Abstract
Crystalline silica has been classified as a human carcinogen by the International Agency for Research on Cancer (Lyon, France); however, few previous studies have provided quantitative data on silica exposure, silicosis, and/or smoking. We investigated a cohort in China (in 1960–2003) of 34,018 workers without exposure to carcinogenic confounders. Cumulative silica exposure was estimated by linking a job-exposure matrix to work history. Cox proportional hazards model was used to conduct exposure-response analysis and risk assessment. During a mean 34.5-year follow-up, 546 lung cancer deaths were identified. Categorical analyses by quartiles of cumulative silica exposure (using a 25-year lag) yielded hazard ratios of 1.26, 1.54, 1.68, and 1.70, respectively, compared with the unexposed group. Monotonic exposure-response trends were observed among nonsilicotics (P for trend < 0.001). Analyses using splines showed similar trends. The joint effect of silica and smoking was more than additive and close to multiplicative. For workers exposed from ages 20 to 65 years at 0.1 mg/m3 of silica exposure, the estimated excess lifetime risk (through age 75 years) was 0.51%. These findings confirm silica as a human carcinogen and suggest that current exposure limits in many countries might be insufficient to protect workers from lung cancer. They also indicate that smoking cessation could help reduce lung cancer risk for silica-exposed individuals.
Keywords: cohort, exposure-response analysis, lung cancer, risk assessment, silica, silicosis
Crystalline silica is one of the most common minerals and a common particulate air pollutant in both working and living environments. Occupational exposure frequently occurs in a variety of industries, such as metal and coal mining, construction, and clay manufacturing. Recent reports have indicated that more than 1.7 million workers in the United States (1), more than 2 million in Europe (2, 3), and more than 23 million in China (4) have been occupationally exposed to crystalline silica dust. In ambient air, crystalline silica can be easily generated from industrial operations, volcanic explosions, and sandstorms. The adverse health effects of silica exposure represent an important global public health concern.
Lung cancer is considered one of the serious consequences of silica exposure. The association has been studied for many decades (5–9).In 1997, the International Agency for Research on Cancer (Lyon, France) classified silica as “carcinogenic to humans” (10). However, the working group also stated that the carcinogenicity was not found in all industrial circumstances, and the conclusion remained somewhat controversial (11) because few published studies could provide quantitative exposure-response trends to support causal inference. In 2009, another working group from the International Agency for Research on Cancer focused on exposure-response studies and a pooled analysis of 10 cohort studies (12) and concluded that “crystalline silica in the form of quartz or cristobalite dust causes cancer of the lung” (13). Nonetheless, some critics persist in the view that the weight of evidence from occupational epidemiology does not support a casual association of lung cancer and silica exposure (14).
The role of silicosis in the development of lung cancer associated with silica exposure remains controversial (15). Most epidemiologic studies have consistently observed higher risk of lung cancer among silicotics but detected no higher risk or slightly higher risk among nonsilicotics (16–19). When silicosis cases are excluded, epidemiologic data from many studies might be insufficient to detect elevated lung cancer risk due to silica exposure (7, 19). Thus, the carcinogenic role of silica in the absence of silicosis needs further evaluation (16, 17, 20, 21).
Cigarette smoking is an important potential confounding factor in the evaluation of the carcinogenicity of crystalline silica. However, many studies have not been able to adequately control for its confounding effect because of difficulty collecting detailed smoking data for each participant (22, 23). Furthermore, the joint effect of smoking and silica exposure remains unclear (24). Studies with smoking data often have too few lung cancer deaths among never smokers to adequately investigate this issue.
In the late 1980s, a large cohort of 74,040 workers from 29 Chinese metal mines and pottery factories was established in China (9, 25). Here, we focus on a subcohort of 34,018 workers who were unlikely to have been exposed to other carcinogenic confounders, such as radon, polycyclic aromatic hydrocarbons, and arsenic. We extended prior analyses to 2003 and conducted a quantitative exposure-response analysis and risk assessment for lung cancer, taking into consideration smoking, as well as silicosis. In addition, we investigated the joint effect of silica exposure and smoking in the development of lung cancer.
MATERIALS AND METHODS
Study population
The Chinese silica cohort has been described elsewhere (9, 25). Briefly, the cohort included 74,040 workers who worked at 29 metal mines and pottery factories for 1 year or more between 1960 and 1974. All participants were followed up until they were lost to follow-up, died, or survived to 2003. Data on demography, lifestyle, work history, silicosis status, and cause of death were collected by trained investigators from 1986 onward. Monitoring of dust concentrations, polycyclic aromatic hydrocarbons, radon, and other occupational hazards was conducted (9).
In this study, we excluded 8,268 workers without detailed work histories. Participants without detailed smoking data were also excluded (n = 23,200). To minimize the effects of other carcinogenic confounders, we excluded 8,554 participants from copper mines (where exposure to radon and carcinogenic polycyclic aromatic hydrocarbons may occur) and tin mines (where exposure to arsenic may occur) (26). Finally, this study includes 34,018 participants from 7 metal mines and 4 pottery factories with an average of 34.5 years of follow-up.
Ascertainment of lung cancer deaths and silicosis cases
All participants were traced for vital status during the followup period. Information on underlying causes of death was obtained on the basis of the following 3 levels of evidence: medical records from a hospital (60.5%); employment registers, accident records, or death certificates (35.2%); or oral reports from relatives (4.3%) (9). For participants who died from lung cancer, the diagnostic information was reconfirmed by using hospitals records (9, 27).
