /G cleave synaptobrevin-2 at 76Q8, 59K11, 58Q10,11, and 81A9,12, respectively. The exception is BoNT/F5, first reported in 201014, which has a different enzymatic activity than other BoNT/Fs; it cleaves synaptobrevin-2 at 54L7. Cleavage of the peptide substrates is monitored by mass spectrometry, which allows for a determination of the location of cleavage based on fragment masses, which can be critical for identification of the serotype of toxin present. Our laboratory has also reported on our ability to obtain amino acid sequence information on the toxin itself through digestion of the toxin into peptides and sequencing of those peptides by MS/MS analysis combined with database searching15–17.
Typically a sample or culture supernatant contains a C. botulinum strain that produces only one serotype of toxin, but it is important to be able to detect more than one serotype in a single sample because some strains of C. botulinum produce more than one serotype of BoNT. These are termed bivalent toxin producers and are notated as Ab, Ba, Af, or Bf, with the capital letter indicating the presence of what is believed to be the dominant serotype produced. There are also some strains of C. botulinum that have more than one toxin gene, but only produce a single toxin type with the other gene being silent. An example is CDC5134818 that produces BoNT/A1 type toxin but has a silent B gene and this is noted as A(B) with the silent gene in parenthesis. Several assays have demonstrated the ability to detect more than one serotype of toxin from a single sample14,19,20, indicating the ability to detect neurotoxins produced by a bivalent C. botulinum strain. Our laboratory reported on the ability to detect both toxins produced by Ab, Ba, Af, and Bf bivalent strains using either serotype-specific antibodies with multiple extractions from the same sample21, or using a single antibody which binds to multiple serotypes that cause human botulism, such as BoNT/A, /B, /E, and /F22. Both of these techniques of antibody extraction can be followed by enzymatic cleavage characterization that is specific for each toxin type to detect and differentiate the toxin.
In 1970, Gimenez and Ciccarelli reported on a C. botulinum strain, Af84, that produced both A and F toxins as determined by the mouse bioassay19. The authors reported that in their mouse bioassay, the toxicity of the A and F toxins was in a ratio of about 90/10, respectively. Recently, it was reported by Dover et al. that C. botulinum strain Af84 contains gene clusters for three neurotoxins: BoNT/A2, /F4, and /F523. It was the first report of a C. botulinum strain containing more than two neurotoxin gene clusters, with both bont/A2 and bont/F4 genes located on the chromosome and a bont/F5 gene within a plasmid. These findings were based solely on genetic analyses. Dover et al also performed toxicity testing via the mouse bioassay and showed that both A and F toxins were produced at a ratio of 99/123. However, neither of these reports was able to determine if the BoNT/F toxicity was due to the F4 on the chromosome, the F5 on the plasmid, or both. In this work, we identified the neurotoxins produced by C. botulinum Af84 and determined their relative quantitative ratios through a mass spectral proteomics analysis of neurotoxins extracted from culture supernatants. The presence of the three neurotoxins was confirmed by examining their enzymatic action upon peptide substrates which mimic the toxins’ natural targets and monitoring cleavage of the peptide substrates by mass spectrometry.
Materials and Methods
Materials
Botulinum neurotoxin is highly toxic and requires appropriate safety measures. All neurotoxins were handled in a class 2 biosafety cabinet equipped with HEPA filters. Monoclonal antibodies to BoNT/A, CR2 and RAZ1 and monoclonal antibodies 6F5, 6F8, 6F9, and 6F10, cross-reactive antibodies which recognize BoNT/A, /B, /E, and /F, were obtained from Dr. James Marks at the University of California at San Francisco. Dynabeads® (M-280/Streptavidin) were purchased from Invitrogen (Carlsbad, CA) at 1.3 g/cm3 in phosphate buffered saline (PBS), pH 7.4, containing 0.1% Tween®-20 and 0.02% sodium azide. Polyclonal rabbit anti-BoNT/A antibodies, BoNT/A2 complex, and BoNT/F1 complex were purchased from Metabiologics (Madison, WI). All chemicals were from Sigma-Aldrich (St. Louis, MO) except where indicated. Peptide substrates Acetyl-RGSNKPKIDAGNQRATRXLGGR-NH2 (abbreviated as SubA) for assessment of BoNT/A activity (where X is norleucine) and TSNRRLQQTQAQVDEVVDIMRVNVDKVLERDQKLSELDDRADAL for assessment of BoNT/F activity (abbreviated as SubF) were synthesized by Midwest Bio-tech Inc. (Fishers, IN). The peptide substrate for BoNT/A has been modified from the original sequence of SNAP-25 to produce a more efficient substrate for BoNT/A cleavage24.
Production and Immunocapture of C. botulinum Af84 culture supernatant
Crude culture supernatants representing the C. botulinum Af84 strain originating in Argentina and housed at USARMIID were produced by incubating a subculture for 5 days at 35°C in TPGY medium. After centrifugation, the supernatants were removed and filtered through 0.22 μm filters. Monoclonal antibodies RAZ1 and CR2, provided by Dr. James Marks at the University of California at San Francisco, were used for extraction of BoNT/A25 and 6F5 was used for BoNT/F extraction7. Mabs were immobilized separately to streptavidin Dynabeads® after being rinsed three times with HBS-EP buffer (GE Healthcare, Piscataway, NJ.). A 2 μg aliquot of antibody was used per 100 μL of beads. A standard orbital shaker was used to bind antibody onto the beads for 1.5 hours. For extraction of BoNT/A, an aliquot of 20 μL of antibody-coated beads was added to a solution of 400 μL of HBS-EP buffer and 100 μL of culture supernatant from C. botulinum Af84. For extraction of BoNT/F, an aliquot of 40 μL of antibody-coated beads was added to a solution of 300 μL of HBS-EP buffer and 200 μL of culture supernatant from C. botulinum Af84. After mixing for 1 hr with constant agitation at room temperature, the beads were washed once in 1 mL of HBS-EP buffer. Negative controls consisted of culture supernatant medium with no organism or toxin, with the remainder of the extraction protocol as above.
