Abstract
Our previous phase I/II trial of pegylated liposomal doxorubicin (PLD), low-dose dexamethasone, and lenalidomide in patients with relapsed and refractory myeloma showed an overall response rate of 75%, with 29% achieving ≥VGPR. Here, we investigated this combination (PLD 30 or 40 mg/m2 intravenously, day 1; dexamethasone 40 mg orally, days 1-4; lenalidomide 25 mg orally, days 1-21; administered every 28 days) in a phase II study in patients with newly diagnosed symptomatic multiple myeloma to determine its efficacy and tolerability (ClinicalTrials.gov NCT00617591). At best response, patients could proceed with high-dose melphalan or with maintenance lenalidomide and dexamethasone. In 57 patients, we found that the overall response rate and rate of very good partial response and better on intent-to-treat, our primary endpoints, were 77.2% and 42.1%, respectively, with responses per the International Myeloma Working Group. Median progression-free survival was 28 months (95% CI 18.1-34.8), with 1- and 2-year overall survival rates of 98.1 and 79.6%. During induction, grade 3/4 toxicities were neutropenia (49.1%), anemia (15.8%), thrombocytopenia (7%), fatigue (14%), febrile neutropenia (8.8%), and venous thromboembolic events (8.8%). During maintenance, grade 3/4 toxicities were mainly hematologic. We found this combination to be active in patients with newly diagnosed myeloma, with results comparable to other lenalidomide-based induction strategies without proteasome inhibition. In addition, maintenance therapy with lenalidomide was well tolerated.
Keywords: Lenalidomide, liposomal doxorubicin, multiple myeloma, newly diagnosed, maintenance
Introduction
Multiple myeloma is a neoplasm of plasma cells characterized by the production of a monoclonal protein in the serum and/or urine and end organ dysfunction (lytic bone lesions, renal insufficiency, hypercalcemia, and/or anemia). Advances in the field during the past decade have resulted in the approval by the Food and Drug Administration of six agents (thalidomide, bortezomib, lenalidomide, pegylated liposomal doxorubicin, and most recently carfilzomib and pomalidomide [1-9].
The cellular targets of lenalidomide appears to be cereblon [10], and lenalidomide has been shown to have a direct effect on multiple myeloma cells by activating tumor suppressor genes, as well as indirect effects that are mediated via reduction of supporting cytokine secretion in the bone marrow microenvironment and stimulation of effector T and natural killer cells [11]. Lenalidomide in combination with dexamethasone has been shown to improve the survival of patients with relapsed refractory multiple myeloma [7, 8]. In addition, lenalidomide and low-frequency dexamethasone have resulted in a superior survival rate compared to lenalidomide and high-dose dexamethasone [2]. Specifically, the overall response rate of lenalidomide + low-frequency dexamethasone was 70%, with the rate of very good partial response (VGPR) or better, of 40% and median progression-free survival of 25.3 months [2].
Although the combination of pegylated liposomal doxorubicin (PLD) and bortezomib did not statistically improve the response rate compared to that shown with bortezomib alone in patients with relapsed refractory multiple myeloma, this combination was associated with a higher rate of ≥VGPR and ultimately improvement in time to progression [1]. We have previously reported the promising activity of the combination of PLD, vincristine, dexamethasone, and lenalidomide in patients with relapsed and refractory multiple myeloma [12]. Moreover, Berenson et al. reported on the combination of lenalidomide, bortezomib, liposomal doxorubicin and dexamethasone resulting in a 49% response rate in heavily pretreated patients with relapsed and refractory myeloma [13]. We found that the overall response was 53% and the median progression-free survival was 10.5 months in a group of mostly refractory patients, suggesting synergy between lenalidomide and doxorubicin. In this study, we investigated this combination in newly diagnosed patients with symptomatic multiple myeloma.
