Abstract
This study evaluated the alveolar bone response to testosterone and the impact of Resolvin D2 (RvD2) on testosterone-induced osteoblast function. For the in vivo characterization, 60 male adult rats were used. Treatments established sub-physiologic (L), normal (N), or supra-physiologic (H) concentrations of testosterone. Forty rats were subjected to orchiectomy; 20 rats received periodical testosterone injections while 20 rats received testicular sham-operation. Four weeks after the surgeries, 10 rats in each group received a subgingival ligature around the lower first molars to induce experimental periodontal inflammation and bone loss. In parallel, osteoblasts were differentiated from neonatal mice calvariae and treated with various doses of testosterone for 48 h. Cell lysates and conditioned media were used for the determination of alkaline phosphatase, osteocalcin, RANKL, and osteoprotegerin. Micro-computed tomography linear analysis demonstrated that bone loss was significantly increased for both L and H groups compared to animals with normal levels of testosterone. Gingival IL-1β expression was increased in the L group (p < 0.05). Ten nM testosterone significantly decreased osteocalcin, RANKL, and OPG levels in osteoblasts; 100 nM significantly increased the RANKL:OPG ratio. RvD2 partially reversed the impact of 10 nM testosterone on osteocalcin, RANKL, and OPG. These findings suggest that both L and H testosterone levels increase inflammatory bone loss in male rats. While low testosterone predominantly increases the inflammatory response, high testosterone promotes a higher osteoblast-derived RANKL:OPG ratio. The proresolving mediator RvD2 ameliorates testosterone-derived downregulation of osteocalcin, RANKL, and OPG in primary murine osteoblasts suggesting a direct role of inflammation in osteoblast function.
Keywords: testosterone, osteoblasts, docosahexaenoic acids, RANK ligand, osteocalcin, osteoprotegerin
Introduction
Sex hormones are potent modulators of inflammation and bone turnover [1–3]. Variations in their systemic levels lead to dysregulation of biological processes. Testosterone is the main androgenic hormone involved in a variety of activities in humans [4]. Low levels of testosterone have been associated with a number of chronic inflammatory diseases, including an increase in cardiovascular disease markers [5], mortality [6], diabetes mellitus [7, 8], metabolic syndrome [9], and increased risk for bone fracture [10–15]. High levels of testosterone, due to the use of anabolic androgenic steroids, have also been linked to severe medical consequences including cardiovascular, endocrine, and psychiatric complications [16, 17] suggesting that both sub- and supra-physiologic serum concentrations of testosterone may be pathological.
Testosterone potentially impacts bone metabolism through inflammation. Proposed mechanisms include cytokine regulation and direct actions on osteoblast and osteoclast precursors [12, 18]. Both testosterone and its nonaromatizable metabolite 5α-dihydrotestosterone have been shown to decrease osteoclast differentiation or activity in avian, mouse, rat, and human cells [18–20]. This impact has been, in part, linked to the osteoblastic regulation of osteoclastogenesis [18, 21], suggesting that the bone turnover and osteoblast/osteoclast coupling are targeted by the changing levels of testosterone in circulation. As a part of this process, androgens may stimulate the proliferation of the osteoblast progenitors, differentiation of mature osteoblasts, but inhibit apoptosis [21]. While these activities suggest the involvement of several pathways of bone turnover, the data is still inconclusive. For example, actions of androgens on osteoblast-derived Receptor Activator of Nuclear Kappa B Ligand (RANKL) and osteoprotegerin (OPG) are controversial [18, 22, 23]. Therefore, more work is needed for elucidation of the impact of changes in testosterone levels and bone metabolism and how inflammation influences this scenario.
Bone response to the testosterone may also represent a failure of the resolution of the inflammatory process. Indeed, some studies have suggested that endogenous testosterone actively regulates tissue healing, inhibiting cutaneous repair associated with an increased inflammatory response [24, 25]. Inflammation resolution is a highly coordinated process [26] and requires the local biosynthesis and activity of endogenous specialized proresolving lipid mediators [27]. Resolvin D2 (RvD2) is a potent regulator of excessive inflammatory responses via multiple cellular targets to stimulate resolution and preserve immune vigilance [28]. RvD2 could thus play a role in the stabilization of bone metabolism in response to variations in testosterone levels.
As a chronic inflammatory disease, periodontitis affects teeth-surrounding tissues and results in extensive bone loss. The etiology is infectious and involves complex interactions between the host and the microbial biofilm [29]. It is not fully clear how fluctuations in levels of testosterone are associated with periodontal disease progression; however, potent risk factors such as aging and male gender suggest that there is a common link [30]. In this study, our aim was to test the hypothesis that changes in testosterone levels enhance inflammatory-induced bone loss. We have evaluated the impact of low and high levels of testosterone on ligature-induced periodontitis and bone loss and assessed markers of bone metabolism and cytokine expression. In addition, we have studied the impact of testosterone on primary murine osteoblasts in vitro. Finally, we have tested the impact of RvD2 on testosterone-treated osteoblasts.
Materials and Methods
Materials
A commercially available mixture of testosterone esters including 30 mg testosterone propionate, 60 mg testosterone phenylpropionate, 60 mg testosterone isocaproate, and 100 mg testosterone decanoate (Durateston) was purchased from MSD (Campinas, SP, Brazil). The biochemical colorimetric tests of calcium (Ca+2), alkaline phosphatase (ALP), and phosphorus (P) were purchased from Bioclin, Quibasa (Belo Horizonte, MG, Brazil). Minimal Essential Medium (MEM)-α was purchased from Life Technologies (Grand Island, NY, USA), Fetal Bovine Serum (FBS) from ATCC (Manassas, VA, USA), penicillin/streptomycin from Corning (Corning, NY, USA), and testosterone was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). A single plex OPG and multiplex RANKL-osteocalcin kits were purchased from EMD Millipore (Billerica, MA, USA). Minimum detectable concentration for the OPG was 2.3 pg/ ml; 2.7 pg/ml for RANKL, and 4.7 pg/ml for osteocalcin. Analyses were done on a Bio-Plex 200 from Bio-Rad Laboratories Inc. (Hercules, CA, USA). Enzyme-Linked Immunosorbent Assay (ELISA) kit for detection of ALP was purchased from Life Sciences Advanced Technologies Inc. (Saint Petersburg, FL, USA). Commercial kits for the detection of interleukin (IL)-1β and IL-6 were purchased from R & D Systems (Minneapolis, MN, USA). All protocols were performed according to the manufacturers’ instructions.
Animal model
Holtzman rats were obtained from Unesp Animal Facility (Araraquara, SP, Brazil). Sixty male adult Holtzman rats weighing 300–400 g were kept in cages under controlled conditions (controlled temperature 23 ± 2 °C, humidity 65–75 %, and 12-h lightdark cycles). Food and water were provided ad libitum. All experimental protocols were approved by the Institutional Ethics Committee for Animal Experimentation (protocol #25/2010) and performed in accordance with the guidelines of the National Council for Animal Experimentation Control (CONCEA).