Endopep-MS analysis
The beads were reconstituted in 18 μL of reaction buffer consisting of 0.05 M Hepes (pH 7.3), 25 mM dithiothreitol, and 20 μM ZnCl2 and 2 μL of SubA for beads used to extract BoNT/A or SubF for beads used to extract BoNT/F. The final concentration of substrate was 50 pmol/μL. All samples then were incubated at 37°C for 4 hr with no agitation. A 2-μL aliquot of each reaction supernatant was mixed with 18 μL of matrix solution consisting of α-cyano-4-hydroxy cinnamic acid (CHCA) at 5 mg/mL in 50% acetonitrile, 0.1% trifluoroacetic acid (TFA), and 1 mM ammonium citrate. A 0.5-μL aliquot of this mixture was pipeted onto one spot of a 384-spot matrix-assisted laser desorption/ionization (MALDI) plate (Applied Biosystems, Framingham, MA). Mass spectra of each spot were obtained by scanning from 1100 to 4800 m/z in MS-positive ion reflector mode on an Applied Biosystems 5800 Proteomics Analyzer (Framingham, MA). The instrument uses an Nd-YAG laser at 355 nm, and each spectrum is an average of 2400 laser shots.
Digestion of BoNT
Following Endopep-MS analysis, the beads were washed once with 100 μL of HBS-EP buffer and then reconstituted in 15 μL of 50 mM ammonium bicarbonate, pH of 7.5 (tryptic buffer) and 2 μL of stock trypsin, and digested for 5 min at 52°C. Following digestion, the supernatant was then removed from the beads and 1 μL of 10% TFA was added to the supernatant. A second, independent digestion (5 min at 52°C) was performed by reconstitution of the beads in 15 μL of tryptic buffer and addition of 1 μL of chymotrypsin diluted to 0.2 mg/mL in the tryptic buffer. After digestion, each supernatant was then removed from the beads and 1 μL of 10% TFA was added. Each digested sample was analyzed separately.
LC-MS/MS Qualitative Analysis
After digestion, 5 μL of the sample was injected onto a Waters NanoAcquity C18 5 μm UPLC trap column (180 μm ID and 2 cm long) and separated on a Waters NanoAcquity C18 1.7 μm UPLC column, 100 μm ID and 10 cm long (Waters, Milford, MA). After a trap-loading flow rate of 5 μL/min for 5 min, the peptides were eluted from the column at a flow rate of 600 nL/min using the following gradient conditions, where A is water with 0.1% formic acid and B is 100% acetonitrile with 0.1% formic acid: A=95% and B=5% at 0 min; A= 85% and B=15% at 10 min; A=65% and B=35% at 60 min; A=30% and B=70% at 62 min; hold for 5 minutes, A=95% and B=5% at 70 min; hold for 10 minutes.
Peptides eluting from the column were introduced into a LTQ-Orbitrap Elite instrument (Thermo Fisher Scientific, San Jose, CA) using a Bruker CaptiveSpray ion source (Bruker-Michrom Inc, Auburn, CA). The instrument was operated in data-dependent acquisition mode to automatically record both MS and MS/MS spectra. The orbitrap was used for MS acquisition with a resolution of 60,000 from 400 to 1600 m/z. Simultaneously, the linear ion trap was used for MS/MS analysis of the fifteen most abundant ions in each MS scan. Automatic gain control was used to accumulate ions for orbitrap analysis with a target value of 1,000,000 and 10,000 for MS and MS/MS analysis respectively. Collision energy of 35% was utilized in the linear ion trap to fragment the tryptic and chymotryptic digest fragments. Database searches were conducted using Mascot (Matrix Science Inc, Boston, MA, version 2.2.0) against a database generated by extracting entries from the NCBI non-redundant database using “clostridia” as the extraction parameter with 9 maximum missed cleavages, non-specific enzyme, peptides with up to +4 charge, MS1 tolerance of 20 ppm and MS/MS tolerance of 0.8 Da.
LC-MS/MS Quantitative Analysis
BoNT digests were spiked with 1.4 pmol of MassPREP digestion standard yeast alcohol dehydrogenase (Waters, Milford, MA) as a control for quantitative purposes. NanoESI LC-MS/MS quantitative analysis was performed on a Synapt hybrid tandem mass spectrometer (Waters, Milford, MA) connected to a nano-Acquity UPLC (Waters, Milford, MA) using the same buffers and gradient conditions for separation as described above. Analysis was performed using an MSE (Protein Expression) method consisting of alternating low and high collision energy scans in a data-independent format with m/z range of 50–1990 in 1.8 sec. Lockmass data was also obtained on a separate channel to acquire accurate mass measurement using GluFib. Data were processed with ProteinLynx Global Server (PLGS v2.5; Milford, MA) with a database search against the same database as described above. The relative protein quantification of individual replicates (three) was determined based on the average MS signals of the three most intense tryptic peptides per protein, through use of the PLGS IdentityE software. Once processed, the data sets were exported from PLGS and clustered according to digestion number for further evaluation by use of Excel. The femtomole on column values were calculated by averaging the technical replicates, excluding outliers with 30% or greater variation. These values were then averages on the basis of lot grouping. The lot grouping averages values were used to determine the ratio of each toxin type.