Patients and Methods
Eligibility
This study was approved by the Institutional Review Board at the University of South Florida (ClinicalTrials.gov: NCT00617591). Written, informed consent was obtained from all patients before study entry. Eligible patients had previously untreated symptomatic multiple myeloma as defined by the International Myeloma Working Group [14]. In addition, patients had measurable paraprotein levels in serum (≥ 0.5 g/dL) or urine (≥ 0.2 g in a 24-hour urine collection sample) or by free light chain (involved free light chain >100 mg/L and abnormal serum free kappa-to-lambda ratio). Notable exclusion criteria included serum creatinine levels >2.5 mg/dL, ECOG performance status >2 (if not due to bone disease), and ejection fraction <50%. Patients must have a platelet count of ≥ 50,000/mm3 and an absolute neutrophil count of ≥ 1.0 × 109/L unless they had ≥ 50% bone marrow plasmacytosis, where lower peripheral blood counts were allowed.
Treatment Regimen
Patients received lenalidomide 25 mg orally on days 1-21, dexamethasone 40 mg orally on days on 1-4, and PLD 40 mg/m2 intravenously on day 1 (reduced to 30 mg/m2 after the initial 29 patients were treated). Cycles were repeated every 28 days. Granulocyte colony-stimulating factor (G-CSF) support was at the discretion of the treating physician. Thromboprophyalxis was mandated with aspirin, low-molecular-weight heparin, or warfarin [15]. Patients received prophylactic acyclovir and fluoroquinolones (given our experience with similar regimens combining liposomal doxorubicin and immunomodulatory agents) [16]. At the best response (4-8 cycles of induction), patients could proceed with either high-dose therapy or maintenance with lenalidomide and dexamethasone at the tolerated doses on the same schedule until disease progression.
Response Criteria
Response to therapy was defined per the uniform response criteria of the International Myeloma Working Group [14].
Statistical Considerations
This phase II study had the following primary endpoints: overall response rate (partial response or better with induction regimen) and rate of VGPR or better. Secondary endpoints included progression-free survival in patients who received induction therapy, overall survival, and tolerability of therapy as assessed by the NCI CTC version 3.0.
A two-stage MiniMax design was used. In the first stage, 22 eligible patients would be enrolled. If 16 patients or greater had a response, an additional 35 eligible patients would be enrolled. If 45 patients or greater achieve a response, the combination was deemed effective. The combination is considered effective if the overall response rate is significantly higher than 70%. This design provides a power of at least 0.85 (if the true overall response is at least 85%) with an alpha of 0.05. For VGPR or better, which was another primary end point, this design provides a power of 0.91 at a one-sided alpha of 0.035 to test if the percent VGPR or better is higher than 30% (assuming an alternative response rate of 50%) at the end of stage 2.
Primary efficacy analyses were performed according to the intent-to-treat approach, which included all enrolled patients. Response rates and toxicity profiles with their 95% confidence intervals were calculated based on the exact binomial distribution. Associations between two categorical variables were evaluated using Fisher's exact test. Survival time was analyzed using the Kaplan-Meier method with survival log-rank test for stratified analysis. Progression-free survival was defined as the time from study entry to progression/relapse or death, and overall survival was defined as time from study entry to death of any cause. Patients with non-events were censored to their last observation or follow-up date. Univariate and multivariable Cox proportional hazards models for progression-free survival were fitted to explore the following potential risk factors: age, gender, heavy chain type, cytogenetics (high risk versus low risk), and baseline β2-microglobulin and albumin levels.
All P values were 2 sided, with statistical significance set at 0.05.
Results
Patients
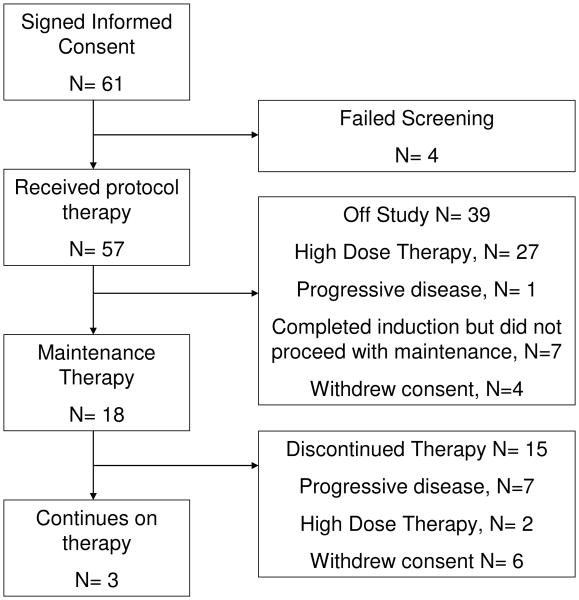
Between February 2008 and February 2011, 61 patients were consented and screened and 57 patients were eligible and treated. Of the 57 patients, after induction, 18 proceeded with maintenance therapy and 27 patients proceeded with high-dose therapy. Of the 12 remaining patients, 8 had stable disease or progressive disease at the end of induction (and received other therapies) and 4 withdrew consent (3 refused either maintenance or high dose therapy and 1 withdrew consent during induction). The decision to proceed with high-dose therapy was at the discretion of the treating physician and patient. Figure 1 summarizes the patient disposition after enrollment. The data cutoff date for this analysis was July 2012. The median age was 63 years (range 36-78), and 31 (54%) patients were males. Table 1 lists patient characteristics at study entry. The median β2-microglobulin level was 3.2 mg/L (range 1.4-11.3). Cytogenetics and FISH studies were available on 50 patients, of which 11 (22%) had high-risk disease (17p deletion, t(4;14), or hypodiploidy). In addition, 17 patients (34%) had 13q deletion by FISH.
