Abstract
Childhood cancer survivors are at risk for development of subsequent neoplasms of the central nervous system (CNS). Better understanding of the rates, risk factors for and outcomes of subsequent neoplasms of the CNS among survivors of childhood cancer may lead to the development of more informed screening guidelines. Two independent investigators independently performed a systematic search of studies from the MEDLINE and EMBASE databases (1966 – 2012) for studies examining subsequent neoplasms of the CNS among childhood cancer survivors. Articles were selected to answer 3 questions: What is the risk of CNS tumors following radiation to the cranium for a pediatric cancer as compared with the general population? What are the outcomes in children with subsequent neoplasms of the CNS who have been treated with CNS directed radiation for a pediatric cancer? Are outcomes of subsequent neoplasms different from primary neoplasms of the same histology? Our search identified 72 reports, of which 18 publications were included in this review. These studies reported that childhood cancer survivors have an 8.1 – 52.3 times higher incidence of subsequent CNS neoplasms compared with the general population. Nearly all cancer survivors who developed a CNS neoplasm had been exposed to cranial radiation; some studies demonstrate a correlation between radiation dose and risk of subsequent CNS tumors. Five year survival rates for subsequent high-grade gliomas and meningiomas range from 0 – 19.5% and 73 – 100%, respectively, which are similar to those observed in patients with primary gliomas or meningiomas. The quality of evidence was limited by variation in study design, heterogeneity of details regarding treatment and outcomes, limited follow-up and relatively small sample sizes. We concluded that survivors of childhood cancer who were treated with cranial radiation therapy have an elevated risk for subsequent CNS neoplasms. The current literature is insufficient to comment about the potential harms and benefits of routine screening for subsequent CNS neoplasms.
INTRODUCTION
An estimated 10,700 children between the ages 0 – 14 years are diagnosed with cancer in the United States every year.1 Although cancer among children is rare, the development of effective treatments for childhood cancer is one of the most impressive success stories of modern medicine. With contemporary therapies, greater than 80% of all children who are diagnosed with cancer are expected to become long-term survivors.1 For survivors, the occurrence of a subsequent neoplasm is among the most devastating sequelae following cancer therapy and is often associated with considerable morbidity and risk for mortality.
Children exposed to cranial radiation are at increased risk for developing subsequent neoplasms of the central nervous system (CNS). The risk was first recognized among children who developed brain tumors following treatment with radiation therapy during the 1940s and 1950s for tinea capitis2 or tonsillitis3, and was observed among children who were exposed to as little as 2·5 Gray (Gy) (or dose equivalent) radiation. A recent study by Pearce and colleagues demonstrated an almost three-fold increased risk of brain tumors among children following CT scans with exposures doses of approximately 60 mGy radiation.4 Furthermore, a dose-response relationship with an increased risk of CNS tumors was identified among atomic bomb survivors.5 In the modern era, children with acute lymphoblastic leukemia (ALL) and CNS tumors, the two types of childhood cancer that are most often treated with cranial radiation therapy, are also at risk of subsequent neoplasms of the CNS.6–10
Childhood cancer survivors with subsequent neoplasms of the CNS are at high risk for death.11–13 A study utilizing the Surveillance, Epidemiology, and End Results (SEER) database reported a 10 year survival rate of 13·6% after subsequent CNS tumors after a primary diagnosis of pediatric solid tumors.14 A report by Morris and co-workers reported that death due to subsequent neoplasms of the CNS was the second most common cause of death among five year survivors of CNS tumors, ranking only behind recurrence of the primary tumor.15
The Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers,16 published by the Children’s Oncology Group (COG), recommend that childhood cancer survivors exposed to cranial radiation therapy undergo annual history and physical examinations with screening MRI of the brain “as clinically indicated for symptomatic patients.” The Long-Term Follow-Up Guidelines also suggest that clinicians consider brain MRI every other year for patients with neurofibromatosis beginning 2 years after radiation therapy.”16 Nevertheless, the benefit derived from screening MRI for subsequent neoplasms among childhood cancer survivors treated with cranial radiation therapy is incompletely understood and approaches to surveillance are variable. A study of surveillance imaging by pediatric oncologists revealed that 57·4% did not routinely obtain MRIs beyond 10 years after diagnosis for survivors of childhood brain tumors treated with cranial radiation.17 The objectives of this systematic review is to examine rates of, risk factors for, and outcomes of subsequent CNS tumors with the intention of establishing more informed guidelines for screening for these tumors.
METHODS
Study Population
This manuscript is a review of studies describing survivors of childhood, adolescent or young adult cancer (aged < 21 years at diagnosis) who are diagnosed with subsequent neoplasms of the CNS.
Key Questions
The review focused upon three key questions. (1) What is the risk of CNS tumors following radiation to the cranium for a pediatric cancer as compared with the general population? (2) What are the outcomes in children with subsequent neoplasms of the CNS who have been treated with CNS directed radiation for a pediatric cancer? (3) Are outcomes of subsequent neoplasms different from primary neoplasms of the same histology?