MSD immunoassays
BoNT/A2 and total BoNT/F concentrations were determined using quantitative electrochemiluminescent sandwich immunoassays. For the BoNT/A determinations, special plates were coated with monoclonal antibodies CR2 and RAZ1. A regression curve was generated using serial dilutions of BoNT/A2 complex as a standard. Sample dilutions of crude Af84 culture supernatants were applied to test wells in duplicate. Polyclonal rabbit anti-BoNT/A antibodies were used to detect bound toxin. A secondary antibody of goat anti-rabbit antibodies conjugated to SULFO-TAG (Meso Scale Diagnostics, Gaithersburg, MD) bound to polyclonal rabbit antibodies if present and provided a response signal when the plates were electronically processed. Signal responses ranged from a maximum of approximately 800,000 MSD units with background signals of ~500 units, providing a dynamic range of greater than three logs for the assay. The BoNT/F determinations used a similar assay protocol. Plates were coated with equine anti-BoNT/F antibodies (PerImmune, Rockville, MD). The regression curve was generated using dilutions of BoNT/F1 complex. A combination of three BoNT/F-specific, cross-reactive mouse monoclonal antibodies (6F8, 6F9, and 6F10) were used for detection, followed by goat anti-mouse/SULFO-TAG (Meso Scale Diagnostics, Gaithersburg, MD). For the BoNT/F assay, maximum responses of ~900,000 were recorded, with background signals of less than 1500. A dynamic range that was similar to that of the BoNT/A assay was obtained. Regression curves were generated and analyzed using four parameter logistic curve fitting (Discovery Workbench 4.0). Results were used to calculate BoNT/A2 and total BoNT/F concentrations and relative percentages for the toxins in the same crude culture supernatants that were used for mass spectrometry studies.
Results
In this work, we sought to first determine if all three neurotoxins (BoNT/A2, /F4, and /F5) were present and then determine if the neurotoxins present were enzymatically active. We used a mass spectrometry proteomics approach to detect and differentiate the protein neurotoxins produced by C. botulinum Af84, independent of their activity. In this process, the neurotoxin is digested into peptide fragments and introduced into the mass spectrometer. Once inside the mass spectrometer, the peptide is fragmented, resulting in a MS/MS spectrum.
An analysis of the peptide fragments following a digestion of material extracted from C. botulinum Af84 using antibodies specific for BoNT/A indicated that BoNT/A2 was present in this sample. Figure 1 lists the amino acid sequence for BoNT/A2 predicted from its gene sequence. Residues for which MS/MS amino acid sequence information was obtained in this experiment are marked in red font. MS/MS sequence data was obtained from 154 peptides, and comprises 79.4% of the protein. Residues which are unique to only BoNT/A2 are underlined. We obtained MS/MS information on 13 peptides unique to BoNT/A2 which encompasses 11 of the amino acid residues found only in BoNT/A2, as compared to all published sequences of BoNT/A. Collectively, these data demonstrate that the bont/A2 gene is expressed as the BoNT/A2 neurotoxin in C. botulinum Af84.
Figure 1.

Amino acid sequence of BoNT/A2 from C. botulinum strain Af84. MS/MS evidence was found for amino acids marked in red, and residues unique to BoNT/A2 are underlined.
Next, we examined BoNT/F following an extraction of a culture supernatant from C. botulinum Af84 using antibodies for BoNT/F. MS/MS analysis indicated the presence of two BoNT/F proteins; BoNT/F4 and /F5. Figure 2 is an alignment of the predicted amino acid sequences of BoNT/F4 and /F5. Residues for which MS/MS amino acid sequence information was obtained are marked in red font. We obtained MS/MS information on 19.7 % of the BoNT/F4 protein and 51.1 % of the /F5 protein. Seven of the peptides were common between BoNT/F4 or BoNT/F5, 11 peptides were specific for BoNT/F4 and 34 peptides were specific for BoNT/F5. The presence of these peptides found only in BoNT/F4 or /F5 indicates that both BoNT/F4 and /F5 are in the culture supernatant and that both the bont/F4 and bont/F5 genes are expressed as neurotoxins in C. botulinum Af84. Additionally, experiments carried out with C. botulinum Af84 obtained from a different laboratory (data not shown) also yielded evidence for the existence of all three proteins with 80.2% sequence coverage for BoNT/A2, 49.5% sequence coverage for /F4, and 68.6% sequence coverage for BoNT/F5.
Figure 2.

Sequence alignment of BoNT/F4 and /F5 from C. botulinum Af84. MS/MS evidence was found for amino acids marked in red. The underlined portion is the epitope of binding to mAb 6F5.
As a further demonstration of the existence of both BoNT/F4 and /F5, Figure 3 is the MS/MS spectra from similar peptides generated from the tryptic digest of BoNT/F4 and /F5. The peptide 1067VFDTELDKTEIETLYSDEPDPSILK1091 is from BoNT/F4 and is found only in /F4 and the peptide 1067VFNTELDKTEIEILYSNEPDPSILK1091 is from /F5 and is also found only in /F5. The y ions are marked in blue and b ions are marked in red. The two peptide sequences are closely related and the spectra appear similar. The y ions show that the y5 and y7 are the same in both spectra at m/z 557.5 and 769.4; however the y9 of the peptide from BoNT/F5 is 1 Da less than the y9 from BoNT/F4. This is because the y9 ion contains an asparagine in BoNT/F5 rather than an aspartic acid in /F4, and asparagine is 1 Da less than aspartic acid. This pattern continues for the remainder of the y ions.