Yearly radiographs for workers exposed to silica dust have been required by the Chinese government since 1963, and silicosis diagnoses were included in a silicosis registry. National diagnostic criteria for silicosis were standardized as stage I, II, or III. Silicosis was defined as stage I or higher. The agreement was 89.3% between the presence of radiological silicosis diagnosed by the International Labour Office (28) and Chinese criteria (29).
Silica exposure assessment
We produced quantitative estimates of silica exposure by using historical data on dust concentrations and work histories (9). Total dust concentrations were available since 1950. A field study was conducted to convert Chinese total dust concentrations to silica concentrations (9, 30). A job-exposure matrix with facility-, job-, and year-specific silica concentrations was then created. Lifetime work histories were retrospectively collected in 1986 and then updated yearly by industrial hygienists using employee rosters during follow-up. By linking the job-exposure matrix with the work history, cumulative silica exposure (in mg/m3-years) was calculated as follows:
where n is the total number of job titles, Ci is the silica concentration for the ith job title, and Ti is the working years for the ith job title (9).
Smoking information
Detailed lifetime smoking data were collected in 1986, 1995, and 2004. Overall, smoking data from next-of-kin or colleagues accounted for 11% of the study subjects. Data reliability was examined for 1,990 randomly selected subjects in 2004. The agreement on smoking status (yes or no) from next-of-kin and colleagues of decedents (n = 602) was 89.1%, and the agreement on smoking status from self-report and next-of-kin (or colleagues) for living subjects was 93.6%. The smoking data included the average number of cigarettes per day and the corresponding start and end dates, taking into consideration smoking intensity. The smoking amount for ever smokers of all smoking intensities was calculated by multiplying packs per day by smoking duration.
Statistical analysis
Quantitative exposure-response analyses for silica exposure and lung cancer were conducted by using Cox proportional hazard models. We used age to define the risk set for each lung cancer death (31). The association was quantified by hazard ratios and their 95% confidence intervals with adjustment for potential confounding factors including facility, sex, year of birth, and smoking amount. We considered lag periods of 0, 5, 10, 15, 20, and 25 years for cumulative silica exposure. We used a minimized Akaike’s information criterion statistic to select the optimal exposure-response models.
We conducted categorical analysis by quartiles of cumulative silica exposure. The overall risk of silica exposure was examined by including silica exposure as a dichotomous variable (exposed/unexposed). Continuous models were conducted by using unlogged or logged cumulative silica exposure. In a nested case-control sample, we used penalized splines to investigate the shape of the exposure-response relationship, avoiding parametric assumptions (32, 33). The association was also evaluated after exclusion of silicotics.
To investigate the joint effect of silica and smoking, we estimated hazard ratios by crossed dichotomized silica exposure (exposed = A+, unexposed = A−) and smoking (ever smokers = B+, never smokers = B−). As suggested by Li and Chambless (34), the relative excess risk due to interaction (calculated as hazard ratio (HR)A+B+ – HRA+B− – HRA–B+ + 1) was used to evaluate departure from additivity (35). Departure from multiplicativity was examined by adding an interaction term of silica exposure (A+/A−) and smoking (B+/B−) to the model. A model with an interaction term of continuous exposure and smoking (B+/B−) was used to assess the multiplicative joint effect.
Risk assessment was conducted by using the results from the models and converting rates to risk. Excess lifetime risk of lung cancer was estimated by assuming an exposure of 0.1 mg/m3 for 45 years (from ages 20 to 65 years), and a lifetime was defined as 55 years (from ages 20 to 75 years). The 0.1-mg/m3 level is the compliance level for respirable silica exposure in the workplace published by the US Occupational Safety and Health Administration (Washington, DC). We also considered risks at 0.02 and 0.01 mg/m3, as well as the occupational exposure limits in China, which range from 0.07 to 0.35 mg/m3 depending on the percentage of crystalline silica. Age specific background mortality rates for lung cancer (4) and all causes of death in the general population were adjusted (36). The penalized splines were fitted in S-PLUS, version 8.0, software (Insightful Corporation, Seattle, Washington); all other statistical analyses were conducted by using SAS, version 9.3, software (SAS Institute Inc., Cary, North Carolina). All statistical tests were 2-sided.
RESULTS
Table 1 presents selected characteristics of the cohort subjects. The cohort included 34,018 participants from 6 tungsten mines (n = 19,007), 1 iron mine (n = 7,663), and 4 pottery factories (n = 7,348), 23,628 of whom were silica-exposed workers. More than 86% of the cohort was male. A total of 1,527 (4.5%) workers were lost to follow-up; their person-time was accordingly truncated at time of loss.
Table 1.