In order to test the impact of “low testosterone (Low)”, we have used an orchiectomy model based on the literature [31]. After 1 week of acclimatization, 20 rats received orchiectomy to suppress the testosterone production and study the impact of decreased testosterone on bone metabolism. Briefly, a scrotal incision was performed for bilateral testicular removal and the incision was sutured under anesthesia using ketamine [1 ml/kg/body weight (bw)] and xylazine (0.4 ml/kg/bw) under sterile conditions. The rats were given acetaminophen (300 mg/kg/bw; orally) for postoperative pain relief and an intramuscular dose of penicillin and streptomycin (1 ml/kg/bw). After the procedure, the animals were kept in individual cages for 7 days for recovery. As a model for “high testosterone (High)”, we have used a method that was previously described [32–34]. This approach involves orchiectomy followed by an exogenous administration of testosterone. In order to reproduce this model, 20 rats were orchiectomized as described above and received supra-physiologic testosterone injections. Starting 3 days after the orchiectomy, a 250 mg/kg long-lasting mixture of testosterone esters was diluted to 0.1 ml in corn oil and injected intramuscularly every 7 days until sacrifice. In order to control for the stress of the surgery itself, 20 rats underwent sham-surgery and received the same surgical procedure except for the testicular removal. This group was used as the “normal testosterone” control group (Normal). Table 1 demonstrates the confirmation of low and high levels of testosterone in these experimental groups compared to the normal controls.
Table 1.
Mean (±SD) serum testosterone concentration and weight for animals in each experimental group.
| Normal testosterone | Low testosterone | High testosterone | |
|---|---|---|---|
| Final serum testosterone concentration (nM) | 4.42 ± 2.9 | 0.74 ± 0.2AA** | 111.10 ± 32.4* |
| Baseline weight (g) | 384.5 ± 10.5 | 385 ± 19.9 | 384.5 ± 15.0 |
| Final weight – nonligated animals (g) | 445±26.6 | 442±28.2A | 404±10.7** |
| Final weight – ligated animals (g) | 445±15.1 | 417.8±23.9A* | 378.9±24.2** |
p < 0.05;
p < 0.01 when compared to Normal testosterone group
p < 0.01;
p < 0.001 when compared to High testosterone group
In order to induce experimental periodontitis as a model for inflammatory bone loss, half of the rats in each group (n = 10/group) were anesthetized and received 3.0 cotton ligatures in a subgingival position, bilaterally, around the lower first molar teeth 4 weeks after the surgery. Ligatures enabled bacterial accumulation around the teeth, resulting in inflammation and bone loss. The other 10 animals in each group served as nonligated controls. The ligatures were maintained for 2 weeks and after that all rats were sacrificed.
Bone markers and inflammatory cytokines in circulation
Morning blood samples were collected from every animal at the end of the experiment and after clotting for 45 min at room temperature the sample was centrifuged for 10 min at 3 000 rpm to obtain blood serum. Each serum sample was analyzed for: total testosterone levels using a chemiluminescence-based immunoassay (Immulite 2000, Diagnostic Products Corporation, Gwynedd, UK.); calcium (Ca+2), alkaline phosphatase (ALP), and phosphorus (P) by biochemical colorimetric tests; and interleukin (IL)-1β and IL-6 by ELISA.
Expression of cytokines in gingiva
Total protein was extracted in T-per lysis buffer (Pierce, Thermo-Fisher) supplemented with proteinase inhibitors (Complete, Roche) from gingivomucosal tissues encircling the first molars of 5 animals per group used in the evaluation of IL-1β and IL-6 concentrations. Total protein concentrations in each sample were determined using the Bradford method and used for normalization.
Micro-computed tomography (µCT)
For quantitative and qualitative three-dimensional (3D) analysis of the alveolar bone, hemi-maxillae of 5 animals per group were scanned dorsum ventrally using a microfocus X-ray CT system (Skyscan, Aartselaar, Belgium). The sagittal plane of the specimens was set parallel to the X-ray beam axis. The specimens were scanned at a resolution of 8.8 µm in all 3 spatial dimensions. The scans were Gaussian filtered and segmented using a multilevel global thresh holding procedure for the segmentation of bone. We used CTan/CTvol software (Skyscan) for imaging and analysis. A standardized rectangular region of interest (ROI) measuring 1.13 × 0.97 mm was positioned at the furcation area (region among the roots of the teeth), in a slice-based method. The histogram settings were standardized at 90–130 and 101 serial slices were selected in each sample. Bone volume fraction (BV/TV) was analyzed by the CT-scan software. The linear distance between cementoenamel junction (CEJ)–a reference point on the tooth–and alveolar bone (AB), which can be more sensitive to detect local bone loss, was measured in the mesial surface of the first molars using software (Dataviewer 1.4.3, Skyscan). The measurement was performed 3 times by a calibrated individual, who was blinded to the treatment groups, and under the same background conditions. The mean of all 3 measurements was considered one sample and used for statistical analysis.
Primary murine osteoblast culture
In order to study the role of osteoblast response to testosterone, BalbC mice were obtained from Charles River Laboratories (Wilmington, MA, USA) and bred. Neonatal mouse calvarial osteoblasts were isolated from litters (7–8 mice) by dissection of the scalp skin and removal of the calvariae as previously described [35]. Ascorbic acid (50 µg/ml) and β-glycerophosphate (10 mM) were added to the medium every other day for 10 days to allow osteoblast differentiation.
Testosterone was diluted in DMSO to a stock solution of 100 mM. A 0.1 % DMSO solution was used as control. Further testosterone dilutions were done using culture medium. After 10 days of osteoblast differentiation with ascorbic acid and β-glycerophosphate, cells were treated with 10-fold increasing concentrations of testosterone (1–100 nM) for 48 h. All experiments were performed in quadruplicate and repeated at least 3 times. To assess the impact of RvD2 on testosterone-treated osteoblasts, RvD2 was added at 10 nM. Data were normalized to the control group (value 1) and shown as fold-change.
Conditioned media and cell lysates were collected 48 h after testosterone treatment. Cells were recovered using the ELISA kit lysis buffer containing 1 % protease inhibitor cocktail (P8340, Sigma-Aldrich). Samples were stored at −80 °C until analysis. Conditioned media was used for the detection of ALP, osteocalcin and OPG, whereas RANKL expression was assessed in the cell lysates by Luminex. The RANKL:OPG ratio for each sample was calculated.