Figure 3.
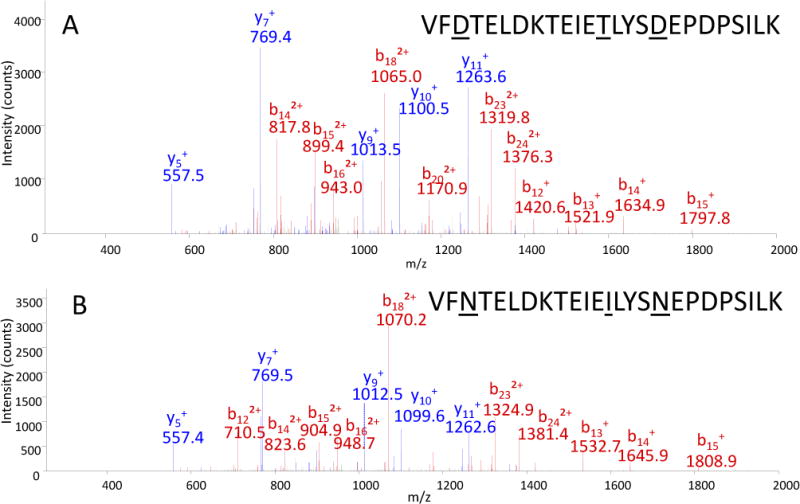
MS/MS of peptides VFDTELDKTEIETLYSDEPDPSILK from BoNT/F4 (A) and VFNTELDKTEIEILYSNEPDPSILK from BoNT/F5 (B). Each peptide is unique to the toxin with b ions are marked in blue with y ions marked in red.
Examining the b ions, the smallest b ion common to both spectra is the b13 ion. The b13 ion encompasses two differences in the peptides: the aspartic acid in position 3 of the peptide from BoNT/F4 becomes an asparagine in BoNT/F5 for a decrease of 1 Da, and the threonine in position 13 of the peptide from BoNT/F4 becomes an isoleucine in /F5 for an increase of 12 Da. These changes explain the increase in 11 Da of the b13 ion from BoNT/F5 compared to that of /F4. The doubly-charged b ions from b14 to b16 also display this same pattern; however, because these are doubly-charged ions, the m/z difference is approximately 5.5 Da. The doubly-charged b18 ion is slightly different, accounting for the third difference between these two peptides; the residue at position 17 is an aspartic acid in BoNT/F4 and an asparagine in /F5. Therefore, the doubly-charged b ions at b18 and higher are only separated in mass by 5 Da. These MS/MS spectra prove the existence of both peptides in the digest, indicating the presence of both BoNT/F4 and /F5 in this sample. It is also important to note that the peptides discussed in Figure 3 are just one example and that there are several other peptides that confirm the presence of both BoNT /F4 and /F5 in this sample, indicated in red font in Figure 2.
C. botulinum strain Af84 was reported to be a bivalent strain, producing more BoNT/A toxin than BoNT/F toxin; hence, the designation Af. We used a label-free quantitative mass spectrometric method to estimate the amount of all three toxin types present in mAb extractions from three culture supernatants grown in TPGY medium (Table 1). Through this work, we determined that the toxins are present in a mixture consisting of 88% BoNT/A2, 1% BoNT/F4, and 11% BoNT/F5. These culture supernatants had roughly 7 times as much BoNT/A2 as total BoNT/F (/F4+/F5) which is in agreement with the designation of Af for this toxin. To ensure that the ratio of toxin on the antibody-coated beads was representative of the ratio of toxin in the culture supernatant, we analyzed antibody-coated beads representing a second extraction of the sample. This second extraction yielded no evidence for toxin (data not shown).
Table 1.
Determination of the composition of BoNT in C. botulinum Af84 by MSE.
| Sample #1 | Sample #2 | Sample #3 | |
|---|---|---|---|
| Concentration of BoNT/A2 (fmol/μL) | 88 | 117 | 99 |
| Concentration of BoNT/F4 (fmol/μL) | 1.2 | 1.3 | 1.1 |
| Concentration of BoNT/F5 (fmol/μL) | 12 | 14 | 11 |
| BoNT/A2 % | 86.7 | 88.4 | 88.4 |
| BoNT/F4 % | 1.2 | 0.9 | 1.0 |
| BoNT/F5 % | 12.1 | 10.6 | 10.6 |
| Standard deviation of BoNT/A measurement | 0.97 | 0.97 | 0.97 |
| Standard deviation of BoNT/F4 measurement | 0.85 | 0.85 | 0.85 |
| Standard deviation of BoNT/F5 measurement | 0.12 | 0.12 | 0.12 |
| Coefficient of variation of BoNT/A measurement | 1.1 % | 1.1 % | 1.1 % |
| Coefficient of variation of BoNT/F4 measurement | 7.7 % | 7.7 % | 7.7 % |
| Coefficient of variation of BoNT/F5 measurement | 11.4 % | 11.4 % | 11.4 % |
Toxin concentrations for the BoNT/A2 and BoNT/F components were also determined using an electrochemiluminescence immunoassay. The type A2 toxin was present at 101 μg/ml in the crude culture supernatant and the combined concentration of the BoNT/F toxins was 16.4 μg/ml. Thus, the sample contained 87% type A and 13% type F toxin, which corresponds closely to the toxin composition determined using label free MS quantitation. While these results are based on toxin concentration, not activity ratios, the results also align closely with the reported results when comparing toxin amounts using mouse bioassays.