Selected Characteristics of the Cohort by Cumulative Silica Exposure, China, 1960–2003
| Characteristic | Entire Cohort |
Cumulative Silica Exposurea, mg/m3-years |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | No. | % | 0 |
0.01 to <1.12 |
1.12 to <2.91 |
2.91 to <6.22 |
≥6.22 |
||||||||||||
| Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | |||||
| No. of subjects | 34,018 | 10,390 | 7,687 | 5,420 | 4,866 | 5,655 | |||||||||||||
| No. of male subjects | 29,297 | 86.1 | 7,444 | 71.6 | 7,247 | 94.3 | 5,045 | 93.1 | 4,342 | 89.2 | 5,219 | 92.3 | |||||||
| Year of birth | |||||||||||||||||||
| Before 1930 | 8,602 | 25.3 | 1,938 | 18.7 | 951 | 12.4 | 1,105 | 20.4 | 1,581 | 32.5 | 3,027 | 53.5 | |||||||
| 1930 to before 1940 | 12,400 | 36.5 | 3,465 | 33.4 | 1,926 | 25.1 | 2,290 | 42.3 | 2,627 | 54.0 | 2,092 | 37.0 | |||||||
| 1940 to before1950 | 8,654 | 25.4 | 3,157 | 30.4 | 3,159 | 41.1 | 1,445 | 26.7 | 507 | 10.4 | 386 | 6.8 | |||||||
| 1950 or later | 4,362 | 12.8 | 1,830 | 17.6 | 1,651 | 21.5 | 580 | 10.7 | 151 | 3.1 | 150 | 2.7 | |||||||
| Age at entry, years | 27.1 (8.2) | 25.6 (8.0) | 24.4 (6.4) | 26.1 (7.1) | 28.9 (7.6) | 32.7 (8.9) | |||||||||||||
| Age at end of follow-up, years | 61.5 (11.7) | 59.6 (13.0) | 58.9 (10.4) | 62.6 (9.9) | 64.6 (10.8) | 65.0 (11.7) | |||||||||||||
| Duration of follow-up, years | 34.5 (10.1) | 34.1 (10.5) | 34.5 (8.5) | 36.6 (8.5) | 35.8 (10.4) | 32.3 (12.0) | |||||||||||||
| Smoking amountb, packyears | |||||||||||||||||||
| 0 | 12,171 | 35.8 | 4,960 | 47.7 | 2,312 | 30.1 | 1,660 | 30.6 | 1,518 | 31.2 | 1,721 | 30.4 | |||||||
| 0.01 to <26.76 | 8,299 | 24.4 | 1,988 | 19.1 | 1,977 | 25.7 | 1,215 | 22.4 | 1,248 | 25.6 | 1,871 | 33.1 | |||||||
| 26.76 to <34.93 | 4,676 | 13.7 | 1,169 | 11.3 | 1,492 | 19.4 | 749 | 13.8 | 585 | 12.0 | 681 | 12.0 | |||||||
| 34.93 to <44.68 | 4,549 | 13.4 | 1,113 | 10.7 | 1,050 | 13.7 | 931 | 17.2 | 710 | 14.6 | 745 | 13.2 | |||||||
| ≥44.68 | 4,323 | 12.7 | 1,160 | 11.2 | 856 | 11.1 | 865 | 16.0 | 805 | 16.5 | 637 | 11.3 | |||||||
| Mean pack-years for ever smokers |
32.8 (15.9) | 33.0 (15.9) | 32.1 (15.9) | 34.9 (16.3) | 34.0 (16.5) | 30.5 (14.4) | |||||||||||||
| Duration of silica exposure, years |
13.4 (12.4) | NA | 15.2 (10.1) | 19.5 (9.6) | 19.5 (9.6) | 24.3 (9.7) | |||||||||||||
| No. of silicosis cases | 5,297 | 15.6 | 0 | 0 | 236 | 3.1 | 663 | 12.2 | 1,566 | 32.2 | 2,832 | 50.1 | |||||||
Abbreviations: NA, not applicable; SD, standard deviation.
Cutpoints defined by quartiles of cumulative silica exposure among lung cancer cases who were ever exposed to silica.
Cutpoints defined by quartiles of smoking amount among lung cancer cases who ever smoked.
At the end of follow-up, 85.9% of all participants had died or retired; only 1,376 workers (4.0%) were still working. We identified 11,377 deaths, including 546 deaths from lung cancer, 418 of which were ever exposed to silica. The overall crude mortality rate of lung cancer was 46.5 per 100,000 person-years, with mortality rates of 51.0 and 36.2 per 100,000 person-years among workers with or without silica exposure, respectively. We identified 5,297 silicosis cases during the follow-up period.
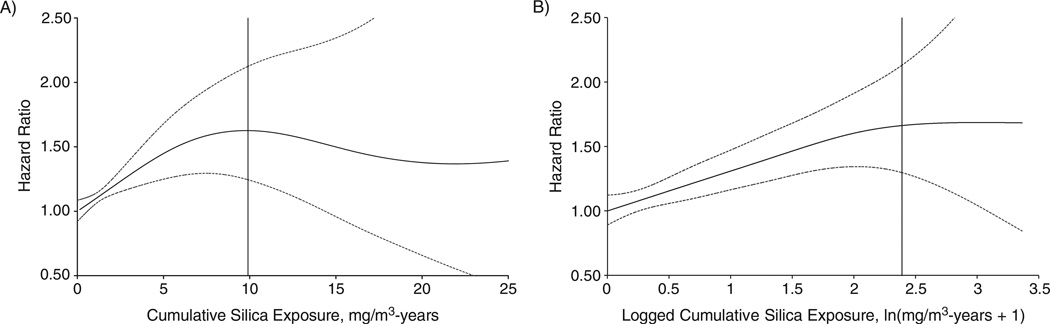
As shown in Table 2, both continuous and categorical analyses suggested positive exposure-response associations between silica exposure and lung cancer. The strongest gradient in risk was observed for 25-year lagged silica exposure. In continuous models, there were significantly positive trends. The logged cumulative silica exposure fit those data better than cumulative exposure itself, which is typical of exposure-response trends, which attenuate at higher exposures (37). The categorical analysis showed increasing hazard ratios with increasing quartiles of cumulative silica exposure (hazard ratios = 1.26, 1.54, 1.68, and 1.70, respectively). Compared with the unexposed group, ever-exposed workers had an overall 44% (95% confidence interval (CI): 18%, 76%) increase in lung cancer risk. Adjustment for smoking did not materially change the association. The penalized spline model using cumulative silica exposure showed similar monotonically increased risk when cumulative silica exposure was lower than approximately 8 mg/m3-years and plateaued afterward (Figure 1A); the spline model produced a linear trend (Plinear = 0.002; Pnonlinear = 0.21) when the logged cumulative silica exposure was used (Figure 1B). Overall, we observed similar associations of silica exposure and lung cancer risk in the absence of silicosis (Table 3) after exclusion of 15% of the cohort who had silicosis, among whom there were 119 lung cancer deaths.