Statistical analysis
One-way analysis of variance (ANOVA) was used for group comparisons. Tukey post-hoc test or an unpaired t-test was used for pairwise comparisons. When data did not present normal distribution or homogeneous variances the nonparametric Kruskal– Wallis test and Dunn’s post-test were used. All data were analyzed using GraphPad Prism version 5.0 for Mac OS X software, San Diego, California, USA. The significance level was set at α = 0.05. All data were expressed as mean ± SD.
Results
All animals survived the procedures. In nonligated healthy animals, baseline weight was similar among all groups (p > 0.05). At the end of the experiment, the mean weight in nonligated animals was significantly lower in the High group (p < 0.01 compared to Normal and Low groups), demonstrating that supra-physiologic levels lead to weight loss probably through increased fat metabolism.
In ligated animals where periodontal inflammation and bone loss was experimentally induced, the mean weight was statistically significantly reduced in the Low group (p < 0.05 compared to Normal) and in the High group (p < 0.01 compared to Normal and Low), suggesting that inflammation combined with abnormal levels of testosterone results in decreased body weight (Table 1).
Periodontal disease and low or high testosterone levels
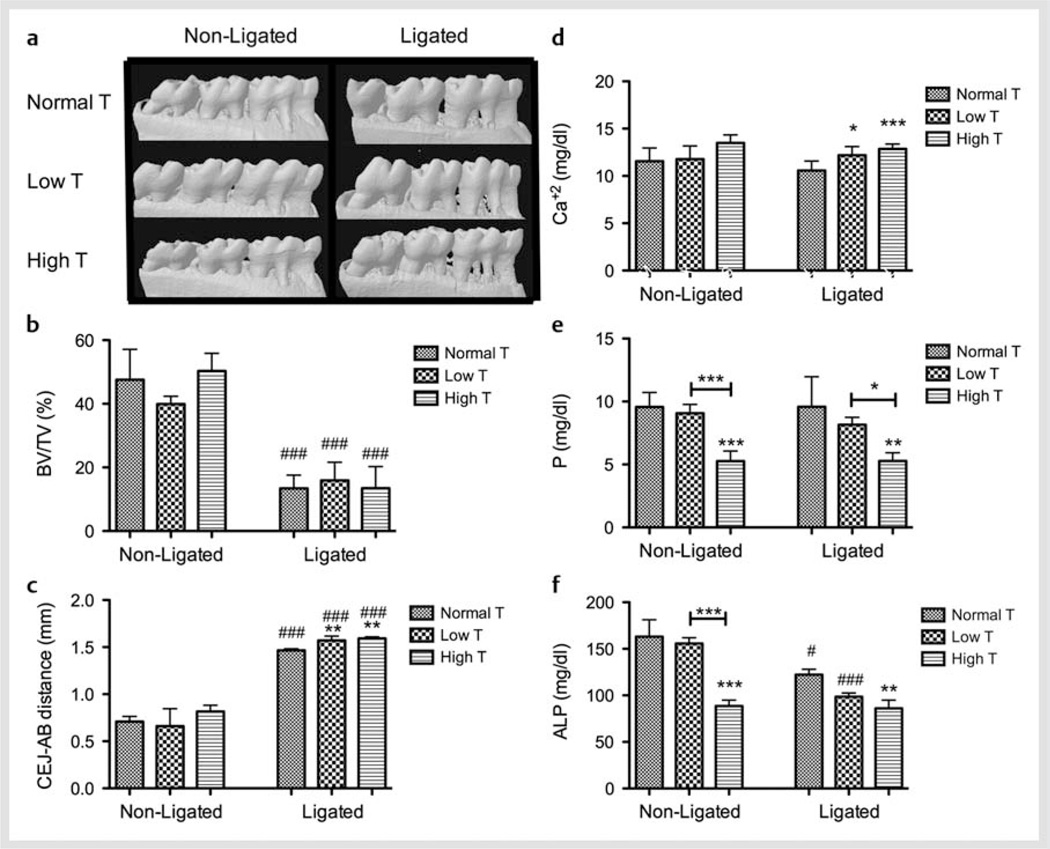
In nonligated animals, Low or High testosterone levels were not associated with any significant variations in alveolar bone volume or height. Ligature-induced periodontitis resulted in statistically significant bone loss, observed as lower BV/TV, in Normal, Low, and High groups compared to the nonligated animals (p < 0.001). Periodontitis in Low and High testosterone groups led to an increased loss in alveolar bone height compared to the Normal animals (p < 0.01). Fig. 1a–c show representative images, bone volume fraction at the furcation area (region among the roots of the tooth), and linear bone loss, respectively. Serum levels of phosphorus and ALP were significantly reduced in the High testosterone group when compared to both Normal and Low testosterone groups (p < 0.001) in nonligated animals. The presence of ligature-induced periodontal inflammation significantly reduced ALP concentration for Normal (p < 0.05) and High testosterone groups (p < 0.001), but not for Low testosterone when compared to their nonligated controls. In periodontitis animals, Low testosterone significantly increased calcium concentration (p < 0.05), while High testosterone increased calcium (p < 0.001) and decreased phosphorus and ALP levels (p < 0.01) suggesting an uncoupling between bone formation and resorption. Fig. 1d–f demonstrate the serum levels of calcium, phosphorus and ALP, respectively.
Fig. 1.
Testosterone (T) modulation affects bone metabolism in vivo. a Representative µCT images; b Bone volume fraction (BV/TV); c linear distance between cemento-enamel junction (CEJ) and alveolar bone (AB) on the mesial surface of the first molars; and serum concentration of d calcium (Ca+2); e phosphorus (P), and f alkaline phosphatase (ALP) of each experimental group in each experimental condition. *p < 0.05; **p < 0.01; ***p < 0.001 when compared to the respective non-ligated or ligated normal T group, unless otherwise connected. # p < 0.05; ### p < 0.001 when compared to the same treatment nonligated group.