Fig. 1.
Consort diagram.
Table 1. Patient Characteristics (n = 57).
| Characteristic | Value or Number of Patients | % |
|---|---|---|
|
| ||
| Age, median (range), years | 63 (36-78) | |
|
| ||
| Male | 31 | 54.4 |
|
| ||
| Heavy Chain | ||
| IgG | 33 | 58.9 |
| IgA | 15 | 26.3 |
| IgD | 2 | 3.5 |
| IgM | 1 | 1.8 |
| Light chain only | 6 | 10.5 |
|
| ||
| Light Chain, Lambda | 18 | 31.6 |
|
| ||
| International staging system | ||
| I | 13 | 22.8 |
| II | 34 | 59.6 |
| III | 7 | 12.3 |
| Missing | 3 | 5.3 |
|
| ||
| β2-microglobulin level, median (range), mg/L | 3.2 (1.4-11.3) | |
| Patients with β2-microglobulin > 5.5 mg/L | 6 | 10.5 |
| Creatinine level, median (range), mg/dL | 1.0 (0.6-2.5) | |
| Albumin level, median (range), g/dL | 4.1 (3.1-4.8) | |
|
| ||
| Cytogenetics | ||
| Deletion 13q | 17 | 29.8 |
| Deletion 17p | 8 | 14 |
| t(4;14) | 3 | 5.3 |
| t(11;14) | 6 | 10.5 |
| High risk | 11 | 19.3 |
| Missing, not done | 7 | 12.3 |
Induction Toxicities
The median number of induction cycles delivered was 6 (range 1-8). After the first 29 patients were enrolled, high rates of grade 3/4 neutropenia and fatigue were noted (48% and 20%, respectively). Of these 29 patients, 7 required dose reductions in PLD and 6 received less than 4 cycles of therapy, with 4 patients discontinuing therapy after only one cycle. The protocol was therefore amended, with the starting dose of PLD decreased from 40 to 30 mg/m2. After the dose adjustment, 28 patients were accrued, with 10 requiring further dose reduction of PLD; however, only 3 patients received less than 4 cycles of therapy and 2 discontinued therapy after 1 cycle. After the dose adjustment in PLD, we noted a lower incidence of grade 3 or 4 neutropenia (46.4% versus 58.6%), anemia (10.7% versus 20.7%), fatigue (3.6% versus 20.6%), and febrile neutropenia (3.6% versus 13.8%) (Table S1). Grade 3 and 4 adverse events were mainly hematologic in nature, with 49% of patients experiencing at least one episode of grade 3/4 neutropenia (Table 2). However, only 5 patients had febrile neutropenias, possibly due to the concurrent use of G-CSF and prophylactic antibiotics. Gastrointestinal adverse events were mainly grade 1 and 2 and were manageable with supportive measures. Although 25 patients reported mild to moderate rashes while on study (grades 1 and 2), four patients had grade 3 rashes (none was a true Steven–Johnson syndrome).