Having determined that C. botulinum strain Af84 contains BoNT/A2, /F4, and /F5, we then determined the enzymatic activity of these toxins, or the light chain functionality of the neurotoxin. Beads coated with antibodies to BoNT/A were used to extract the BoNT/A portion from C. botulinum Af84 and were incubated with a peptide substrate (SubA) which mimics the natural target of the light chain of BoNT/A, SNAP-25 (Figure 4A). BoNT/A cleaves this peptide at the equivalent of 196Q of SNAP-25, which results in peptides of Acetyl-RGSNKPKIDAGNQ with m/z of 1426.8 and RATRXLGGR-NH2 with m/z of 998.7. Figure 4A has peaks corresponding to both of these cleavage products (marked with asterisks), indicating that the BoNT/A in this sample is enzymatically active. Peaks at m/z 1911.1 and 1282.8 correspond to cleavage of the peptide substrate following arginines by non-specific proteases.
Figure 4.
Mass spectra indicating the light chain enzymatic activity of BoNT/A2 (A) and BoNT/F4 and /F5 (B) from C. botulinum Af84. Cleavage products indicating the active toxin are labeled as * for BoNT/A2, ˆ for BoNT/F4, and # for BoNT/F5.
In order to determine the activity of BoNT/F, beads coated with antibodies to BoNT/F were used to extract the BoNT/F portion from C. botulinum Af84 and were incubated with a peptide substrate (SubF) which mimics BoNT/F’s natural target, synaptobrevin-2. BoNT/F1-/F4 and /F6-/F7 cleave this peptide in the same location, corresponding to 58Q of synaptobrevin-213, resulting in cleavage products TSNRRLQQTQAQVDEVVDIMRVNVDKVLERDQ with m/z of 3782.9 and KLSELDDRADAL with m/z of 1345.621,26. However, BoNT/F5 cleaves this peptide in a different location, which is equivalent to 54L of synaptobrevin-27. This results in cleavage products TSNRRLQQTQAQVDEVVDIMRVNVDKVL at m/z 3254.5 and ERDQKLSELDDRADAL at m/z 1872.8.
Figure 4B is the resultant spectrum after incubating this peptide substrate with the BoNT/F extracted from C. botulinum Af84. Peaks are visible at m/z 1345.6, 1872.8, and 3254.5. The peaks at m/z 1872.8 and 3254.5 indicate the presence of enzymatically active BoNT/F5 in this sample and the peak at m/z 1345.6 indicates the presence of enzymatically active BoNT/F4 in this sample. The N-terminal cleavage product produced by cleavage of the substrate by BoNT/F4 is a long peptide with a molecular weight of 3783 Da and historically is rarely seen by MALDI-TOF/MS. However, the absence of peak could also be explained by cleavage of the BoNT/F4 N-terminal cleavage product by /F5, resulting in the peak at 1872.8 m/z.
To test this possibility, we tested the N-terminal cleavage product of BoNT/F4 as a substrate for BoNT/F5 activity. Cleavage of the peptide TSNRRLQQTQAQVDEVVDIMRVNVDKVLERDQ by BoNT/F5 should result in peaks at m/z 3254.5 and 546.3. Conversely, we also tested the C-terminal cleavage product of BoNT/F5 as a substrate for BoNT/F4 activity. BoNT/F4 cleavage of the peptide ERDQKLSELDDRADAL would result in peaks at m/z 546.3 and 1345.6. After incubation with BoNT/F4 and /F5, respectively, both peptides remain unmodified (data not shown), indicating that BoNT/F5 is not able to cleave the BoNT/F4 N-terminal cleavage product. Collectively, these peaks indicate that both forms of BoNT/F in this sample, BoNT/F4 and /F5, are enzymatically active, and that once cleaved by one subtype, the peptide substrate is not able to be cleaved by the other subtype. Additional peptide substrate cleavage experiments with C. botulinum Af84 obtained from a different laboratory also yielded evidence for the presence of three enzymatically active neurotoxins (data not shown).
Discussion
Typically, one strain of C. botulinum produces only one toxin type of BoNT. A few strains, known as bivalent strains, produce more than one serotype of toxin. In all cases prior to this report, the bivalent strains produce two different serotypes of toxin, with more of one serotype produced than the other. The known bivalent strains of C. botulinum produce BoNT/A and /B, /A and /F, or /B and /F of a single subtype. This work represents the first report of one strain of C. botulinum which produces more than two types of neurotoxins. Additionally, this is also the first report of two different subtypes of neurotoxin produced within the same strain.
Although it was reported recently that the genes for all three neurotoxins were present in C. botulinum Af8423, it was not known until this report if these genes were expressed as proteins, and if so, whether the proteins were enzymatically active. Although sequencing of the genes in a strain of C. botulinum is important, it is also important to determine the presence of the proteins produced by C. botulinum. For instance, BoNT A1(B) C. botulinum strains are abundant in the United States. These strains contain genes for both BoNT/A and BoNT/B; however, only the protein for BoNT/A is produced and functionally active. It is possible that genes may not be expressed for a variety of reasons, such as lack of a promoter, so it is important to determine which toxin proteins are actually produced and if they are enzymatically active.