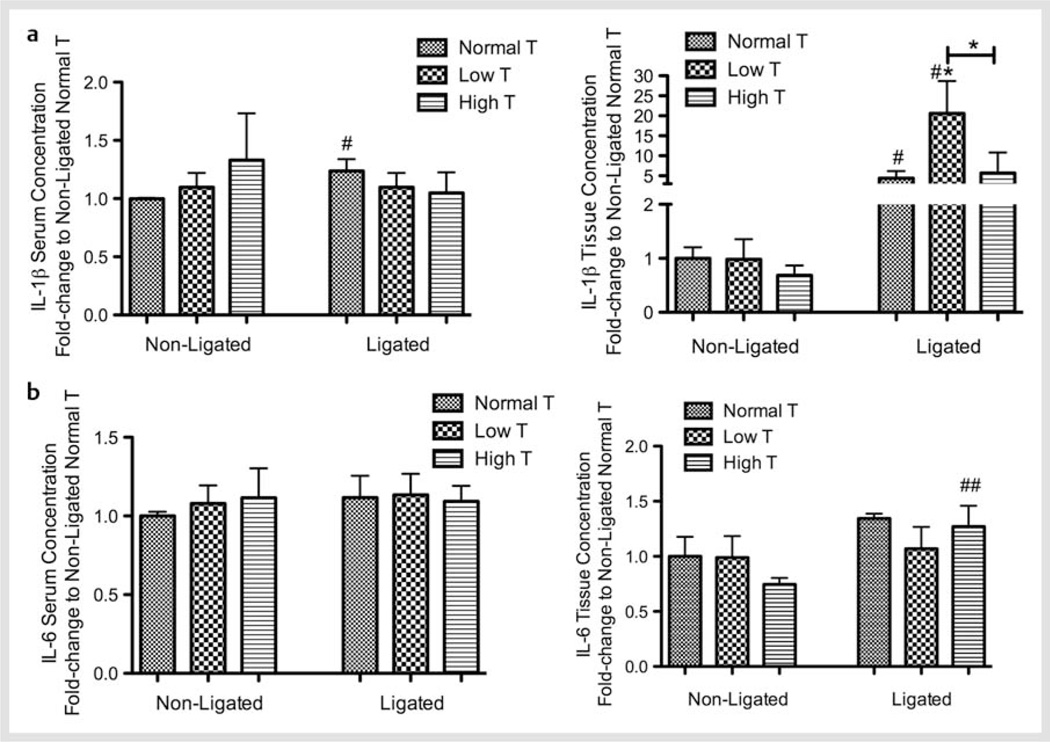
In order to study the impact of the testosterone levels on periodontal disease in an inflammatory environment, we measured the circulating and local expression of IL-1β and IL-6. In nonligated (and noninflamed) animals, changes in testosterone levels did not affect the systemic and gingival concentrations of IL-1β and IL-6. Periodontal inflammation significantly increased serum IL-1β for Normal testosterone animals (p < 0.05) and serum and gingival IL-1β levels for Normal and Low testosterone when compared to their respective nonligated controls (p < 0.05). High testosterone increased gingival IL-6 in the presence of ligature when compared to their nonligated controls. In the presence of inflammation, local IL-1β was significantly increased in the Low testosterone group when compared to Normal and High testosterone groups (p < 0.05). Fig. 2 shows serum and gingival levels of a IL-1β and b IL-6.
Fig. 2.
Testosterone (T) modulation affects cytokines expression in vivo. Fold-change in the serum and tissue concentration of a Interleukin (IL)-1β; and b IL-6 for each experimental group in each experimental condition. *p < 0.05 when compared to the respective non-ligated or ligated normal T group, unless otherwise connected. # p < 0.05; ## p < 0.01 when compared to the same treatment non-ligated group.
Impact of testosterone on osteoblasts
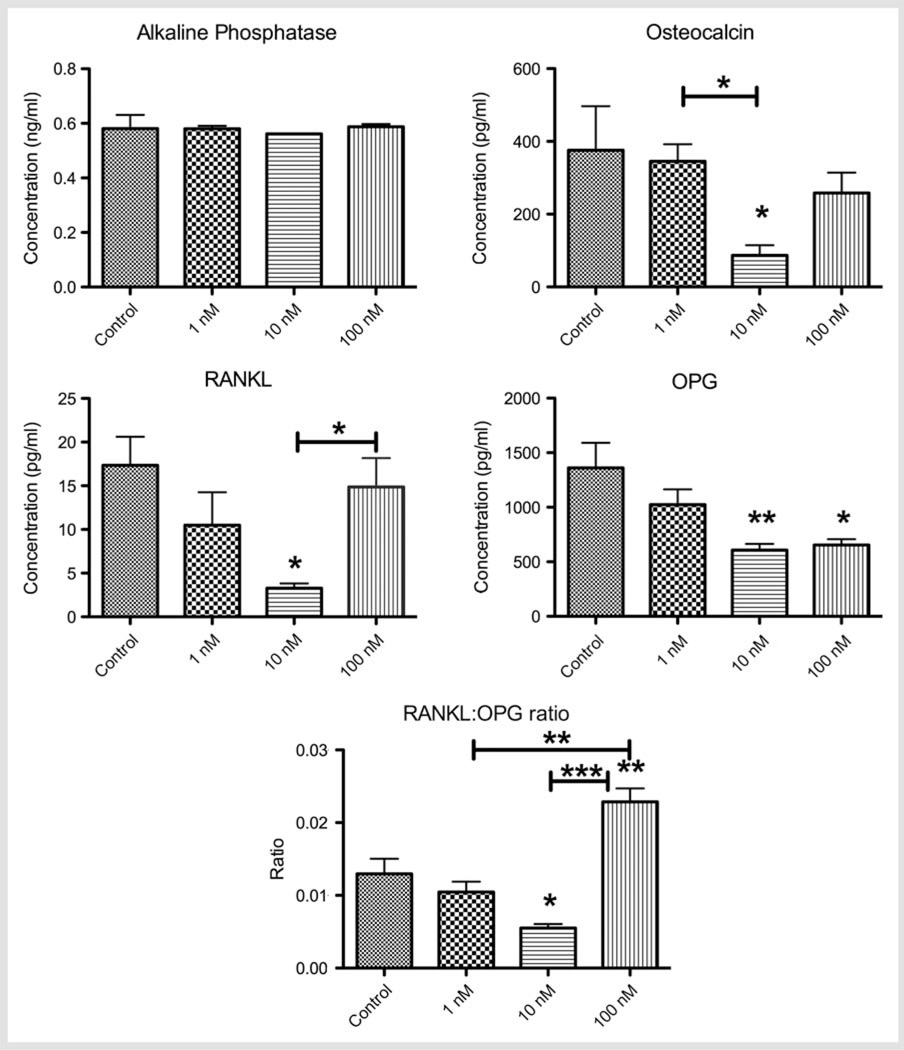
The impact of testosterone on the levels of ALP, osteocalcin, RANKL, and OPG generated by the osteoblasts in culture and RANKL:OPG ratio is shown in Fig. 3. Testosterone treatment did not significantly change the ALP production at any concentration. Osteocalcin concentration was significantly decreased by 10 nM testosterone when compared to the control (375.90 ± 69.75 pg/ml) or 1 nM groups (p < 0.05). Osteocalcin levels in response to 100 nM of testosterone was not significantly different compared to control or 1 nM while there was an increase over the 10 nM dose (p = 0.053). Testosterone resulted in a reduction of RANKL at 1 nM and 10 nM; the decrease was statistically significant at 10 nM testosterone compared to control (p < 0.05). At the higher dose (100 nM) of testosterone, there was a significant increase in RANKL compared to 10 nM, fully restoring the RANKL levels to nontreated levels (p < 0.05). Testosterone dose-dependently decreased OPG levels when compared to the control group; the difference was statistically significant for 10 nM (p < 0.01) and 100 nM (p < 0.05). The RANKL:OPG ratio was significantly decreased in response to 10 nM testosterone when compared to the control group (p < 0.05). High dose testosterone (100 nM), significantly increased the RANKL:OPG ratio when compared to control, 1 nM (p < 0.01) and 10 nM doses (p < 0.001).