Table 2.
Hazard Ratios for Cumulative Silica Exposure Associated With Lung Cancer Death, China, 1960–2003
| Exposure Lag Period | No. | Continuous |
Quartilesa , mg/m3-years |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 mg/m3-years | 1 ln(mg/m3- years + 1) |
0.01 to <1.12 | 1.12 to <2.91 | 2.91 to <6.22 | ≥6.22 | ||||||||||||
| HR | 95% CI | HR | 95% CI | No. | HR | 95% CI | No. | HR | 95% CI | No. | HR | 95% CI | No. | HR | 95% CI | ||
| No lag | |||||||||||||||||
| Lung cancer deaths | 546 | 105 | 104 | 104 | 105 | ||||||||||||
| Subjects at risk | 34,018 | 7,687 | 5,420 | 4,865 | 5,656 | ||||||||||||
| Cumulative silica exposureb | 1.02 | 1.00, 1.04 | 1.20 | 1.07, 1.35 | 1.12 | 0.86, 1.46 | 1.55 | 1.18, 2.04 | 1.63 | 1.22, 2.17 | 1.52 | 1.13, .06 | |||||
| 15-Year lag | |||||||||||||||||
| Lung cancer deaths | 546 | 115 | 99 | 96 | 98 | ||||||||||||
| Subjects at risk | 34,018 | 8,127 | 4,851 | 4,862 | 5,094 | ||||||||||||
| Cumulative silica exposureb | 1.02 | 1.00, 1.05 | 1.20 | 1.07, 1.35 | 1.14 | 0.88, 1.47 | 1.59 | 1.21, 2.09 | 1.50 | 1.12, 2.00 | 1.61 | 1.19, 2 .19 | |||||
| 25-Year lag | |||||||||||||||||
| Lung cancer deaths | 546 | 128 | 84 | 96 | 75 | ||||||||||||
| Subjects at risk | 34,018 | 8,765 | 4,179 | 4,659 | 3,907 | ||||||||||||
| Cumulative silica exposureb | 1.03 | 1.00, 1.06 | 1.24 | 1.10, 1.40c | 1.26 | 0.98, 1.60 | 1.54 | 1.16, 2.05 | 1.68 | 1.26, 2.24 | 1.70 | 1.23, 2 .34 | |||||
Abbreviations: CI, confidence interval; HR, hazard ratio.
Cutpoints of cumulative silica exposure were defined by quartiles of cumulative silica exposure among lung cancer cases who were ever exposed to silica.
Cumulative silica exposure was included in all models as a time-dependent variable. All models were adjusted for sex, facility, year of birth (continuous, time-dependent) and smoking amount (categories of 0, 0.01 to <26.76, 26.76 to <34.93, 34.93 to <44.68, and ≥44.68 pack-years, time-dependent). Subjects without crystalline silica exposure were considered the reference category.
Model with lowest Akaike’s information criterion statistic.
Figure 1.
Hazard ratios of lung cancer as a smooth function of A) unlogged and B) logged cumulative silica exposure estimated by penalized spline models (df = 2), China, 1960–2003. Solid lines represent hazard ratios of 25-year lagged cumulative silica exposure, with dotted lines indicating the 95% confidence interval. The vertical solid line represents the 95th percentile of cumulative silica exposure. For simplicity of presentation, the reference value of silica exposure was set to 0.
Table 3.
Hazard Ratios for Cumulative Silica Exposure Associated With Lung Cancer Death in the Absence of Silicosis, China, 1960–2003
| Exposure Lag Period | No. | Continuous |
Quartilesa, mg/m3-years |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 mg/m3-years | 1 ln(mg/m3- years + 1) |
0.01 to <1.12 | 1.12 to <2.91 | 2.91 to <6.22 | ≥6.22 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HR | 95% CI | HR | 95% CI | No. | HR | 95% CI | No. | HR | 95% CI | No. | HR | 95% CI | No. | HR | 95% CI | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| No lag | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lung cancer deaths | 427 | 89 | 75 | 70 | 65 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Subjects at risk | 28,721 | 7,451 | 4,757 | 3,300 | 2,823 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cumulative silica exposureb | 1.02 | 0.99, 1.05 | 1.17 | 1.03, 1.34 | 1.02 | 0.77, 1.35 | 1.26 | 0.93, 1.70 | 1.40 | 1.02, 1.93 | 1.50 | 1.07, 2.13 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 15-Year lag | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lung cancer deaths | 427 | 92 | 77 | 62 | 59 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Subjects at risk | 28,721 | 7,797 | 4,124 | 3,272 | 2,574 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cumulative silica exposureb | 1.02 | 0.99, 1.06 | 1.18 | 1.03, 1.35 | 0.99 | 0.75, 1.29 | 1.42 | 1.06, 1.92 | 1.28 | 0.92, 1.78 | 1.65 | 1.16, 2.34 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 25-Year lag | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lung cancer deaths | 427 | 102 | 63 | 65 | 41 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Subjects at risk | 28,721 | 8,325 | 3,478 | 3,140 | 1,970 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cumulative silica exposureb | 1.03 | 1.00, 1.07 | 1.25 | 1.08, 1.44c | 1.12 | 0.86, 1.46 | 1.41 | 1.03, 1.93 | 1.58 | 1.14, 2.19 | 1.70 | 1.15, 2.52 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Abbreviations: CI, confidence interval; HR, hazard ratio.
