Abstract
Background
The Notch signaling pathway is activated in a variety of malignancies and has been implicated in colorectal cancer progression. One of the first steps in the Notch pathway activation is mediated by γ-secretase, a proteolytic enzyme which produces an activated intracellular Notch (ICN). RO4929097 is a selective inhibitor of γ-secretase. We tested the activity of RO4929097 in patients with metastatic, refractory colorectal cancer.
Patients and Methods
Patients with metastatic colorectal cancer who had received at least two prior lines of systemic chemotherapy were enrolled on the study. Patients were treated with RO4929097 at its recommended phase II dose of 20mg daily, 3 days on and 4 days off continuously. Cycle length was 28 days. Imaging was performed every two cycles. Archival tissue specimens were stained immunohistochemically for components of the notch pathway: Notch1, ICN, and the downstream target HES1.
Results
37 patients were enrolled of whom 33 were evaluable for toxicity and response. Immunohistochemical analysis of archival tissues demonstrated positive staining for the notch receptor as well as intracellular notch and the downstream gene HES1 in the majority of patients. Nevertheless, no objective radiographic responses were observed in this group and only 6 patients had stable disease as their best response. Median PFS was 1.8 months and median OS was 6.0 months.
Conclusion
In this study of RO4929097 in patients with refractory metastatic colorectal cancer, no radiographic responses were seen and time to progression was short, which suggests that RO4929097 at the study dose and schedule has minimal single agent activity in this malignancy.
Introduction
Colorectal cancer is the second leading cause of cancer-related mortality in the United States with nearly 50,000 deaths each year.1 Combination chemotherapy with 5-fluorouracil, oxaliplatin, irinotecan2, bevacizumab3, and the EGFR inhibitors cetuximab4 and panitumumab5 have led to improvements in longevity,6 with median survival rates now approaching 24 months in patients with stage IV disease.7 However, response rates beyond the first line of treatment remain disappointingly low and new systemic agents are needed for patients who are resistant or intolerant of currently available therapies.
New therapeutic targets include signaling pathways that regulate proliferation and differentiation of stem cells. During development and tissue remodeling, pluripotent stem cells serve as the source of differentiating cells, giving rise to non-proliferating specialized cell types. The fate of these cells appears to depend on primordial regulatory pathways that are active during development. Deregulation of these pathways is linked to the rapid and uncontrolled proliferation of tumors.
The Notch pathway is one of the major developmental signaling pathways.8,9 Notch, represented by four homologs in mammals (Notch1-Notch4), is a cell surface protein receptor involved in transmitting growth and proliferation signals to the cell.10 Activation of Notch occurs through ligand binding. Two Notch ligand families, Jagged and Delta, have been described in mammals with five ligands identified to date (Jagged 1 and 2, and Delta 1, 3, and 4). After ligand binding, two successive proteolytic cleavage steps occur. The first cleavage step is mediated by ADAM/TACE (a disintegrin and metalloprotease/tumor-necrosis factor α converting enzyme) and occurs at the S2 cleavage site. The second cleavage step occurs at the S3 cleavage site and is mediated by the γ-secretase complex, consisting of a catalytic subunit (presenilin 1 or 2), and accessory subunits (nicastrin, Pen-2, and Aph-1). The resulting active form of Notch called IntraCellular Notch (ICN), translocates to the nucleus where it binds a transcriptional repressor known as C-promoter-binding factor (CBF-1), or CSL (CBF-1/Suppresor of Hairless/Lag1), thus activating the Notch target genes, Myc, p21, and Hes (hairy/enhancer of split).11-13. Blocking Notch signaling via γ-secretase inhibition produces a slower growing, less transformed phenotype in human cancer cells in vivo.