Table 2. Adverse events possibly related to therapy (occurring in more than 5% of patients during maintenance and induction).
| Toxicity | Induction (N = 57) | Maintenance (N = 18) | ||
|---|---|---|---|---|
|
| ||||
| Grade 1/2 N (%) | Grade 3/4 N (%) | Grade 1/2 N (%) | Grade 3/4 N (%) | |
|
| ||||
| Hematologic | ||||
| Neutropenia | 10 (17.5) | 28 (49.1) | 2 (11.2) | 8 (44.4) |
| Anemia | 17 (29.8) | 9 (15.8) | 3 (16.7) | 1 (5.6) |
| Thrombocytopenia | 20 (35.1) | 4 (7.0) | 4 (22.2) | 2 (11.1) |
|
| ||||
| Infectious | ||||
| Febrile neutropenia | 0 | 5 (8.8) | 0 | 0 |
| Non-neutropenic fever | 11 (19.3) | 0 | 1 (5.6) | 0 |
|
| ||||
| Gastrointestinal | ||||
| Nausea | 19 (33.3) | 1 (1.7) | 2 (11.1) | 0 |
| Vomiting | 12 (21.0) | 1 (1.7) | 1 (5.6) | 0 |
| Diarrhea | 22 (38.6) | 1 (1.7) | 4 (22.2) | 0 |
| Anorexia | 15 (24.3) | 1 (1.7) | 3 (16.7) | 0 |
|
| ||||
| Cardiovascular | ||||
| Deep venous thrombosis/pulmonary embolus* | 0 | 5 (8.8) | 1 (5.6) | 0 |
|
| ||||
| Neurologic | ||||
| Peripheral neuropathy** | 11 (19.3) | 0 | 3 (16.7) | 0 |
| Dizziness | 11 (19.3) | 0 | 1 (5.6) | 0 |
|
| ||||
| Dermatologic | ||||
| Rash | 25 (43.9) | 4 (7) | 1 (5.6) | 0 |
| Alopecia | 4 (7) | 0 | 0 | 0 |
|
| ||||
| Constitutional | ||||
| Fatigue | 27 (47.4) | 8 (14.0) | 3 (16.7) | 1 (5.6) |
| Weight loss | 4 (7.0) | 0 | 0 | 0 |
| Weight gain | 0 | 0 | 2 (11.2) | 0 |
|
| ||||
| Second primary malignancies | 0 | 0 | 0 | 2 (11.2)*** |
Two patients had a pulmonary embolus in addition to deep venous thrombosis.
Only grade 1.
Two patients had squamous cell carcinomas of skin. The maximum grade of an individual toxicity seen in any given patient is presented. If a patient had multiple occurrences of a particular toxicity, only the highest grade is presented.
Stem Cell Collection and High-Dose Chemotherapy and Autologous Transplant
Thirty-one patients had stem cell collection attempts, and all were successful after the first attempt. Of the 31 patients who had stem cell collection, 27 had high-dose therapy and 4 elected to store stem cells for future use. Stem cell collection occurred after the completion of induction therapy or a median 6 cycles of induction. The stem cell collection method was at the discretion of the transplant physician. G-CSF was used alone in 16 patients (median number of CD34+ cells collected: 4.23; range: 2-8.27 × 106/kg), in combination with plerixafor in 12 (median number of CD34+ cells collected: 4.25; range: 3.63-15.19 × 106/kg), with cyclophosphamide in 2 (median number of CD34+ cells collected: 5.07; range: 4.33-5.81 × 106/kg), and with both plerixafor and cyclophosphamide in 1 (number of CD34+ cells collected: 4.47 × 106/kg) patient.
Maintenance Toxicities
Overall maintenance therapy was well tolerated; despite 8 patients (44%) having grade 3 neutropenia, no patients had neutropenic fevers. Table 2 summarizes the main toxicities reported in maintenance. In addition, 2 patients developed squamous cell carcinoma of the skin, which required surgical excision and could be considered a second primary malignancy.
Response
Based on intent to treat, all patients were counted in the response assessment, although 2 patients were not evaluable for response (received 1 cycle of therapy and discontinued therapy and did not have repeated electrophoretic testing). Table 3 summarizes the response to therapy by treatment dose level (PLD at 40 mg/m2 and at 30 mg/m2). After a median of 6 cycles (range 1-8), the overall response for the entire study was 77.2% and the VGPR and better rate was 42.1%. Patients who started on therapy with PLD at 40 mg/m2 had an overall response rate of 72% and had a VGPR and better rate of 48%. Patients who were administered PLD at 30 mg/m2 had 82% and 36% for overall response rate and VGPR rate, respectively.