Additionally, we sought to determine the amount of each toxin produced using label free MSE quantification. This method involves mAb extraction of BoNTs followed by digestion of the BoNTs into peptides and addition of a known amount of a standard protein (yeast alcohol dehydrogenase) digest. The digest of the standard protein is used as a control to calculate the quantity of specific BoNT peptides present in the digest, which potentially relates to the amount of BoNT. Because BoNT/A2, /F4, and /F5 have differences in their amino acid sequences, some of the peptides resulting from a digest of these three toxins are unique, and these peptides were used for quantification.
In this report, we demonstrate through a proteomics method that BoNT/A2, /F4, and /F5 are all produced in C. botulinum Af84. The botulinum neurotoxin protein is composed of an amino-terminal light chain polypeptide and a carboxy-terminal heavy chain polypeptide. Examining the regions for which we have MS/MS information (Figures 1 and 2), it is apparent that amino acid sequences from both the light chain and the heavy chain of the neurotoxins BoNT/A2, /F4, and /F5 are present. We do not have MS/MS information on the entire protein for any of the proteins produced by this strain of C. botulinum, but we do have sufficient information to determine that both the light and heavy chains of the three proteins (two of which are closely related) are present. The high degree of sequence variation between the two F subtypes aids in accurate determination of these two subtypes; it should be noted that this high degree of sequence variation is unlike anything else at the subtype level as BoNT/F5 is the most divergent of any of the subtypes14.
Not having MS/MS information on the entire protein is a typical situation and high sequence coverage for a protein in low abundance from complex matrices is rare. Our laboratory has previously reported on our ability to distinguish BoNT/A1 from /A2. BoNT/A1 and /A2 are more closely related than BoNT/F4 and /F5 and yet we have been able to confidently differentiate those toxins using similar sequence coverages as reported here. Furthermore, our quantification of the amount of toxins in this sample helps to explain why we have varied MS/MS information for all three toxins. Our estimation of approximately 88 times more BoNT/A2 than /F4 in this sample, determined using a label-free mass spectrometric quantitative method, is reflected in the difference in sequence coverage of these toxins. We were able to obtain sequence information on approximately 80% of the BoNT/A2 protein whereas we were only able to obtain sequence information on about 20% of the BoNT/F4 protein. As a general rule, higher sequence coverages are seen for proteins in greater abundance. This also explains why we have approximately 50% sequence coverage for BoNT/F5, whose level falls between that of BoNT/A2 and /F4.
After determining the presence of all three neurotoxin proteins in C. botulinum strain Af84, we next sought to determine the amount of each of the neurotoxins. MS-based quantification methods have often necessitated the creation of an internal standard in the form of an isotopically-labeled peptide which behaves the same as the native peptide in the mass spectrometer, but can be differentiated from the native peptide due to its increase in mass. Recently, a new quantitative label-free MS-based method known as MSE has been developed27. This method uses alternating high-energy and low-energy scan measurements to obtain information on the intact peptide in the low-energy mode and the peptide’s fragment ions in the high-energy mode. Bioinformatics software then matches the fragment ions to the corresponding intact peptide. This method has demonstrated linearity of quantification over a concentration range of 0.061–62.5 pmol/mL for a four protein mixture, and 0.48–62 pmol/mL for a mixture of the same four proteins spiked into serum27.
Through label free MSE quantification, we determined that the toxin composition of this sample is 88% BoNT/A2, 1% BoNT/F4, and 11% BoNT/F5, or 88% BoNT/A and 12% BoNT/F. Additionally, toxin composition of 87% BoNT/A and 13% BoNT/F was also determined using an electrochemiluminescence immunoassay. These findings are similar to results reported by Giménez and Ciccarelli, in which BoNT/A was found to constitute about 90% of the total toxicity of C. botulinum Af8419. It should be noted that we present here a ratio of the amounts of toxin present in the sample and not a ratio of toxicity of toxins, as was presented in earlier studies19,23. There can be difficulties in using toxin activities to determine toxin amounts. For instance, the specific activity/mg of some serotypes are known to vary by up to an order of magnitude and the specific activities of the toxins in this report, BoNT/F4 and /F5, are not known. Nevertheless, our estimation of approximately 88% BoNT/A based on toxin amount is similar to the earlier report of about 90% BoNT/A based on toxicity.
This label free MSE quantification was performed on BoNT extracted from the supernatant of C. botulinum Af84 using monoclonal antibodies to BoNT. It could be argued that the ratio of BoNT extracted from the supernatant is not the ratio of BoNT in the supernatant. However, we provide evidence that all toxin in the supernatant is extracted with the mAbs because there is no toxin post-extraction upon testing the supernatant with a second set of mAbs. Additionally, BoNT/F4 and /F5 bind equally to mAb 6F5. The epitope to 6F5 is known and is underlined in Figure 2 as peptide YNSYTSDE in BoNT/F4 and YNNYTSDE in /F5. There is only one difference in this epitope comparing /F4 to /F5, and this difference is in a location which is known to be unimportant for 6F5 binding; the four residues critical for 6F5 binding are the Y, Y, D, and E in that epitope22.
Moreover, similar results obtained from a separate assay (the MSD immunoassay) using a polyclonal equine anti-BoNT/F antibody for extraction, further support the theory that all BoNT/F was extracted from the culture supernatant. It is important to note that different culture conditions affects toxin production; sometimes in dramatic fashion28–30. In this work, we present data from two separate assays which support the BoNT composition as approximately 88% BoNT/A2 and 12% BoNT/F; however, we fully acknowledge that this composition is dependent upon culture conditions and would likely change using different culture conditions.