Several studies highlight the association between Notch signaling and tumorigenesis. Inappropriate activation of Notch signaling in T-cell acute lymphoblastic leukemia14,15, breast cancer16,17, melanoma18-20, and lung cancer21-23, has been shown to result in stimulation of tumor cell proliferation, restriction of cell differentiation and prevention of apoptosis. Overexpression of Notch also occurs in other hematologic malignancies, including B-cell malignancies.11 Antiproliferative effects of a γ-secretase inhibitor in a hepatoma cell line have been reported.24 Furthermore, the ability of breast cancer stem cells to form mammospheres was attenuated by inhibition of the Notch pathway suggesting that Notch inhibition can specifically target cancer stem cells.25
The expression of Notch ligands, receptors and downstream genes has been studied in colorectal cancer tissue specimens.26 One study found that levels of Jagged, Notch1 and Hes1 are comparable to or greater than those found in proliferative intestinal crypts, indicating that the Notch pathway is activated in colorectal adenocarcinomas.26 Another study demonstrated that ICN and its downstream target Hes1 were implicated in colon cancer progression.27
RO4929097 is a potent and selective oral inhibitor of γ-secretase. In a phase I dose escalation study of 89 patients with advanced solid tumors, a single objective partial response was documented in a neuroendocrine carcinoma and a minor response in a patient with melanoma. Common mild toxicities included fatigue, nausea, diarrhea, hypophosphatemia, pruritis, and rash (92% grade 1-2).28 The recommended phase II dose of RO4929097 was subsequently established as 20mg daily for 3 days every 7 days29, and multiple early-phase clinical trials are currently enrolling patients with solid and hematological malignancies (table 1).
Table 1.
Current development of RO4929097: active clinical trials
| Malignancy | Combination w/ | Phase | ClinicalTrials.gov Identifier |
|---|---|---|---|
| Colorectal | Cetuximab | I/II | NCT01198535 |
| Brain Metastases | Whole-Brain Radiotherapy | I/II | NCT01217411 |
| Breast | Exemestane | I/II | NCT01149356 |
| Colorectal | 5-FU/LV/Oxaliplatin(FOLFOX), Bevacizumab | II | NCT01270438 |
| Breast (triple negative) | Paclitaxel, Carboplatin | I | NCT01238133 |
| Renal Cell Carcinoma | II | NCT01141569 | |
| Breast (hormone receptor positive) | Letrezole | Ib | NCT01208441 |
| Melanoma | Cisplatin, Vinblastin, Temozolomide | I/II | NCT01196416 |
| Sarcoma | GDC-0449 | I/II | NCT01154452 |
| Non-Small Cell Lung | Erolotinib | I | NCT01193881 |
| Melanoma (resectable) | II | NCT01216787 | |
| Glioma | I | NCT01269411 | |
| Multiple Myeloma | Autologous Stem-Cell Transplant | II | NCT01251172 |
| Glioblastoma Multiforme | II | NCT01122901 | |
| Endometrial and Renal Cell | I | NCT01198184 | |
| Non-Small Cell Lung | II | NCT01193868 |
We conducted an open-label phase II study to test the activity of RO4929097 in patients with metastatic, refractory (3rd line and beyond) colorectal cancer. To our knowledge, this study represented the first trial of a γ-secretase inhibitor in colorectal cancer. The study also offered an opportunity to investigate the expression of the Notch receptor and downstream target genes in patients with colorectal cancer.
Patients and Methods
Patient Selection
This study was an open-label, single-arm, phase II prospective clinical trial. The trial was supported by the Southeast Phase II Consortium and approved by the Quorum institutional review board. Written informed consent was obtained from participants.
Subjects were adults (≥age 18) with stage IV colorectal cancer who had received at least two prior lines of treatment in the metastatic setting. Eligibility requirements mandated prior treatment with 5-fluorouracil (or capecitabine), oxaliplatin and irinotecan, either in the adjuvant or metastatic setting. Other key eligibility criteria were measureable disease, ECOG performance status ≤2, absolute neutrophil count ≥1,000cells/μL, platelets ≥100,000 cells/μL, total bilirubin ≤1.5 × upper limit of normal, AST and ALT ≤2.5 × upper limit of normal, and creatinine ≤1.5 × upper limit of normal. Key exclusion criteria included brain or leptomeningial metastases, major electrolyte abnormalities, and QTcF on baseline ECG > 450msec (males) or 470msec (females).