Cutpoints of cumulative silica exposure were defined by quartiles of cumulative silica exposure among lung cancer cases who were ever exposed to silica.
Cumulative silica exposure was included in all models as a time-dependent variable. All Cox proportional hazards models were conducted by using risk sets with only observations that were not diagnosed as silicosis before the index age. All models were adjusted for sex, facility, year of birth (continuous, time-dependent) and smoking amount (categories of 0, 0.01 to <26.76, 26.76 to <34.93, 34.93 to <44.68, and ≥44.68 pack-years, time-dependent). Subjects without crystalline silica exposure were considered the reference category.
Model with lowest Akaike’s information criterion statistic.
After adjustment for potential confounders, including smoking, but without silica exposure in the model, results indicated that the presence of silicosis was associated with an overall 61% (95% CI: 29%, 103%) increase in lung cancer risk. In our study, the mean cumulative silica exposures for silicotic and nonsilicotic subjects were 7.4 (standard deviation, 5.1) and 3.1 (standard deviation, 3.7) mg/m3-years, respectively, indicating that the presence of silicosis is a marker for high silica exposure.
Table 4 shows the results of the joint effect of silica exposure and smoking in relationship to lung cancer death. In dichotomized analyses, the relative excess risk due to interaction was 0.98 (95% CI: 0.23, 1.74), indicating a joint effect that is more than additive. The interaction term of silica and smoking was not significant (P = 0.25), suggesting that the hypothesis of multiplicative interaction between silica exposure and smoking cannot be rejected. Similar results were found when exposed and unexposed levels were defined as 1.12 mg/m3-years or more and as less than 1.12 mg/m3-years, respectively. Inclusion of an interaction of smoking (never/ever smoking) and continuous unlogged or logged cumulative silica exposure in the model caused the interaction terms again to fall short of statistical significance (P = 0.48 and P = 0.64, respectively).
Table 4.
Joint Effect of Silica Exposure and Smoking on Lung Cancer Risk, China, 1960–2003
| Cumulative Silica Exposure, mg/m3-years |
Smoking Status |
Test for Multiplicativitya | Test for Additivityb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Never Smokers |
Ever Smokers |
|||||||||||
| No. of Lung Cancer Deaths |
No. of Subjects at Risk |
HR | 95% CI | No. of Lung Cancer Deaths |
No. of Subjects at Risk |
HR | 95% CI | Point Estimate |
95% CI | RERI | 95 % CI | |
| 0 | 27 | 4,960 | 1.0 | NA | 101 | 5,430 | 2.75 | 1.74, 4.35 | ||||
| >0 | 50 | 7,211 | 1.10 | 0.68, 1.78 | 368 | 16,417 | 3.83 | 2.48, 5.90 | 1.27 | 0.75, 2.15 | 0.98 | 0.23 , 1.74 |
| <1.12 | 34 | 7,285 | 1.0 | NA | 199 | 10,850 | 3.42 | 2.32, 5.05 | ||||
| ≥1.12 | 43 | 4,886 | 1.60 | 1.01, 2.55 | 270 | 10,997 | 5.07 | 3.41, 7.52 | 0.93 | 0.57, 1.51 | 1.05 | 0.05, 2.05 |
Abbreviations: CI, confidence interval; HR, hazard ratio; NA, not applicable; RERI, relative excess risk due to interaction.
Estimated by adding an interaction term of ever-exposed and ever-smoking in the Cox proportional hazards model. A point estimate greater than 1 indicates a joint effect more than multiplicative. All models were adjusted for sex, facility, and year of birth (continuous, time-dependent).
Estimated by using RERI. RERI greater than 0 indicates a joint effect more than additive. All models were adjusted for sex, facility, and year of birth (continuous, time-dependent).
Based on results of the spline model (25-year lag), the excess lifetime risk (through age 75 years) of lung cancer, with exposure to 0.1 mg/m3 of silica from ages 20 to 65 years, was estimated to be 0.51% (95% CI: 0.34%, 0.68%) above a background risk of 3.78% in China in 2010. If silica exposure was assumed to be 0.02 or 0.01 mg/m3 for 45 years, the excess lifetime risks decreased to 0.10% and 0.05%, respectively. The estimated excess lifetime risks ranged from 0.35% (95% CI: 0.23%, 0.48%) to 1.60% (95% CI: 0.83%, 2.45%) for respirable silica levels between 0.07 mg/m3 and 0.35 mg/m3 (the occupational silica exposure limit in China). The exposure level should be under 0.04 mg/m3 to keep the excess lifetime risk within 0.1%. The use of the result from the best-fitting model using logged cumulative silica exposure (25-year lag) showed slightly higher lifetime excess risk (0.74%) at an exposure of 0.1 mg/m3.
DISCUSSION
In this study, we conducted quantitative exposure-response analyses on the basis of 546 lung cancer deaths with detailed data on historical silica exposure and smoking, and we minimized possible bias caused by carcinogenic confounders. We found a positive exposure-response association between silica exposure and lung cancer risk, although the positive trend was relatively moderate. A positive exposure-response trend was also found among subjects without silicosis, indicating that silicosis was not an essential prerequisite for silica-induced lung cancer. We also found a joint effect of silica exposure and smoking, which is more than additive and close to multiplicative. The excess lifetime risk of death from lung cancer due to silica exposure was much higher than the 0.1% standard suggested by the US Occupational Safety and Health Administration (38).