Table 3. Response by treatment dose level.
| All patients (N = 57) | PLD 40 mg/m2 (N = 29) | PLD 30 mg/m2 (N = 28) | ||||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Complete response | 4 | 7 | 4 | 13.8 | 0 | 0 |
| Stringent complete response | 4 | 7 | 1 | 3.4 | 3 | 10.7 |
| Very good partial response | 16 | 28.1 | 9 | 31 | 7 | 25 |
| Partial response | 20 | 35.1 | 7 | 24.1 | 13 | 46.4 |
| Stable disease | 9 | 15.8 | 5 | 17.2 | 4 | 14.3 |
| Progressive disease | 2 | 3.5 | 2 | 6.9 | 0 | 0 |
| Not evaluable | 2 | 3.5 | 1 | 3.4 | 1 | 3.6 |
Survival and Progression-Free Survival
The median progression-free survival rate for all patients was 28 months (95% CI 18.1-34.8) (Fig. 2A). Although the median overall survival was not reached, the 1- and 2-year overall survival rates were 98% (95% CI 87.6-99.7) and 79.6% (95% CI 65.4-88.5), respectively (Fig. 2B). Patients with high-risk cytogenetics (17p deletion or t(4;14)) had a median progression-free survival of 18.1 months (95% CI 6.48-30.9), whereas patients without high-risk cytogenetic abnormalities had a median progression-free survival of 30.6 months (95% CI 18.6-44.8); this difference was not statistically significant (P = 0.38) (Fig. 2C). Similarly, the overall survival of high-risk and non-high-risk (standard) patients was not statistically different (P = 0.18) (Fig. 2D). For patients who proceeded with maintenance therapy, median progression-free survival was 34.8 months (95% CI 18.6 months; not reached). For patients who proceeded with high-dose therapy, median progression-free survival was 28 months (95% CI 17.3-33.6), which was not statistically significant (P = 0.15) (Fig. 2E). Similarly, there was no difference in overall survival among patients who proceeded with high-dose therapy and those who proceeded with maintenance (P = 0.77) (Fig. 2F). Both univariate and multivariable Cox proportional hazards models including all covariates listed in Table 1 showed that only age (in years) was statistically significant in regard to progression-free survival (hazard ratio = 1.08; P = 0.04) (Table S2).
Fig. 2.
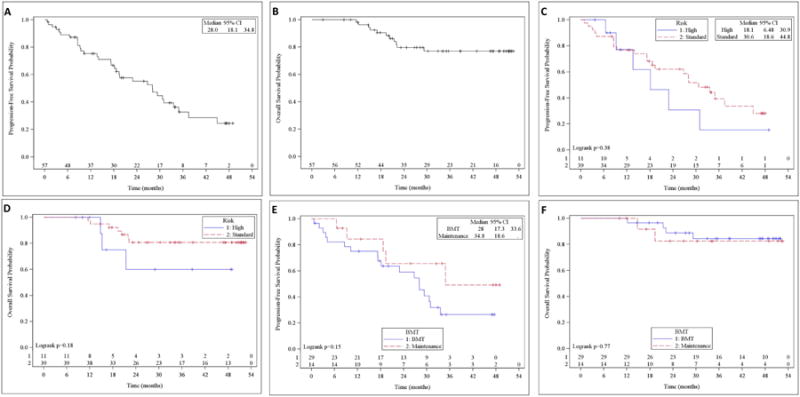
(A) Progression-free survival. (B) Overall survival. (C) Progression-free survival in high-risk versus low-risk patients. (D) Overall survival in high-risk versus low-risk patients. (E) Progression-free survival in patients on maintenance versus bone marrow transplant (BMT) therapy. (F) Overall survival in patients on maintenance versus BMT therapy.