In order to facilitate recruitment, patients were prescreened for the trial using the Total Cancer Care® (TCC) database, a Moffitt Cancer Center and affiliate registry. This registry consists of over 75,000 cancer patients who have prospectively consented for lifetime clinical follow-up. Among the goals of TCC is to match patients with appropriate clinical trials based on clinicopathological inclusion criteria. We sought to assess whether use of this registry to identify eligible patients would enable recruitment of 37 patients in less than eight months.
Treatment and Evaluation
RO4929097 was administered as a 20mg tablet by mouth daily on an empty stomach, 3 days on and 4 days off continuously. A single 50% dose reduction was allowed for recurrent grade 3 or 4 toxicity (10mg administered 3 days on and 4 days off continuously). Patients who experienced recurrent grade 3 or 4 toxicities after dose reduction were to be removed from the study. Evaluation visits were scheduled every 4 weeks along with standard blood tests (complete blood count, comprehensive metabolic panel) and CEA. Radiologic assessment of tumor burden (CT scans of the chest, abdomen and pelvis, or MRI of the abdomen and pelvis and CT of the chest) was scheduled every 8 weeks. Response Evaluation Criteria in Solid Tumors (RECIST version 1.1) were used for evaluation of the primary endpoint.
Immunohistochemical Analysis of Archival Specimens
Archival paraffin-embedded pathology specimens were requested on all subjects for immunohistochemical analysis of components of the Notch pathway: Notch-1, Intracellular Notch (ICN) and Hes-1 proteins. Pretreatment for antigen retrieval was performed using the DAKO Antigen Retrieval kit (Cat#S1700). Samples were preheated to 98°C for 15 min. After cooling for 20 min @ room temperature in solution, the samples were washed in milliQ-water for 4-5 min and incubated in quenching buffer for 10 min, washed in milliQ-water for 4-5 min, and reincubated for 10 min with Avidin blocking buffer (Vector Labs Cat#SP-2001). After washing with PBS for 5 min, the slides were blocked with Biotin blocking buffer, and placed in blocking buffer (1% BSA, 0.2% Milk) for an hour. This is followed by incubation with the rat anti-Hes-1 primary antibody (2 ug /ml), mouse anti-Notch-1 monoclonal antibody (dilution: 1:50 ), and mouse Anti-Human Notch-1, intracellular domain, aa 2428-2556 monoclonal antibody, Unconjugated, Clone 433802 (R&D system, 1 - 2 μg/mL) in blocking buffer overnight at 4°C (MBL Cat#D134-3 L#11). After rinsing with PBS for 5 min, the slides were incubated with a biotinylated goat anti-Rat secondary antibody, diluted in blocking buffer (0.334 ug/ml) for 1 hr at room temperature. After washing with PBS for 5 min, the slides were incubated with streptavidin–HPR diluted in blocking buffer 1:100 for 30 min (Invitrogen TSA Kit #21 cat# T20931), and washed again. The slides were next incubated in biotin –XX tyramide (in kit amplification buffer/0.0015% H2O2) for 10 min. After an additional washing, the samples were incubated with ABC for 30 min (Vector labs Cat# PK-6100) and developed with 3,3-diaminobenzidine (DAB) for 1-7 min (Vector labs Cat# SK-4100). All of the slides were lightly counterstained with hematoxylin for 10s before dehydration and mounting. Immunostaining was observed with a Leitz Orthoplan 2 microscope and images are captured by a CCD camera with the Smart Capture Program (Vysis, Downers Grove, IL). Positive controls were run with each set of slides. Negative controls were included by omitting the primary antibody during the primary antibody incubation.
The stained slides were scored for the presence of Notch 1, ICN and Hes-1 protein. The positive antibody reaction was scored into four grades, according to the intensity of the staining: 0, 1+, 2+, and 3+. The percentages of positive cells were also scored into four categories: 0 (0%), 1 (1-33%), 2 (34-66%), and 3 (67-100%). The product of the intensity and the percentage scores was used as the final score. The final scores were classified as: 0 negative; 1-3, weak; 4-6, moderate; and 7-9, strong.