We found a similar association between silica exposure and lung cancer in this study as that found by Steenland et al. (12) in a pooled analysis of 10 cohort studies. The model with continuous logged cumulative silica exposure fit the best in both studies. The coefficient for logged cumulative exposure (15-year lag) in the study by Steenland et al. (12) was 0.062, whereas here, the corresponding coefficient was 0.055 (0.065 with a 25-year lag). Our categorical results were also similar, producing hazard ratios of 1.5–1.7 for the highest exposure category (>6.2 mg/m3-years) compared with the unexposed category. The pooled analysis produced odds ratios of 1.5 and 1.7, respectively, for the highest 2 categories of exposure (5.4–12.8 and >12.8 mg/m3-years) compared with a low-exposure referent (<0.4 mg/m3-years). Another quantitative analysis by Rice et al. (39) produced a rate ratio of 1.6 for lung cancer for those with mean cumulative silica exposure (2.16 mg/m3-years); in contrast, our best-fitting model produced a slightly lower hazard ratio of 1.3 for the same cumulative silica exposure. When comparing exposed with unexposed groups, we showed that silica exposure was associated with an overall 44% increase in lung cancer risk, which was slightly higher than that of other studies. A 37% increase in lung cancer deaths due to silica exposure was reported in a multicenter case-control study by Cassidy at al. (40). Kurihara et al. (17) estimated an analogous 32% increase in lung cancer risk in their study.
We found clear exposure-response trends between silica exposure and lung cancer at lower silica exposure levels, but the trends were attenuated at higher levels (12, 32). There are several possible reasons, including the healthy worker survivor effect, which refers to a depletion of the number of susceptible people in the population at high exposure levels and less reliable estimates at those levels (12, 37). However, the monotonic increase in risk covered the first percentile to greater than the 95th percentile of cumulative silica exposure (Figure 1).
Whether silicosis is necessary for silica to induce lung cancer has been a controversial topic for many years. Previous studies suggested that it was difficult to distinguish any causal role of silicosis independent of silica exposure, because silicosis serves as a marker of high silica exposure (19, 20). Most of the previous studies focusing on silica and lung cancer have not excluded silicosis, which, if removed from the analysis, might have resulted in lower risk estimates (12, 24). With sufficient data, our study breaks new ground in showing that positive exposure-response trends exist between lung cancer and silica exposure in the absence of silicosis.
The joint effect of silica and smoking on lung cancer risk has seldom been quantitatively evaluated in previous studies. Our study indicated that the joint effect between silica exposure and smoking was greater than additive. This result is consistent with a study of South African gold miners, which suggested that the 2 factors played synergistic roles (6). Furthermore, our results suggest that the joint effect was close to multiplicative. Similarly, a multicenter case-control study did not observe any joint effect beyond a multiplicative model between smoking and silica exposure (40). Our results are very similar to a more recent pooled analysis, which concluded that the joint effect of silica and smoking was between additive and multiplicative, perhaps closer to the latter; however, the authors could not ascertain the joint effect of silica exposure and smoking because of small numbers of lung cancer among never smokers (8). The assessment of the joint effect of silica exposure and smoking has important public health implications. If a joint effect does exist, smoking cessation would probably be an effective approach to lowering lung cancer risk for silica-exposed workers, especially for those with high silica exposure.
We found that the excess lifetime risk of lung cancer was 0.51% for those exposed to respirable silica dust at the US Occupational Safety and Health Administration standard of 0.1 mg/m3 for 45 years. The excess risk was lower than the 1.1% estimated by Steenland et al. (12), which may be because of the lower background mortality rates for lung cancer and all causes and the longer lag periods (25 vs. 15 years). Nonetheless, the estimated excess lifetime risk in both China and the United States was much higher than 0.1%, which is the acceptable excess risk suggested by the US Occupational Safety and Health Administration. Our study suggests that the current occupational silica exposure limits used by many countries might be insufficient to protect workers from lung cancer.
Our study has several major strengths. First, the cohort was large and was followed up for a long period (34.5 years). Second, we minimized possible carcinogenic confounders by excluding those who worked in tin or copper mines. Third, we collected detailed data on silica exposure, silicosis, and smoking and included these data in the analyses as time-dependent variables. The job-exposure matrix provided sufficient information for the exposure assessment of crystalline silica as demonstrated by a monotonic exposure-response trend in our prior study of silicosis (41). The sufficient data allowed us to investigate the association of silica with lung cancer with consideration of silicosis and smoking.
One limitation of our study is that the silica concentrations before 1950 were estimated by using the concentrations in 1950. This might have led to underestimates of silica exposure for those who started working before 1950. However, when we conducted the same analyses after excluding the subjects whose crystalline silica exposure occurred before 1950 (n = 3,738), the model with logged cumulative silica exposure fit the data best, producing a hazard ratio of 1.23, which was very close to the hazard ratio of 1.24 for all subjects. Second, the cigarette smoking data for deceased subjects were obtained from next-of-kin or colleagues, and recall bias might apply. However, smoking did not appear to be a confounder in our data. The hazard ratio for logged cumulative silica exposure (25-year lag) without adjustment for smoking was 1.27, which was very close to the hazard ratio of 1.24 with adjustment for smoking. Third, exposure to 3 types of silica dust (from tungsten mines, iron mines, and pottery factories) is examined in this study, although the separate associations were likely to be homogenous. Caution should be taken when considering the association of silica exposure and lung cancer in different circumstances. Finally, we did not consider the use of personal protective equipment. However, personal protective equipment were rarely used (by <5% of subjects) or used improperly, indicating that the use of personal protective equipment had little effect on the results.