After determining that BoNT/A2, /F4, and /F5 proteins were produced by C. botulinum Af84, we next tested their function. This is important as function cannot be assumed from the discovery of the presence of the protein. Different factors can affect the function of a protein, only some of which can be ascertained through knowledge of the protein’s amino acid sequence. For example, the presence or absence of post-translational modifications can influence the function of a protein, and these post-translational modifications are not encoded in the amino acid sequence of the protein. Through this work, we determined that the all three neurotoxins; BoNT/A2, /F4, and /F5, are enzymatically active, displaying the expected function of these proteins.
It should be noted that the route to this discovery was simplified because all three neurotoxins have different enzymatic activity. Although BoNT/F4 and /F5 both cleave the same substrate, they cleave it in different places, producing cleavage products of different masses which can be easily identified and differentiated with the mass spectrometer. Alternate methods which monitor only cleavage of the substrate without knowledge of the location of cleavage could not distinguish between BoNT/F4 and /F5 activity. Actually, previous enzymatic activity studies with C. botulinum Af84 were performed using the mouse bioassay, and this assay was unable to determine the location of BoNT cleavage. Similarly, if BoNT/F4 and /F5 had the same enzymatic activity upon the substrate, it would have been considerably more difficult to ascertain the enzymatic activity of both toxins.
It should also be noted that, although we report here that this sample contains almost an order of magnitude less BoNT/F4 than /F5, this difference does not necessarily translate to a difference in the enzymatic activity of the light chains upon our peptide substrate. Indeed, looking at the spectrum in Figure 4B, it appears that the light chain enzymatic activity of BoNT/F4 is approximately equal to that of BoNT/F5. Ionization differences in the C-terminal cleavage products are generated by the activity of BoNT/F4 and /F5 upon the peptide substrate. Because these cleavage products seen at m/z 1345.6 and 1872.8 ionize differently in the mass spectrometer, we cannot truly estimate the ratio of BoNT/F4 to /F5 by estimating the ratio of those two peaks, but the results do suggest that perhaps BoNT/F5 has greater enzymatic activity for cleavage of this peptide mimic than BoNT/F4.
We did not test the functionality of the heavy chain in this work; however, it should be noted that the antibodies used for extraction of BoNT/A2, /F4, and /F5 are all directed against the heavy chain of the toxin, and all three toxins were successfully extracted with these antibodies. Therefore, the portion of the heavy chain which serves as the epitopes for these antibodies has the expected conformation and ability to bind antibodies. Additionally, because we observe light chain enzymatic activity from material extracted using antibodies to the heavy chain, we know that the light chain is connected to the heavy chain as expected. Based on these findings, we fully expect that the heavy chain of all three neurotoxins is also functional, yielding three completely functional BoNTs in C. botulinum Af84.
In summary, we report here that C. botulinum strain Af84 produces three neurotoxins: BoNT/A2, /F4, and /F5. This determination was made through a mass spectrometry proteomics study, carefully examining the amino acid sequence of all three neurotoxins, two of which are related as they are from the same serotype, and comparing them to the data generated during this study to ensure confident protein identifications. MSE Label free quantification of the three toxins showed that the toxin composition is 88% BoNT/A2, 1% BoNT/F4, and 11% BoNT/F5. In addition, we report that the light chains of all three neurotoxins are enzymatically active. This was determined by immunoaffinity extraction of the neurotoxins from C. botulinum Af84 by antibodies specific to the heavy chain and the addition of peptide substrates known to be cleaved by the neurotoxins. Because the cleavage of the peptide substrates was monitored by mass spectrometry, the enzymatic activity of BoNT/F4 could be distinguished from /F5, successfully proving that all three neurotoxins produced by C. botulinum strain Af84 are enzymatically active.
Acknowledgments
The authors greatly appreciate the kind gift of an additional supernatant of C. botulinum Af84 from Dr. Susan Maslanka and Dr. Brian Raphael at the Centers for Disease Control and Prevention, National Center of Emerging and Zoonotic Infectious Diseases.
Abbreviations
- BoNT
Botulinum Neurotoxin
- SNAP
synaptosomal-associated protein
- VAMP-2
vesicle-associated membrane protein
- CHCA
α-cyano-4-hydroxy cinnamic acid
- TFA
trifluoroacetic acid
- MALDI
matrix-assisted laser desorption ionization
Footnotes
The opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Centers for Disease Control and Prevention or the U.S. Army.