Discussion
Overall, the addition of PLD resulted in manageable toxicities, consisting mainly of neutropenia and fatigue in excess of what is expected with lenalidomide and low-dose dexamethasone. In fact, grade 3/4 neutropenia was noted in almost 50% of patients (with 8.8% of patients experiencing febrile neutropenia) compared to 20% with lenalidomide and low-dose dexamethasone [2]. In this study, neutropenia was managed with the use of G-CSF and dose reduction of PLD. Interestingly, after dose reduction of PLD to 30 mg/m2, therapy was better tolerated, and the response rates were not different, highlighting the importance of improved tolerability in myeloma therapy similar to what was observed with the use of high-dose dexamethasone versus low-dose dexamethasone or once or twice weekly bortezomib in combination with melphalan and prednisone [2, 17]. Stem cell collection after lenalidomide-based induction therapy in this trial was possible for all patients for whom this was attempted, and maintenance therapy was generally well tolerated, as we did not observe clinically significant second primary malignancies in contrast to earlier reports that suggested that an induction therapy that included anthracycline increased the rate of second primary malignancies. Finally, we noted venous thromboembolic events in 5 (8.8%) patients despite prophylaxis. Overall, this is consistent with a baseline rate of thromboembolic events noted in patients with plasma cell dyscrasia [18].
In this study, the combination of lenalidomide, low-dose dexamethasone, and PLD resulted in an overall response rate of 77% and a VGPR or better rate of 42%, with median progression-free survival of 28 months. Although comparisons across studies on frontline therapy for multiple myeloma should be regarded cautiously, this is comparable to other lenalidomide-based combinations, including those that combined low- or high-dose dexamethasone with lenalidomide and cyclophosphamide (Table 4) [2, 19].
Table 4. Comparisons of primary and secondary endpoints for lenalidomide-based induction regimens studies for newly diagnosed multiple myeloma.
| Overall response rate, % | VGPR, % | Median progression-free survival (95% CI), months | 2-year overall survival, % | |
|---|---|---|---|---|
| Lenalidomide + high-dose dexamethasone | ||||
| Zonder et al [25] | 78% | 63% | NR | 87% |
| Rajkumar et al [2] | 81% | 50% | 19.1 | 75% |
| Lenalidomide + low-dose dexamethasone | ||||
| Rajkumar et al [2] | 70% | 40% | 25.3 | 87% |
| Lenalidomide + cyclophosphamide + dexamethasone | ||||
| Kumar et al [18] | 85% | 47% | 28 | 87% |
| Clarithromycin + lenalidomide + dexamethasone | ||||
| Niesvizky et al [26] | 90%* | 74%* | NR | NR |
| Lenalidomide + bortezomib + dexamethasone | ||||
| Richardson et al [20] | 100% | 67% | NR | NR |
| Kumar et al [19] | 85%* | 51%* | NR | NR |
| Lenalidomide + bortezomib + dexamethasone + liposomal doxorubicin | ||||
| Jakubowiak et al. [22] | 96% | 57% | NR | NR |
| Carfilzomib + lenalidomide + dexamethasone | ||||
| Jakubowiak et al [21] | 98%* | 81%* | NR | NR |
| Present study | 77% | 42% | 28 (18.1-34.8) | 80% |
Abbreviations: VGPR, very good partial response and better rate; NR, not reported.
Represents best overall response rate throughout the study.
The addition of bortezomib to lenalidomide-based therapy appears to result in a higher response rate (85-100%) and higher rates of VGPR and better (51-67%) [20, 21] than our combination. Interestingly, Jakubowiak et al. conducted a phase I/II trial and investigated the addition of liposomal doxorubicin to the lenalidomide bortezomib and dexamethasone backbone [22]. They reported response rates and VGPR and better rate of 96% and 57% at 4 cycles (95% and 65% at 8 cycles). These results are not drastically different from what has been observed without the addition of liposomal doxorubicin. However, the addition of bortezomib to lenalidomide-based induction comes often at the cost of increased neurotoxicity, and it remains unclear whether this will translate into a longer progression-free or overall survival over lenalidomide + dexamethasone induction. In fact, the Southwest Oncology Group recently completed accrual of patients to a randomized trial comparing lenalidomide + dexamethasone with or without bortezomib for newly diagnosed multiple myeloma patients; results are still awaited. More recently, Jakubowiak et al reported impressive early results using the combination of carfilzomib, lenalidomide, and dexamethasone with overall response rates of 98% and ≥VGPR responses in 81% of patients without increased neurotoxicity [23]. These results will need to be validated in the context of a randomized controlled trial [23].