Sample Size Calculation
The primary end point was the objective radiographic response rate. Secondary end points included progression-free survival (PFS), overall survival (OS), and toxicity, calculated according to the most recent version of the NCI Common Terminology Criteria for Adverse Events (CTCAE). For sample size calculation, a Simon's two-stage optimal design was used. The information used in the calculations of this design were: P0 <.05, P1 ≥.20, α=.1, power = 90%. This calculation yielded a total sample size of 37 patients. At least four responses (≥11%) were necessary to consider the regimen sufficiently active to pursue in further studies.
A first stage interim analysis was planned after enrollment of 12 patients, with early stopping to occur if no responses were observed (resulting in a 0.54 probability of early stopping if the response rate was ≤5%). However, per-protocol, accrual was allowed to continue beyond the first stage until all initial 12 patients underwent their first two follow-up scans.
Statistical Analysis
The Kaplan-Meier method was used to estimate all time-to-event functions. PFS was defined as time from start of treatment until disease progression or death as a result of any cause. OS was defined as time from start of treatment until death as a result of any cause, with patients censored at the date of last follow-up if still alive. Exact 95% CIs were calculated for each proportion of interest. Statistical analysis was performed using Stata SE 9.0 software and SAS 9.2 software. Parametric survival modeling was implemented as well. Exponential distribution assumption was verified using a reduced piecewise exponential test procedure. Point estimate and the exact 95% CI of the median survival based on the exponential distribution were computed.30 For tissue analysis, Spearman's rank correlation coefficient and Kendall's tau coefficient were computed to assess the correlations among the three immunohistochemical scores (Notch-1, ICN and HES-1).
Results
Patient Population
Thirty-seven patients were enrolled between May 13, 2011 and September 18, 2011. Demographic variables and tumor characteristics are listed in Table 2. Four patients withdrew consent; therefore 33 patients were evaluable for safety and radiologic response. Use of the TCC® registry contributed to the rapid identification of eligible patients and completion of accrual in four months. The conduct of the entire trial, from letter of intent submission to CTEP (N01 contract) through treatment of the last patient required just over 10 months.
Table 2.
Patient Demographics and Clinical Characteristics (N=37)
| Characteristic | No. | % |
|---|---|---|
| Age, years | ||
| Median | 60 | |
| Range | 42-81 | |
| Sex | ||
| Male | 22 | 59 |
| Female | 15 | 41 |
| Race | ||
| White | 30 | 81 |
| Black or African Ancestry | 4 | 11 |
| Other* | 3 | 8 |
| ECOG PS | ||
| 0 | 8 | 22 |
| 1 | 22 | 59 |
| 2 | 7 | 19 |
| Prior lines of systemic treatment† | ||
| 2 | 4 | 11 |
| 3 | 7 | 19 |
| >3 | 26 | 70 |
| Kras status | ||
| Wild type | 13 | 35 |
| Mutated | 18 | 49 |
| Unknown | 6 | 16 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; PS, performance status.
Hispanic, Asian/Pacific Islander, Native American
Excluding adjuvant therapy
Radiologic Response
Among 33 evaluable patients, 27 underwent at least one follow-up scan and 6 progressed clinically within 2 months of enrollment (including 1 disease-related death). No objective radiologic responses (PRs) were observed. Six patients had SD and 21 patients experienced PD as their best response. Figure 1 summarizes the maximum percent change from baseline in the sum of the longest diameters of target lesions.
Figure 1.