References
- 1.Binz T, Blasi J, Yamasaki S, Baumeister A, Link E, Sudhof TC, Jahn R, Niemann H. J Biol Chem. 1994;269:1617. [PubMed] [Google Scholar]
- 2.Blasi J, Chapman ER, Link E, Binz T, Yamasaki S, De Camilli P, Sudhof TC, Niemann H, Jahn R. Nature. 1993;365:160. doi: 10.1038/365160a0. [DOI] [PubMed] [Google Scholar]
- 3.Foran P, Lawrence GW, Shone CC, Foster KA, Dolly JO. Biochemistry. 1996;35:2630. doi: 10.1021/bi9519009. [DOI] [PubMed] [Google Scholar]
- 4.Schiavo G, Rossetto O, Catsicas S, Polverino de Laureto P, DasGupta BR, Benfenati F, Montecucco C. J Biol Chem. 1993;268:23784. [PubMed] [Google Scholar]
- 5.Schiavo G, Santucci A, Dasgupta BR, Mehta PP, Jontes J, Benfenati F, Wilson MC, Montecucco C. FEBS letters. 1993;335:99. doi: 10.1016/0014-5793(93)80448-4. [DOI] [PubMed] [Google Scholar]
- 6.Williamson LC, Halpern JL, Montecucco C, Brown JE, Neale EA. J Biol Chem. 1996;271:7694. doi: 10.1074/jbc.271.13.7694. [DOI] [PubMed] [Google Scholar]
- 7.Kalb SR, Baudys J, Webb RP, Wright P, Smith TJ, Smith LA, Fernandez R, Raphael BH, Maslanka SE, Pirkle JL, Barr JR. FEBS letters. 2012;586:109. doi: 10.1016/j.febslet.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiavo G, Benfenati F, Poulain B, Rossetto O, Polverino de Laureto P, DasGupta BR, Montecucco C. Nature. 1992;359:832. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- 9.Schiavo G, Malizio C, Trimble WS, Polverino de Laureto P, Milan G, Sugiyama H, Johnson EA, Montecucco C. J Biol Chem. 1994;269:20213. [PubMed] [Google Scholar]
- 10.Schiavo G, Shone CC, Rossetto O, Alexander FC, Montecucco C. J Biol Chem. 1993;268:11516. [PubMed] [Google Scholar]
- 11.Yamasaki S, Baumeister A, Binz T, Blasi J, Link E, Cornille F, Roques B, Fykse EM, Sudhof TC, Jahn R, et al. J Biol Chem. 1994;269:12764. [PubMed] [Google Scholar]
- 12.Yamasaki S, Binz T, Hayashi T, Szabo E, Yamasaki N, Eklund M, Jahn R, Niemann H. Biochemical and biophysical research communications. 1994;200:829. doi: 10.1006/bbrc.1994.1526. [DOI] [PubMed] [Google Scholar]
- 13.Barr JR, Moura H, Boyer AE, Woolfitt AR, Kalb SR, Pavlopoulos A, McWilliams LG, Schmidt JG, Martinez RA, Ashley DL. Emerging infectious diseases. 2005;11:1578. doi: 10.3201/eid1110.041279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raphael BH, Choudoir MJ, Luquez C, Fernandez R, Maslanka SE. Appl Environ Microbiol. 2010;76:4805. doi: 10.1128/AEM.03109-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalb SR, Baudys J, Rees JC, Smith TJ, Smith LA, Helma CH, Hill K, Kull S, Kirchner S, Dorner MB, Dorner BG, Pirkle JL, Barr JR. Analytical and bioanalytical chemistry. 2012;403:215. doi: 10.1007/s00216-012-5767-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalb SR, Goodnough MC, Malizio CJ, Pirkle JL, Barr JR. Anal Chem. 2005;77:6140. doi: 10.1021/ac0511748. [DOI] [PubMed] [Google Scholar]
- 17.Wang D, Baudys J, Rees J, Marshall KM, Kalb SR, Parks BA, Nowaczyk L, 2nd, Pirkle JL, Barr JR. Anal Chem. 2012;84:4652. doi: 10.1021/ac3006439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raphael BH, Luquez C, McCroskey LM, Joseph LA, Jacobson MJ, Johnson EA, Maslanka SE, Andreadis JD. Appl Environ Microbiol. 2008;74:4390. doi: 10.1128/AEM.00260-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gimenez DF, Ciccarelli AS. Zentralblatt fur Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene. 1. Abt. Medizinisch-hygienische Bakteriologie, Virusforschung und Parasitologie Originale. 1970;215:212. [PubMed] [Google Scholar]
- 20.Maslanka SE, Luquez C, Raphael BR, Dykes JK, Joseph LA. The Botulinum Journal. 2011;2:72. [Google Scholar]
- 21.Kalb SR, Smith TJ, Moura H, Hill K, Lou J, Geren IN, Garcia-Rodriguez C, Marks JD, Smith LA, Pirkle JL, Barr JR. International Journal of Mass Spectrometry. 2008;278:101. [Google Scholar]
- 22.Kalb SR, Garcia-Rodriguez C, Lou J, Baudys J, Smith TJ, Marks JD, Smith LA, Pirkle JL, Barr JR. PLoS One. 2010;5:e12237. doi: 10.1371/journal.pone.0012237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dover N, Barash JR, Hill KK, Davenport KW, Teshima H, Xie G, Arnon SS. PLoS One. 2013;8:e61205. doi: 10.1371/journal.pone.0061205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang DX, Baudys J, Ye YM, Rees JC, Barr JR, Pirkle JL, Kalb SR. Analytical biochemistry. 2013;432:115. doi: 10.1016/j.ab.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalb SR, Lou J, Garcia-Rodriguez C, Geren IN, Smith TJ, Moura H, Marks JD, Smith LA, Pirkle JL, Barr JR. PLoS ONE. 2009;4:e5355. doi: 10.1371/journal.pone.0005355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalb SR, Baudys J, Egan C, Smith TJ, Smith LA, Pirkle JL, Barr JR. Appl Environ Microbiol. 2011;77:1301. doi: 10.1128/AEM.01662-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levin Y, Hradetzky E, Bahn S. Proteomics. 2011;11:3273. doi: 10.1002/pmic.201000661. [DOI] [PubMed] [Google Scholar]
- 28.Briozzo J, Delagarde EA, Chirife J, Parada JL. Applied and environmental microbiology. 1986;51:844. doi: 10.1128/aem.51.4.844-848.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graham AF, Lund BM. Journal of Applied Bacteriology. 1987;63:387. doi: 10.1111/j.1365-2672.1987.tb04859.x. [DOI] [PubMed] [Google Scholar]
- 30.Lund BM, Graham AF, Franklin JG. International journal of food microbiology. 1987;4:215. [Google Scholar]