In this study, patients generally did not receive post-transplant maintenance as the results of the IFM 2005-002 and CALB 100104 were not available at the time. Accordingly, patients who proceeded with early high-dose therapy had a median progression-free survival of 28 months, which is comparable to the progression-free survival rates shown in the IFM 2005-002 and CALB 100104 control groups (no maintenance, 23 and 27 months) but lower than rates in the maintenance lenalidomide groups (41 and 46 months) [24, 25]. On the other hand, patients who proceeded with a deferred transplant approach and maintenance lenalidomide and dexamethasone following induction therapy had a median progression-free survival of approximately 35 months. In aggregate, the results further highlight the success of maintenance lenalidomide after primary therapy and high-dose therapy.
Patients with high-risk myeloma (17p deletion or t(4;14)) had a median progression-free survival of 18 months in this trial compared with 30.6 months for patients with standard risk myeloma. Although this was not statistically different (likely due to the limited number of patients with high-risk features (11 patients)), it is nonetheless an indicator that the current regimen may not overcome high-risk features. The HOVON-65 trial randomized patients with newly diagnosed myeloma to either VAD or PAD induction followed by high-dose therapy and maintenance with either thalidomide or bortezomib. The bortezomib regimen resulted in a median PFS of 22 months (which was superior to a median of 12 months for non-bortezomib-based therapy) and about 18 months (which was not statistically different than the PFS of the non-bortezomib-based regimen) in patients with 17p deletion and t(4;14), respectively [26]. Our results are comparable with the HOVON-65 outcomes in high-risk patients. Overall, the estimated 2-year overall survival of 60% for high-risk patients is consistent with contemporary myeloma therapy and likely also a reflection of salvage therapies used.
In summary, the addition of PLD to the lenalidomide and low-dose dexamethasone backbone was associated with greater hematologic toxicity but was fairly well tolerated, with significant improvement in toxicities following our dose reduction of PLD to 30 mg/m2. Response rates were also comparable, but not clearly better than other lenalidomide-based induction strategies that did not include a proteasome inhibitor. Given the current therapeutic landscape in newly diagnosed myeloma, the role of this combination is not clearly defined. Lenalidomide maintenance was well tolerated after primary therapy and was associated with a meaningful progression-free survival.
Supplementary Material
Acknowledgments
We thank Rasa Hamilton (Moffitt Cancer Center) for editorial assistance.
Grant support: This work was supported by Orthobiotec and Celgene Corporation.
Footnotes
Author Contributions: Conception and Design: R. Baz, M. A. Hussein, M. Alsina.
Collection and Assembly of Data: R. Baz, K. Shain, M. A. Hussain, D. Sullivan, J. L. Ochoa-Bayona, T. Nishihori, W. Dalton, M. Alsina.
Data Analyses: R. Baz, J-H Lee, E. FinleyOliver, L. Nardelli, L. Nodzon, X. Zhao.
Manuscript Writing: All authors.
Final Approval of Manuscript: All authors.
References
- 1.Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25:3892–3901. doi: 10.1200/JCO.2006.10.5460. [DOI] [PubMed] [Google Scholar]
- 2.Rajkumar SV, Jacobus S, Callander NS, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010;11:29–37. doi: 10.1016/S1470-2045(09)70284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richardson PG, Sonneveld P, Schuster M, et al. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trial. Blood. 2007;110:3557–3560. doi: 10.1182/blood-2006-08-036947. [DOI] [PubMed] [Google Scholar]
- 4.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 5.Siegel DS, Martin T, Wang M, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120:2817–2825. doi: 10.1182/blood-2012-05-425934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vij R, Wang M, Kaufman JL, et al. An open-label, single-arm, phase 2 (PX-171-004) study of single-agent carfilzomib in bortezomib-naive patients with relapsed and/or refractory multiple myeloma. Blood. 2012;119:5661–5670. doi: 10.1182/blood-2012-03-414359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weber DM, Chen C, Niesvizky R, et al. Lenalidomide plus Dexamethasone for Relapsed Multiple Myeloma in North America. N Engl J Med. 2007;357:2133–2142. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