Waterfall plot illustrating best radiographic response (percent change) in each patient
Progression-free and Overall Survival
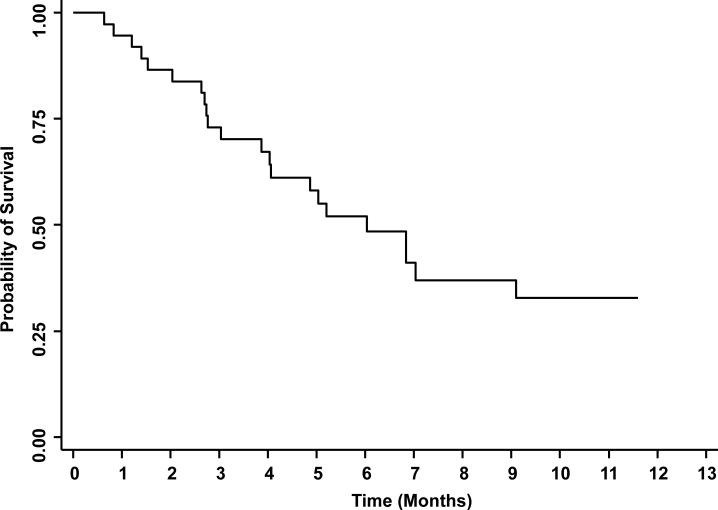
At time of data cutoff, 22 patients had died and 15 were alive, with follow-up duration for the surviving patients ranging from 2.8-11.6 months. The median PFS was 1.8 months (95% CI, 1.8-1.86; fig. 2) and the median OS was 6.0 months (95% CI, 3.9-9.1; fig. 3). The test for exponential survival indicated no violation of the exponential distribution with a single change point p-value from a backward elimination procedure to be 0.196. With the exponential assumption, the median survival was estimated to be 6.35 month (95%CI, 4.31-10.46)
Figure 2.
Kaplan Meier estimate of progression-free survival
Figure 3.
Kaplan Meier estimate of overall survival
Safety Profile
The study-drug was well-tolerated with no drug-related grade 3-4 toxicities observed on the trial. The toxicities considered possibly related to treatment are listed in table 3, and consisted primarily of grade 1-2 nausea.
Table 3.
Treatment-related Toxicity at Least Possibly Related to Therapy (all grade 1-2)
| Toxicity | No. | % |
|---|---|---|
| Nausea | 3 | 8 |
| Vomiting | 2 | 5 |
| Fatigue | 1 | 3 |
| Pruritis | 2 | 5 |
| Rash | 1 | 3 |
| Skin Hyperpigmentation | 1 | 3 |
| Dizziness | 1 | 3 |
| Stomatitis | 1 | 3 |
Tissue Analysis
Archival pathology specimens were available on 29 patients; 7 specimens obtained from primary tumor locations and 22 from distant metastases. These were stained immunohistochemically using antibodies to Notch-1, intracellular notch (ICN), and the target gene Hes-1 (fig. 4). The median Notch-1 IHC intensity score was 2 (range 0-6; 7 patients had absent staining, 12 had weak staining and 9 had moderate staining intensity). The median ICN score was 4 (range 0-9; 4 absent, 9 weak, 14 moderate, 2 strong) and the median Hes-1 score was 3 (range 0-9; 6 absent, 9 weak, 13 moderate, 1 strong). The Spearman's correlations were 0.61 between Notch-1 and Hes-1 (p=0.0001); 0.83 between Hes-1 and ICN (p<0.001); and 0.74 between Notch-1 and ICN (p<0.001). The Kendall's tau B correlations were 0.51 between Notch-1 and Hes-1 (p=0.001); 0.74 between Hes-1 and ICN (p<0.001); and 0.64 between Notch-1 and ICN (p<0.001). These results indicated that scores of Notch-1, ICN and Hes-1 were significantly correlated.
Figure 4.
Examples of positive and negative immunohistochemical staining for HES1, Notch1, and intracellular notch (ICN); (× 100 magnification).
Discussion
To our knowledge, this trial represented the first study of a γ-secretase inhibitor in patients with colorectal cancer. Preclinical data suggested that the Notch pathway was upregulated in patients with metastatic colorectal cancer, and that inhibition of γ-secretase could therefore alter the natural history of disease. However, in our study population, RO4929097 monotherapy, while tolerable, demonstrated no evidence of clinical activity. Not only was there an absence of objective responses, but other signs of drug activity (such as high rate of disease stability) were also lacking. The large majority of patients progressed during or prior to their initial restaging scans.