Fig. 3.
Concentration (mean ± SD) of alkaline phosphatase, osteocalcin, RANKL, OPG, and RANKL:OPG ratio expressed by primary murine osteoblasts with increasing doses of testosterone. *p < 0.05; **p < 0.01; ***p < 0.001 when compared to control, unless otherwise connected.
Impact of RvD2 on 10 nM testosterone-derived downregulation of osteocalcin, RANKL, and OPG
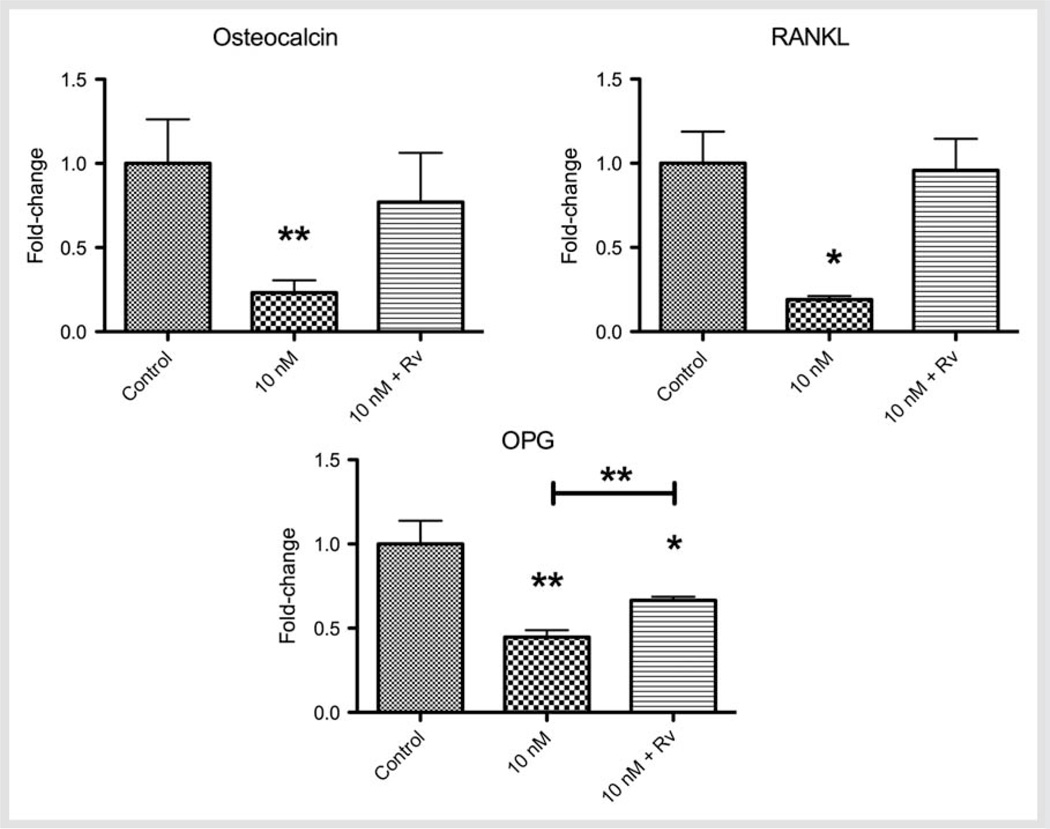
Since the major impact of the testosterone on osteoblast markers was observed at 10 nM, we studied the actions of RvD2 on 10 nM-testosterone-treated cells. The expression of osteocalcin, RANKL, and OPG in testosterone-treated cells with or without RvD2 is illustrated in Fig. 4. Osteocalcin and RANKL expression, which were decreased by 10 nM testosterone, were restored back to baseline levels in response to RvD2 treatment. RvD2 resulted in a more modest (49 %) but statistically significant (p < 0.01) restoration of OPG levels in cultures treated with 10 nM testosterone.
Fig. 4.
Fold-change of the concentration (mean ± SD) of osteocalcin, RANKL, and OPG expressed by primary murine osteoblasts upon testosterone-treated cells (10 nM) with or without RvD2 (10 nM). *p < 0.05; **p < 0.01 when compared to control, unless otherwise connected.
Discussion and Conclusions
The aim of this study was to test the impact of changes in testosterone levels on bone metabolism using a ligature-induced periodontal inflammation model and in vitro characterization of the osteoblast response. RvD2 was used as an active agonist of resolution of inflammation in osteoblast cultures. In the absence of inflammation, low or high levels of testosterone did not result in any bone loss. Inflammation stimulated pronounced bone loss and the impact was substantial in animals with low or high testosterone levels. We have further observed that osteoblast expression of RANKL is significantly decreased in the presence of 10 nM testosterone whereas 100 nM promoted a significant increase in RANKL:OPG ratio, suggesting that osteoclastogenesis is increased in vivo. Lastly, our data suggest that RvD2, a proresolution agonist, acts directly on osteoblasts reversing the actions of testosterone and rescuing the expression of osteocalcin, RANKL, and OPG.
Testosterone levels vary greatly among individuals and in different racial/ethnic populations [36]. High doses of testosterone are observed in bodybuilders and others using products for enhancement of athletic performance. This group of people generally use the compounds at concentrations of 10–100 times higher than normal [37]. We have recently shown that serum concentration of testosterone was approximately 7 nM in rats; a 10-fold reduction in these levels can be obtained following orchiectomy as a model of late onset hypogonadism in a rat model [34]. In parallel, the findings from our previous study and the current work showed that rats can be used for low or high levels of testosterone studies, with the advantage of enabling evaluation of the hormonal imbalances in vivo and ex vivo analyses [34]. Our results also reveal that testosterone modulates periodontal bone loss in an inflammatory environment without resulting in any significant change in healthy animals. This observation suggests that variations in testosterone levels are not associated with bone resorption in the absence of an inflammatory stimulus. When animals were exposed to an inflammatory bone loss, high testosterone levels aggravated the pathology; associated also with decreased phosphorus and ALP levels, and increase calcium levels. One possible explanation for these findings is that testosterone possesses immunosuppressive properties, as demonstrated by the suppressive effect on leukocyte count on orchiectomized mice and young male rats [38, 39]. Along those same lines, a recently published paper demonstrated that men using anabolic androgenic steroids present with more severe forms of periodontitis and higher prevalence of periodontopathogenic microorganisms, including some species of Candida [40]. We demonstrated that Low testosterone upregulates IL-1β production only at the site of inflammation (and not systemically), but it had no impact on IL-6 either locally or systemically. Both IL-1β and IL-6 are important cytokines that regulate osteoclast number and activity, and are therefore related to bone loss [41]. In humans, conflicting results have been reported regarding the relationship between cytokines and testosterone. A cross-sectional study demonstrated an inverse correlation between testosterone and soluble IL-6 receptor in older men (sIL-6r), but no correlation was found for other markers, such as IL-6, IL-1β, or tumor necrosis factor (TNF) [42]. Conversely, induction of 4-week hypogonadism in older men increased both IL-6 and sIL-6r [43], but those results seem not to be maintained in longer periods of hypogonadism [44]. Testosterone replacement therapy in hypogonadal men significantly decreased IL-1β and TNF, but had no effect on IL-6 levels [45]. When testosterone replacement therapy was withdrawn, a significant increase in IL-6 and decrease in TNF levels was observed 2 weeks later [46].