- 8.Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus Dexamethasone for Relapsed or Refractory Multiple Myeloma. N Engl J Med. 2007;357:2123–2132. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- 9.Richardson PG, Siegel D, Baz R, et al. Phase 1 study of pomalidomide MTD, safety, and efficacy in patients with refractory multiple myeloma who have received lenalidomide and bortezomib. Blood. 2013;121:1961–1967. doi: 10.1182/blood-2012-08-450742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu YX, Braggio E, Shi CX, et al. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood. 2011;118:4771–4779. doi: 10.1182/blood-2011-05-356063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies F, Baz R. Lenalidomide mode of action: linking bench and clinical findings. Blood Rev. 2010;24(Suppl 1):S13–19. doi: 10.1016/S0268-960X(10)70004-7. [DOI] [PubMed] [Google Scholar]
- 12.Baz R, Walker E, Karam MA, et al. Lenalidomide and pegylated liposomal doxorubicin-based chemotherapy for relapsed or refractory multiple myeloma: safety and efficacy. Ann Oncol. 2006;17:1766–1771. doi: 10.1093/annonc/mdl313. [DOI] [PubMed] [Google Scholar]
- 13.Berenson JR, Yellin O, Kazamel T, et al. A phase 2 study of pegylated liposomal doxorubicin, bortezomib, dexamethasone and lenalidomide for patients with relapsed/refractory multiple myeloma. Leukemia. 2012;26:1675–1680. doi: 10.1038/leu.2012.51. [DOI] [PubMed] [Google Scholar]
- 14.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 15.Baz R, Li L, Kottke-Marchant K, et al. The role of aspirin in the prevention of thrombotic complications of thalidomide and anthracycline-based chemotherapy for multiple myeloma. Mayo Clin Proc. 2005;80:1568–1574. doi: 10.4065/80.12.1568. [DOI] [PubMed] [Google Scholar]
- 16.Hussein MA, Baz R, Srkalovic G, et al. Phase 2 study of pegylated liposomal doxorubicin, vincristine, decreased-frequency dexamethasone, and thalidomide in newly diagnosed and relapsed-refractory multiple myeloma. Mayo Clin Proc. 2006;81:889–895. doi: 10.4065/81.7.889. [DOI] [PubMed] [Google Scholar]
- 17.Bringhen S, Larocca A, Rossi D, et al. Efficacy and safety of once-weekly bortezomib in multiple myeloma patients. Blood. 2010;116:4745–4753. doi: 10.1182/blood-2010-07-294983. [DOI] [PubMed] [Google Scholar]
- 18.Srkalovic G, Cameron MG, Rybicki L, et al. Monoclonal gammopathy of undetermined significance and multiple myeloma are associated with an increased incidence of venothromboembolic disease. Cancer. 2004;101:558–566. doi: 10.1002/cncr.20405. [DOI] [PubMed] [Google Scholar]
- 19.Kumar SK, Lacy MQ, Hayman SR, et al. Lenalidomide, cyclophosphamide and dexamethasone (CRd) for newly diagnosed multiple myeloma: results from a phase 2 trial. Am J Hematol. 2011;86:640–645. doi: 10.1002/ajh.22053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar S, Flinn I, Richardson PG, et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood. 2012;119:4375–4382. doi: 10.1182/blood-2011-11-395749. [DOI] [PubMed] [Google Scholar]
- 21.Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116:679–686. doi: 10.1182/blood-2010-02-268862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jakubowiak AJ, Griffith KA, Reece DE, et al. Lenalidomide, bortezomib, pegylated liposomal doxorubicin, and dexamethasone in newly diagnosed multiple myeloma: a phase 1/2 Multiple Myeloma Research Consortium trial. Blood. 2011;118:535–543. doi: 10.1182/blood-2011-02-334755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jakubowiak AJ, Dytfeld D, Griffith KA, et al. A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma. Blood. 2012;120:1801–1809. doi: 10.1182/blood-2012-04-422683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366:1782–1791. doi: 10.1056/NEJMoa1114138. [DOI] [PubMed] [Google Scholar]
- 25.McCarthy PL, Owzar K, Hofmeister CC, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366:1770–1781. doi: 10.1056/NEJMoa1114083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sonneveld P, Schmidt-Wolf IG, van der Holt B, et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/GMMG-HD4 trial. J Clin Oncol. 2012;30:2946–2955. doi: 10.1200/JCO.2011.39.6820. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.