The explanation for lack of activity is not clear. One potential mechanism is auto-induction of RO4929097 metabolism (as a CYP3A4 substrate, RO4909097 also increases CYP3A4 activity in vivo). This effect was found to result in significant reduction of steady-state drug levels in a phase I clinical trial investigating multiple dosing schedules. Another possibility is that γ-secretase inhibitors are inactive as monotherapy in the treatment of colorectal cancer. Indeed, one preclinical study suggests a synergistic interaction between cytotoxic agents and γ-secretase inhibitors in colorectal cell lines.27 Consequently, early phase studies investigating combination therapies involving γ-secretase inhibitors may be warranted.
In summary, this study represented the first trial of a γ-secretase inhibitor in metastatic colorectal cancer. The study demonstrated no evidence of objective radiographic response and median PFS was short, indicating a lack of clinical activity at the study dose and schedule. Based on this data, we cannot recommend further investigations of RO4929097 as monotherapy in colorectal cancer.
Acknowledgments
Research support: National Cancer Institute Cancer N01 Contract CM-62208
Footnotes
ClinicalTrials.gov Identifier: NCT01116687
Study was performed at the H. Lee Moffitt Center and Research Institute
No Financial Disclosures
Conflict of Interest Statement:
None of the authors declares a personal or financial conflict of interest which could affect the outcome of this study
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 60(5):277–300. doi: 10.3322/caac.20073. Sep-Oct. [DOI] [PubMed] [Google Scholar]
- 2.Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004 Jan 15;22(2):229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 3.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004 Jun 3;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 4.Van Cutsem E, Kohne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009 Apr 2;360(14):1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 5.Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007 May 1;25(13):1658–1664. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 6.Saltz L. Colorectal Cancer Treatment: What's Next? (or: Is There Life After EGFR and VEGF?). Gastrointest Cancer Res. 2008 Jul;2(4 Suppl):S20–22. [PMC free article] [PubMed] [Google Scholar]
- 7.Grothey A, Sargent D, Goldberg RM, Schmoll HJ. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004 Apr 1;22(7):1209–1214. doi: 10.1200/JCO.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 8.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006 Sep;7(9):678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 9.Bolos V, Grego-Bessa J, de la Pompa JL. Notch signaling in development and cancer. Endocr Rev. 2007 May;28(3):339–363. doi: 10.1210/er.2006-0046. [DOI] [PubMed] [Google Scholar]
- 10.Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998 May 28;393(6683):382–386. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- 11.Nefedova Y, Gabrilovich D. Mechanisms and clinical prospects of Notch inhibitors in the therapy of hematological malignancies. Drug Resist Updat. 2008 Dec;11(6):210–218. doi: 10.1016/j.drup.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shih Ie M, Wang TL. Notch signaling, gamma-secretase inhibitors, and cancer therapy. Cancer Res. 2007 Mar 1;67(5):1879–1882. doi: 10.1158/0008-5472.CAN-06-3958. [DOI] [PubMed] [Google Scholar]
- 13.Rizzo P, Osipo C, Foreman K, Golde T, Osborne B, Miele L. Rational targeting of Notch signaling in cancer. Oncogene. 2008 Sep 1;27(38):5124–5131. doi: 10.1038/onc.2008.226. [DOI] [PubMed] [Google Scholar]