In vitro, we conducted a set of experiments to observe the impact of different testosterone doses on primary murine osteoblasts. We observed no difference in ALP levels after 48 h of testosterone treatment on primary murine osteoblasts, which is in accordance with a previous study using a different anabolic steroids (stanozolol) [47]. On the other hand, Hofbauer et al. [48] observed that 10 nM testosterone decreased ALP levels in human cell lysates. In stanozolol-treated cells, lower doses produced the highest osteocalcin secretion, with decreasing osteocalcin with increasing stanozolol concentration. This is in accordance with our results, which showed that osteocalcin expression is diminished in the presence of 10 nM testosterone. Our results suggest a feedback loop to regulate testosterone production in vivo, since it was recently demonstrated that osteocalcin from murine osteoblasts supernatants enhance the production of testosterone by testis explants as well as Leydig cells [49].
Our results showed that both RANKL and OPG are reduced by 10 nM testosterone, but RANKL reduction is greater, leading to a decreased RANKL:OPG ratio. RANKL and OPG play important roles in osteoclast differentiation; binding of RANKL to RANK on osteoclast precursors induces differentiation. OPG is a scavenger receptor for RANKL; hence, the RANKL:OPG ratio is critical in osteoclastogenesis [50]. OPG was found to be significantly upregulated by androgens in primary murine cells, osteoblastlike cells, and in a co-culture system of human cells [18, 23], but dose-dependently downregulated in human cells [22]. Our study is consistent with these last findings. The authors [22] suggest that these observations partially explain the weaker antiresorptive effects of testosterone as compared to estrogens. Yet, other studies report that RANKL mRNA has not been consistently detected and/or has been described as not regulated by androgens [22, 23]. We were able to detect RANKL expression in cell lysates, but not on cell supernatants. Most importantly, the RANKL:OPG ratio was significantly diminished with the 10 nM dose, suggesting that this dose may be protective against excessive osteoclastogenesis and favors bone homeostasis. The 100 nM dose was demonstrated to be inductive for osteoclastogenesis, as suggested by the significantly increased RANKL:OPG ratio, which can explain, at least partially, our in vivo results which showed high testosterone levels to increase periodontal bone resorption.
Previous studies from our group have already shown that osteoclast differentiation, activity and thus, bone remodeling, can be modulated by RvE1, an analogue of RvD2, with direct actions on bone, rescuing OPG production and restoring a favorable RANKL:OPG ratio [51, 52]. Here, we tested the hypothesis that RvD2 reverses testosterone-derived downregulation of osteocalcin, RANKL and OPG. Similarly to RvE1, RvD2 also had a positive impact on osteoblasts, either by bringing osteocalcin expression back to control levels or by significantly increasing OPG levels. This partial rescue can be explained by the simultaneous addition of the 2 treatments to the media, so that cells have a receptor agonist stimulus to respond to. On the other hand, RvD2 also increased, although not significantly, RANKL expression when combined with 10 nM testosterone. It is important to note that the cells used in the experiment were not subjected to any stimulus [like bacterial lipopolysaccharide (LPS) or IL-6] and thus should reflect physiologic conditions.
Not many studies are available using RvD2, and therefore many connections have to be suggested using RvE1 findings. In vivo, RvE1 was shown to decrease leukocyte infiltration, osteoclast differentiation, and decrease alveolar bone loss on an experimental model of ligature and Porphyromonas gingivalis-induced periodontal disease in rabbits [53]. RvE1 also restored lost periodontal tissue, including bone [54]. The specific binding sites for RvE1 has been identified as the human chemokine-like receptor 1 (ERV aka chemR23 or CMKLR1) and the overexpression of this receptor in transgenic mice indicates that in addition to antiinflammatory actions, RvE1 directly impacts bone remodeling by suppressing bone resorption [52]. The present proof of principle study suggests an interaction between sex hormones and the proresolving mediator RvD2 on the osteoblast level. In vivo experiments should be performed to further explore this issue, not only in physiologic but also in pathological conditions, such as inflammatory diseases.
Taken together, our findings suggest that both low and high testosterone levels increase ligature-induced periodontal bone loss in male rats. The mechanisms, however, may be different: low testosterone increases gingival IL-1β while high testosterone can promote higher osteoblast-derived RANKL:OPG ratio. Additionally, the proresolving mediator RvD2 can ameliorate testosterone-derived downregulation of osteocalcin, RANKL, and OPG by primary murine osteoblasts.
Acknowledgements
JPS was a recipient of a scholarship from the Sao Paulo State Research Foundation (FAPESP), Sao Paulo, SP, Brazil (#2010/09658-0) and from the Coordination for the Improvement of Higher Education Personnel (CAPES), Brasilia, DF, Brazil (#5258-11-1). JPS and LCS held research support grants from FAPESP (#2010/12021-4) and from the National Council for Scientific and Technological Development (CNPq), Brasilia, DF, Brazil (#470870/2011-7). LCS holds a Productivity Scholarship from CNPq. This work was supported by NIH/NIDCR grants DE015566 and DE020906 to AK and TVD.