The results of the present study, which was conducted in a large population with a long period of observation, confirm that silica exposure is associated with a significant increase in lung cancer risk, even in those without silicosis, and that a joint effect greater than additive was detected between silica and smoking in the development of lung cancer. The results have important implications for public health. The current occupational exposure limits in many countries may need to be lowered to protect silica-exposed workers from lung cancer. Also, smoking cessation may be an effective way to reduce lung cancer risk for silica-exposed smokers.
ACKNOWLEDGMENTS
This study was supported by the National Basic Research Program of China (grant 2011CB503804).
We thank Dr. Tongzhang Zheng at the Yale School of Public Health and Dr. Frank B. Hu at the Harvard School of Public Health for providing valuable comments.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the funding organizations.
Abbreviations
- CI
confidence interval
- HR
hazard ratio
Footnotes
Conflict of interest: none declared.
REFERENCES
- 1.National Institute for Occupational Safety and Health. Health Effects of Occupational Exposure to Respirable Crystalline Silica. Washington, DC: National Institute for Occupational Safety and Health; 2002. (DHHS publication no. 2002–129) [Google Scholar]
- 2.European Commission. Millions of workers’ health to be protected by Europe’s first multisector agreement. Brussels, Belgium: European Commission; 2006. [Accessed June 3, 2013]. ( http://europa.eu/rapid/press-release_IP-06-524_en.htm?locale=en) [Google Scholar]
- 3.Maciejewska A. Occupational exposure assessment for crystalline silica dust: approach in Poland and worldwide. Int J Occup Med Environ Health. 2008;21(1):1–23. doi: 10.2478/v10001-008-0010-3. [DOI] [PubMed] [Google Scholar]
- 4.Ministry of Health. China’s Health Statistics Yearbook 2011. Beijing, China: Peking Union Medical College Press; 2011. [Google Scholar]
- 5.Lynge E, Kurppa K, Kristofersen L, et al. Silica dust and lung cancer: results from the Nordic occupational mortality and cancer incidence registers. J Natl Cancer Inst. 1986;77(4):883–889. [PubMed] [Google Scholar]
- 6.Hnizdo E, Sluis-Cremer GK. Silica exposure, silicosis, and lung cancer: a mortality study of South African gold miners. Br J Ind Med. 1991;48(1):53–60. doi: 10.1136/oem.48.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amandus H, Costello J. Silicosis lung cancer in U.S. metal miners. Arch Environ Health. 1991;46(2):82–89. doi: 10.1080/00039896.1991.9937433. [DOI] [PubMed] [Google Scholar]
- 8.Vida S, Pintos J, Parent ME, et al. Occupational exposure to silica and lung cancer: pooled analysis of two case-control studies in Montreal, Canada. Cancer Epidemiol Biomarkers Prev. 2010;19(6):1602–1611. doi: 10.1158/1055-9965.EPI-10-0015. [DOI] [PubMed] [Google Scholar]
- 9.Chen W, Liu Y, Wang H, et al. Long-term exposure to silica dust and risk of total and cause-specific mortality in Chinese workers: a cohort study. PLoS Med. 2012;9(4):e1001206. doi: 10.1371/journal.pmed.1001206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.International Agency for Research on Cancer. Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 68: Silica, Some Silicates, Coal Dust and Para-aramid Fibrils. Lyon, France: International Agency for Research on Cancer; 1997. [PMC free article] [PubMed] [Google Scholar]
- 11.Hessel PA, Gamble JF, Gee JB, et al. Silica, silicosis, and lung cancer: a response to a recent working group report. J Occup Environ Med. 2000;42(7):704–720. doi: 10.1097/00043764-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Steenland K, Mannetje A, Boffetta P, et al. Pooled exposure-response analyses and risk assessment for lung cancer in 10 cohorts of silica-exposed workers: an IARC multicentre study. Cancer Causes Control. 2001;12(9):773–784. doi: 10.1023/a:1012214102061. [DOI] [PubMed] [Google Scholar]
- 13.International Agency for Research on Cancer. Monographs on the Evaluaton of Carcinogenic Risks to Humans. Vol. 100C: Metals, Particles and Fibres. Lyon, France: International Agency for Research on Cancer; 2009. [Google Scholar]
- 14.Gamble JF. Crystalline silica and lung cancer: a critical review of the occupational epidemiology literature of exposure-response studies testing this hypothesis. Crit Rev Toxicol. 2011;41(5):404–465. doi: 10.3109/10408444.2010.541223. [DOI] [PubMed] [Google Scholar]
- 15.Lacasse Y, Martin S, Simard S, et al. Meta-analysis of silicosis and lung cancer. Scand J Work Environ Health. 2005;31(6):450–458. doi: 10.5271/sjweh.949. [DOI] [PubMed] [Google Scholar]
- 16.Pelucchi C, Pira E, Piolatto G, et al. Occupational silica exposure and lung cancer risk: a review of epidemiological studies 1996–2005. Ann Oncol. 2006;17(7):1039–1050. doi: 10.1093/annonc/mdj125. [DOI] [PubMed] [Google Scholar]
- 17.Kurihara N, Wada O. Silicosis and smoking strongly increase lung cancer risk in silica-exposed workers. Ind Health. 2004;42(3):303–314. doi: 10.2486/indhealth.42.303. [DOI] [PubMed] [Google Scholar]