- 14.Aster JC, Pear WS, Blacklow SC. Notch signaling in leukemia. Annu Rev Pathol. 2008;3:587–613. doi: 10.1146/annurev.pathmechdis.3.121806.154300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staal FJ, Langerak AW. Signaling pathways involved in the development of T-cell acute lymphoblastic leukemia. Haematologica. 2008 Apr;93(4):493–497. doi: 10.3324/haematol.12917. [DOI] [PubMed] [Google Scholar]
- 16.Reedijk M, Odorcic S, Chang L, et al. High-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival. Cancer Res. 2005 Sep 15;65(18):8530–8537. doi: 10.1158/0008-5472.CAN-05-1069. [DOI] [PubMed] [Google Scholar]
- 17.Dickson BC, Mulligan AM, Zhang H, et al. High-level JAG1 mRNA and protein predict poor outcome in breast cancer. Mod Pathol. 2007 Jun;20(6):685–693. doi: 10.1038/modpathol.3800785. [DOI] [PubMed] [Google Scholar]
- 18.Massi D, Tarantini F, Franchi A, et al. Evidence for differential expression of Notch receptors and their ligands in melanocytic nevi and cutaneous malignant melanoma. Mod Pathol. 2006 Feb;19(2):246–254. doi: 10.1038/modpathol.3800526. [DOI] [PubMed] [Google Scholar]
- 19.Okuyama R, Tagami H, Aiba S. Notch signaling: its role in epidermal homeostasis and in the pathogenesis of skin diseases. J Dermatol Sci. 2008 Mar;49(3):187–194. doi: 10.1016/j.jdermsci.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 20.Pinnix CC, Herlyn M. The many faces of Notch signaling in skin-derived cells. Pigment Cell Res. 2007 Dec;20(6):458–465. doi: 10.1111/j.1600-0749.2007.00410.x. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, De Marco MA, Graziani I, et al. Oxygen concentration determines the biological effects of NOTCH-1 signaling in adenocarcinoma of the lung. Cancer Res. 2007 Sep 1;67(17):7954–7959. doi: 10.1158/0008-5472.CAN-07-1229. [DOI] [PubMed] [Google Scholar]
- 22.Konishi J, Kawaguchi KS, Vo H, et al. Gamma-secretase inhibitor prevents Notch3 activation and reduces proliferation in human lung cancers. Cancer Res. 2007 Sep 1;67(17):8051–8057. doi: 10.1158/0008-5472.CAN-07-1022. [DOI] [PubMed] [Google Scholar]
- 23.Peacock CD, Watkins DN. Cancer stem cells and the ontogeny of lung cancer. J Clin Oncol. 2008 Jun 10;26(17):2883–2889. doi: 10.1200/JCO.2007.15.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suwanjunee S, Wongchana W, Palaga T. Inhibition of gamma-secretase affects proliferation of leukemia and hepatoma cell lines through Notch signaling. Anticancer Drugs. 2008 Jun;19(5):477–486. doi: 10.1097/CAD.0b013e3282fc6cdd. [DOI] [PubMed] [Google Scholar]
- 25.Farnie G, Clarke RB. Mammary stem cells and breast cancer--role of Notch signalling. Stem Cell Rev. 2007 Jun;3(2):169–175. doi: 10.1007/s12015-007-0023-5. [DOI] [PubMed] [Google Scholar]
- 26.Reedijk M, Odorcic S, Zhang H, et al. Activation of Notch signaling in human colon adenocarcinoma. Int J Oncol. 2008 Dec;33(6):1223–1229. doi: 10.3892/ijo_00000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meng RD, Shelton CC, Li YM, et al. gamma-Secretase inhibitors abrogate oxaliplatin-induced activation of the Notch-1 signaling pathway in colon cancer cells resulting in enhanced chemosensitivity. Cancer Res. 2009 Jan 15;69(2):573–582. doi: 10.1158/0008-5472.CAN-08-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tolcher AW, Mikulski SM, Mesersmith WA, et al. Paper presented at: ASCO 2010. Chicago: A phase I study of RO4929097, a novel gamma secretase inhibitor, in patients with advanced solid tumors. [Google Scholar]
- 29.Investigator's Brochure RO4929097. F. Hoffmann-La Roche LTD.; Jun, 2008. [Google Scholar]
- 30.Epstien B, Sobel M. Some Theorems Relevant to Life Testing from an Exponential Distribution. Annals of Mathematical Statistics. 1954;25:373–381. [Google Scholar]