Footnotes
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1.Clarke BL, Khosla S. Androgens and bone. Steroids. 2009;74:296–305. doi: 10.1016/j.steroids.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vodo S, Bechi N, Petroni A, Muscoli C, Aloisi AM. Testosterone-induced effects on lipids and inflammation. Mediat Inflam. 2013;2013:183041. doi: 10.1155/2013/183041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilliver SC. Sex steroids as inflammatory regulators. J Steroid Biochem Mol Biol. 2010;120:105–115. doi: 10.1016/j.jsbmb.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 4.Bain J. Testosterone and the aging male: to treat or not to treat? Maturitas. 2010;66:16–22. doi: 10.1016/j.maturitas.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Del Fabbro E, Hui D, Nooruddin ZI, Dalal S, Dev R, Freer G, Roberts L, Palmer JL, Bruera E. Associations among hypogonadism, C-reactive protein, symptom burden, and survival in male cancer patients with cachexia: a preliminary report. J Pain Symptom Manage. 2010;39:1016–1024. doi: 10.1016/j.jpainsymman.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 6.Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93:68–75. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stellato RK, Feldman HA, Hamdy O, Horton ES, McKinlay JB. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts male aging study. Diabetes Care. 2000;23:490–494. doi: 10.2337/diacare.23.4.490. [DOI] [PubMed] [Google Scholar]
- 8.Selvin E, Feinleib M, Zhang L, Rohrmann S, Rifai N, Nelson WG, Dobs A, Basaria S, Golden SH, Platz EA. Androgens and diabetes in men: results from the Third National Health and Nutrition Examination Survey (NHANES III) Diabetes Care. 2007;30:234–238. doi: 10.2337/dc06-1579. [DOI] [PubMed] [Google Scholar]
- 9.Laaksonen DE, Niskanen L, Punnonen K, Nyyssonen K, Tuomainen TP, Valkonen VP, Salonen R, Salonen JT. Testosterone and sex hormonebinding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004;27:1036–1041. doi: 10.2337/diacare.27.5.1036. [DOI] [PubMed] [Google Scholar]
- 10.Mellstrom D, Johnell O, Ljunggren O, Eriksson AL, Lorentzon M, Mallmin H, Holmberg A, Redlund-Johnell I, Orwoll E, Ohlsson C. Free testosterone is an independent predictor of BMD and prevalent fractures in elderly men: MrOS Sweden. J Bone Miner Res. 2006;21:529–535. doi: 10.1359/jbmr.060110. [DOI] [PubMed] [Google Scholar]
- 11.Meier C, Nguyen TV, Handelsman DJ, Schindler C, Kushnir MM, Rockwood AL, Meikle AW, Center JR, Eisman JA, Seibel MJ. Endogenous sex hormones and incident fracture risk in older men: the Dubbo Osteoporosis Epidemiology Study. Arch Intern Med. 2008;168:47–54. doi: 10.1001/archinternmed.2007.2. [DOI] [PubMed] [Google Scholar]
- 12.Maggio M, Basaria S. Welcoming low testosterone as a cardiovascular risk factor. Int J Impot Res. 2009;21:261–264. doi: 10.1038/ijir.2009.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Traish AM, Guay A, Feeley R, Saad F. The dark side of testosterone deficiency: I. Metabolic syndrome and erectile dysfunction. J Androl. 2009;30:10–22. doi: 10.2164/jandrol.108.005215. [DOI] [PubMed] [Google Scholar]
- 14.Traish AM, Saad F, Guay A. The dark side of testosterone deficiency: II. Type 2 diabetes and insulin resistance. J Androl. 2009;30:23–32. doi: 10.2164/jandrol.108.005751. [DOI] [PubMed] [Google Scholar]
- 15.Traish AM, Saad F, Feeley RJ, Guay A. The dark side of testosterone deficiency: III. Cardiovascular disease. J Androl. 2009;30:477–494. doi: 10.2164/jandrol.108.007245. [DOI] [PubMed] [Google Scholar]
- 16.Achar S, Rostamian A, Narayan SM. Cardiac and metabolic effects of anabolic-androgenic steroid abuse on lipids, blood pressure, left ventricular dimensions, and rhythm. Am J Cardiol. 2010;106:893–901. doi: 10.1016/j.amjcard.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanayama G, Hudson JI, Pope HG., Jr Long-term psychiatric and medical consequences of anabolic-androgenic steroid abuse: a looming public health concern? Drug Alcohol Depend. 2008;98:1–12. doi: 10.1016/j.drugalcdep.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michael H, Harkonen PL, Vaananen HK, Hentunen TA. Estrogen and testosterone use different cellular pathways to inhibit osteoclastogenesis and bone resorption. J Bone Miner Res. 2005;20:2224–2232. doi: 10.1359/JBMR.050803. [DOI] [PubMed] [Google Scholar]
- 19.Pederson L, Kremer M, Judd J, Pascoe D, Spelsberg TC, Riggs BL, Oursler MJ. Androgens regulate bone resorption activity of isolated osteoclasts in vitro. Proc Natl Acad Sci USA. 1999;96:505–510. doi: 10.1073/pnas.96.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huber DM, Bendixen AC, Pathrose P, Srivastava S, Dienger KM, Shevde NK, Pike JW. Androgens suppress osteoclast formation induced by RANKL and macrophage-colony stimulating factor. Endocrinology. 2001;142:3800–3808. doi: 10.1210/endo.142.9.8402. [DOI] [PubMed] [Google Scholar]
- 21.Vanderschueren D, Vandenput L, Boonen S, Lindberg MK, Bouillon R, Ohlsson C. Androgens and bone. Endocr Rev. 2004;25:389–425. doi: 10.1210/er.2003-0003. [DOI] [PubMed] [Google Scholar]
- 22.Hofbauer LC, Hicok KC, Chen D, Khosla S. Regulation of osteoprotegerin production by androgens and anti-androgens in human osteoblastic lineage cells. Eur J Endocrinol. 2002;147:269–273. doi: 10.1530/eje.0.1470269. [DOI] [PubMed] [Google Scholar]
- 23.Chen Q, Kaji H, Kanatani M, Sugimoto T, Chihara K. Testosterone increases osteoprotegerin mRNA expression in mouse osteoblast cells. Horm Metab Res. 2004;36:674–678. doi: 10.1055/s-2004-826013. [DOI] [PubMed] [Google Scholar]
- 24.Ashcroft GS, Mills SJ. Androgen receptor-mediated inhibition of cutaneous wound healing. J Clin Investig. 2002;110:615–624. doi: 10.1172/JCI15704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilliver SC, Ashworth JJ, Mills SJ, Hardman MJ, Ashcroft GS. Androgens modulate the inflammatory response during acute wound healing. J Cell Sci. 2006;119:722–732. doi: 10.1242/jcs.02786. [DOI] [PubMed] [Google Scholar]
- 26.Serhan CN. A search for endogenous mechanisms of anti-inflammation uncovers novel chemical mediators: missing links to resolution. Histochem Cell Biol. 2004;122:305–321. doi: 10.1007/s00418-004-0695-8. [DOI] [PubMed] [Google Scholar]