- 18.Smith AH, Lopipero PA, Barroga VR. Meta-analysis of studies of lung cancer among silicotics. Epidemiology. 1995;6(6):617–624. doi: 10.1097/00001648-199511000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Checkoway H, Franzblau A. Is silicosis required for silica-associated lung cancer? Am J Ind Med. 2000;37(3):252–259. doi: 10.1002/(sici)1097-0274(200003)37:3<252::aid-ajim2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 20.Erren TC, Glende CB, Morfeld P, et al. Is exposure to silica associated with lung cancer in the absence of silicosis? A meta-analytical approach to an important public health question. Int Arch Occup Environ Health. 2009;82(8):997–1004. doi: 10.1007/s00420-008-0387-0. [DOI] [PubMed] [Google Scholar]
- 21.Peretz A, Checkoway H, Kaufman JD, et al. Silica, silicosis, and lung cancer. Isr Med Assoc J. 2006;8(2):114–118. [PubMed] [Google Scholar]
- 22.Stayner L. Silica and lung cancer: When is enough evidence enough? Epidemiology. 2007;18(1):23–24. doi: 10.1097/01.ede.0000249538.78415.58. [DOI] [PubMed] [Google Scholar]
- 23.Steenland K, Sanderson W. Lung cancer among industrial sand workers exposed to crystalline silica. Am J Epidemiol. 2001;153(7):695–703. doi: 10.1093/aje/153.7.695. [DOI] [PubMed] [Google Scholar]
- 24.Brown T. Silica exposure, smoking, silicosis and lung cancer—complex interactions. Occup Med (Lond) 2009;59(2):89–95. doi: 10.1093/occmed/kqn171. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, McLaughlin JK, Zhang J, et al. Mortality among dust-exposed Chinese mine and pottery workers. J Occup Med. 1992;34(3):311–316. doi: 10.1097/00043764-199203000-00017. [DOI] [PubMed] [Google Scholar]
- 26.Chen W, Bochmann F, Sun Y. Effects of work related confounders on the association between silica exposure and lung cancer: a nested case-control study among Chinese miners and pottery workers. Int Arch Occup Environ Health. 2007;80(4):320–326. doi: 10.1007/s00420-006-0137-0. [DOI] [PubMed] [Google Scholar]
- 27.McLaughlin JK, Chen JQ, Dosemeci M, et al. A nested case-control study of lung cancer among silica exposed workers in China. Br J Ind Med. 1992;49(3):167–171. doi: 10.1136/oem.49.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.International Labour Office. Guidelines for the Use of ILO International Classification of Radiographs of Pneumoconiosis. Geneva, Switzerland: International Labour Office; 1980. [Google Scholar]
- 29.Hodous TK, Chen RA, Kinsley KB, et al. A comparison of pneumoconiosis interpretation between Chinese and American readers and classifications. J Tongji Med Univ. 1991;11(4):225–229. doi: 10.1007/BF02888156. [DOI] [PubMed] [Google Scholar]
- 30.Zhuang Z, Hearl FJ, Odencrantz J, et al. Estimating historical respirable crystalline silica exposures for Chinese pottery workers and iron/copper, tin, and tungsten miners. Ann Occup Hyg. 2001;45(8):631–642. [PubMed] [Google Scholar]
- 31.Breslow NE, Lubin JH, Marek P, et al. Multiplicative models and cohort analysis. J Am Stat Assoc. 1983;78(381):1–12. [Google Scholar]
- 32.Eisen EA, Agalliu I, Thurston SW, et al. Smoothing in occupational cohort studies: an illustration based on penalised splines. Occup Environ Med. 2004;61(10):854–860. doi: 10.1136/oem.2004.013136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steenland K, Deddens JA. A practical guide to dose-response analyses and risk assessment in occupational epidemiology. Epidemiology. 2004;15(1):63–70. doi: 10.1097/01.ede.0000100287.45004.e7. [DOI] [PubMed] [Google Scholar]
- 34.Li R, Chambless L. Test for additive interaction in proportional hazards models. Ann Epidemiol. 2007;17(3):227–236. doi: 10.1016/j.annepidem.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Hosmer DW, Lemeshow S. Confidence interval estimation of interaction. Epidemiology. 1992;3(5):452–456. doi: 10.1097/00001648-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Gail M. Measuring the benefit of reduced exposure to environmental carcinogens. J Chronic Dis. 1975;28(3):135–147. doi: 10.1016/0021-9681(75)90002-8. [DOI] [PubMed] [Google Scholar]
- 37.Stayner L, Steenland K, Dosemeci M, et al. Attenuation of exposure-response curves in occupational cohort studies at high exposure levels. Scand J Work Environ Health. 2003;29(4):317–324. doi: 10.5271/sjweh.737. [DOI] [PubMed] [Google Scholar]
- 38.Rodricks JV, Brett SM, Wrenn GC. Significant risk decisions in federal regulatory agencies. Regul Toxicol Pharmacol. 1987;7(3):307–320. doi: 10.1016/0273-2300(87)90038-9. [DOI] [PubMed] [Google Scholar]
- 39.Rice FL, Park R, Stayner L, et al. Crystalline silica exposure and lung cancer mortality in diatomaceous earth industry workers: a quantitative risk assessment. Occup Environ Med. 2001;58(1):38–45. doi: 10.1136/oem.58.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cassidy A, ‘t Mannetje A, van Tongeren M, et al. Occupational exposure to crystalline silica and risk of lung cancer: a multicenter case-control study in Europe. Epidemiology. 2007;18(1):36–43. doi: 10.1097/01.ede.0000248515.28903.3c. [DOI] [PubMed] [Google Scholar]
- 41.Chen W, Zhuang Z, Attfield MD, et al. Exposure to silica and silicosis among tin miners in China: exposure-response analyses and risk assessment. Occup Environ Med. 2001;58(1):31–37. doi: 10.1136/oem.58.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]