- 27.Serhan CN. Resolution phase of inflammation: novel endogenous antiinflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 28.Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amano A. Host-parasite interactions in periodontitis: microbial pathogenicity and innate immunity. Periodontology 2000. 2010;54:9–14. doi: 10.1111/j.1600-0757.2010.00376.x. [DOI] [PubMed] [Google Scholar]
- 30.Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ Cdc Periodontal Disease Surveillance Workgroup: James Beck GDRP. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dental Res. 2012;91:914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- 31.Borst SE, Conover CF. Orchiectomized Fischer 344 male rat models body composition in hypogonadal state. Life Sci. 2006;79:411–415. doi: 10.1016/j.lfs.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 32.Haggstrom S, Lissbrant IF, Bergh A, Damber JE. Testosterone induces vascular endothelial growth factor synthesis in the ventral prostate in castrated rats. J Urol. 1999;161:1620–1625. [PubMed] [Google Scholar]
- 33.Nolan LA, Levy A. The effects of testosterone and oestrogen on gonadectomised and intact male rat anterior pituitary mitotic and apoptotic activity. J Endocrinol. 2006;188:387–396. doi: 10.1677/joe.1.06508. [DOI] [PubMed] [Google Scholar]
- 34.Steffens JP, Coimbra LS, Ramalho-Lucas PD, Rossa C, Jr, Spolidorio LC. The effect of supra- and subphysiologic testosterone levels on ligatureinduced bone loss in rats-a radiographic and histologic pilot study. J Periodontol. 2012;83:1432–1439. doi: 10.1902/jop.2012.110658. [DOI] [PubMed] [Google Scholar]
- 35.Gao A, Kantarci A, Herrera BS, Gao H, Van Dyke TE. A critical role for suppressors of cytokine signaling 3 in regulating LPS-induced transcriptional activation of matrix metalloproteinase-13 in osteoblasts. Peer J. 2013;1:e51. doi: 10.7717/peerj.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazur A. The age-testosterone relationship in black, white, and Mexican-American men, and reasons for ethnic differences. Aging Male. 2009;12:66–76. doi: 10.1080/13685530903071802. [DOI] [PubMed] [Google Scholar]
- 37.Ozcelik O, Haytac MC, Seydaoglu G. The effects of anabolic androgenic steroid abuse on gingival tissues. J Periodontol. 2006;77:1104–1109. doi: 10.1902/jop.2006.050389. [DOI] [PubMed] [Google Scholar]
- 38.Kamis AB, Ibrahim JB. Effects of testosterone on blood leukocytes in plasmodium berghei-infected mice. Parasitol Res. 1989;75:611–613. doi: 10.1007/BF00930957. [DOI] [PubMed] [Google Scholar]
- 39.Yao G, Liang J, Han X, Hou Y. In vivo modulation of the circulating lymphocyte subsets and monocytes by androgen. Internat Immunopharmacol. 2003;3:1853–1860. doi: 10.1016/j.intimp.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 40.Brusca MI, Verdugo F, Amighini C, Albaina O, Moragues MD. Anabolic steroids affect human periodontal health and microbiota. Clin Oral Investig. 2013 doi: 10.1007/s00784-013-1126-9. [DOI] [PubMed] [Google Scholar]
- 41.Graves DT, Oates T, Garlet GP. Review of osteoimmunology and the host response in endodontic and periodontal lesions. J Oral Microbiol. 2011;3 doi: 10.3402/jom.v3i0.5304. 10.3402/jom.v3i0.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maggio M, Basaria S, Ble A, Lauretani F, Bandinelli S, Ceda GP, Valenti G, Ling SM, Ferrucci L. Correlation between testosterone and the inflammatory marker soluble interleukin-6 receptor in older men. J Clin Endocrinol Metab. 2006;91:345–347. doi: 10.1210/jc.2005-1097. [DOI] [PubMed] [Google Scholar]
- 43.Khosla S, Atkinson EJ, Dunstan CR, O´Fallon WM. Effect of estrogen versus testosterone on circulating osteoprotegerin and other cytokine levels in normal elderly men. J Clin Endocrinol Metab. 2002;87:1550–1554. doi: 10.1210/jcem.87.4.8397. [DOI] [PubMed] [Google Scholar]
- 44.Maggio M, Blackford A, Taub D, Carducci M, Ble A, Metter EJ, Braga-Basaria M, Dobs A, Basaria S. Circulating inflammatory cytokine expression in men with prostate cancer undergoing androgen deprivation therapy. J Androl. 2006;27:725–728. doi: 10.2164/jandrol.106.000141. [DOI] [PubMed] [Google Scholar]
- 45.Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89:3313–3318. doi: 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- 46.Yialamas MA, Dwyer AA, Hanley E, Lee H, Pitteloud N, Hayes FJ. Acute sex steroid withdrawal reduces insulin sensitivity in healthy men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2007;92:4254–4259. doi: 10.1210/jc.2007-0454. [DOI] [PubMed] [Google Scholar]
- 47.Vaishnav R, Beresford JN, Gallagher JA, Russell RG. Effects of the anabolic steroid stanozolol on cells derived from human bone. Clin Sci (Lond) 1988;74:455–460. doi: 10.1042/cs0740455. [DOI] [PubMed] [Google Scholar]
- 48.Hofbauer LC, Hicok KC, Khosla S. Effects of gonadal and adrenal androgens in a novel androgen-responsive human osteoblastic cell line. J Cell Biochem. 1998;71:96–108. [PubMed] [Google Scholar]
- 49.Oury F, Sumara G, Sumara O, Ferron M, Chang H, Smith CE, Hermo L, Suarez S, Roth BL, Ducy P, Karsenty G. Endocrine regulation of male fertility by the skeleton. Cell. 2011;144:796–809. doi: 10.1016/j.cell.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nelson CA, Warren JT, Wang MW, Teitelbaum SL, Fremont DH. RANKL employs distinct binding modes to engage RANK and the osteoprotegerin decoy receptor. Structure. 2012;20:1971–1982. doi: 10.1016/j.str.2012.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herrera BS, Ohira T, Gao L, Omori K, Yang R, Zhu M, Muscara MN, Serhan CN, Van Dyke TE, Gyurko R. An endogenous regulator of inflammation, resolvin E1, modulates osteoclast differentiation and bone resorption. Br J Pharmacol. 2008;155:1214–1223. doi: 10.1038/bjp.2008.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao L, Faibish D, Fredman G, Herrera BS, Chiang N, Serhan CN, Van Dyke TE, Gyurko R. Resolvin E1 and chemokine-like receptor 1 mediate bone preservation. J Immunol. 2013;190:689–694. doi: 10.4049/jimmunol.1103688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hasturk H, Kantarci A, Ohira T, Arita M, Ebrahimi N, Chiang N, Petasis NA, Levy BD, Serhan CN, Van Dyke TE. RvE1 protects from local inflammation and osteoclast-mediated bone destruction in periodontitis. FASEB J. 2006;20:401–403. doi: 10.1096/fj.05-4724fje. [DOI] [PubMed] [Google Scholar]
- 54.Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, Van Dyke TE. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 2007;179:7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]