Search Strategy and Selection Criteria
Before performing the literature review, criteria were defined for inclusion of studies and for assessing the validity of these studies. Inclusion and exclusion criteria for each key question are detailed in Table 1. The MEDLINE and EMBASE databases were searched for literature published from January of 1966 through March of 2012. Limits were set for human only and English language only. The MeSH terms brain neoplasm, CNS neoplasm, meningioma, glioma, glioblastoma, astrocytoma$ and second neoplasm/cancer; child$ or adol$ and neoplasm; radiotherapy and brain or cranium or craniospinal; cancer screening or MRI were used in separate searches, and studies found during each search were combined. Cavernous hemangiomas and other vascular malformations were not considered to be neoplasms and were not examined in this review. Two of the authors (DCB, LB) independently reviewed the full texts of the articles that appeared to meet eligibility criteria based upon abstract review. The STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) reporting criteria18 were utilized to evaluate all studies included in the review. The STROBE criteria were developed by the STROBE Initiative, an international collaboration of epidemiologists, methodologists, statisticians, researchers and journal editors with the aim to assist authors when writing up analytical observational studies, to support editors and reviewers when considering such articles for publication, and to help readers when critically appraising published articles. Relevant articles, abstracts, and review articles were selected and reviewed, and we supplemented this reference list by cross-checking bibliographies of retrieved articles to identify additional studies. The last search was done May 30, 2011.
Table 1.
Inclusion and Exclusion Criteria for the 3 Key Questions*
| Variable | Question 1 | Question 2 | Question 3 |
|---|---|---|---|
| Inclusion criteria | |||
| Population | |||
| Cancer survivors who received a diagnosis of brain tumor after cranial radiation | X | X | X |
| Previous diagnosis of cancer as a child or young adult | X | X | X |
| Study design | |||
| Retrospective cohort | X | X | X |
| Retrospective case:control | X | X | X |
| Prospective cohort or clinical trial | X | ||
| Outcomes | |||
| Risk estimates of subsequent brain tumors: standardized incidence ratios, relative risks, absolute excess risks or cumulative incidence; overall and treatment-based (radiation) risk estimates | X | ||
| Clinical characteristics of subsequent brain tumor: location, pathologic features, interval since primary cancer | X | ||
| Progression-free and overall survival | X | ||
| Exclusion criteria | |||
| Study did not include humans | X | X | X |
| Study was not published in English | X | X | X |
| Case report, review, editorial, or letter | X | X | X |
Question 1: What is the risk of subsequent CNS tumors following radiation to the cranium for a pediatric cancer? Question 2: What are the outcomes in children with subsequent neoplasms of the CNS who have been treated with cranial radiation for a pediatric cancer? Question 3: Are outcomes of subsequent neoplasms different from primary neoplasms of the same histology?
Data Extraction
For key question 1, we extracted measures of subsequent CNS tumor risk [standardized incidence ratios, relative risk, absolute excess risk, and cumulative incidence]. For key question 2, we extracted information regarding age at first cancer diagnosis, exposure to cranial radiation, interval from primary cancer diagnosis to subsequent CNS tumor diagnosis and survival (both overall and 5 year survival rates). For key question 3, we compared survival of cancer survivors with subsequent CNS tumors with young adults with primary CNS tumors of the same histology (high-grade gliomas and meningiomas). Estimates of survival of people from the general population with high-grade gliomas were obtained from the Central Brain Tumor Registry of the United States (CBTRUS).19 The CBTRUS report from the years 2004 – 2008 was selected as a comparison group for survivors of childhood cancer with subsequent CNS tumors because this database contains the largest aggregation of population–based data for the incidence of all primary CNS tumors in the United States.19 The 2012 CBTRUS Report contains data provided by forty-nine population-based cancer registries from the National Program of Cancer Registries of the Centers for Disease Control and Prevention of the United States and selected participating states of the National Cancer Institutes’ Surveillance, Epidemiology, and End Results (SEER) program. Non-malignant meningiomas have only been included in the SEER registry since 2004;20 therefore, the estimates of survival of people from the general population with meningiomas from 1985 – 1992 was derived from the study by McCarthy and colleagues of the National Cancer Data Base, which includes tumors from approximately 1000 hospitals participating in the American College of Surgeons’ tumor registry program.21
RESULTS
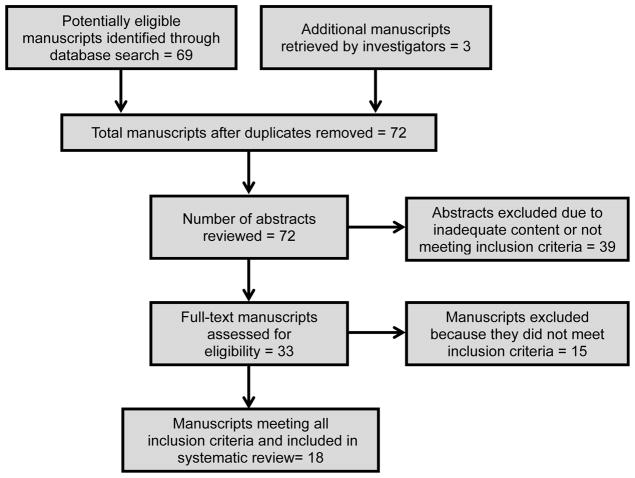
Sixty-nine articles were initially identified from the MEDLINE and EMBASE searches. Thirty nine studies were excluded after review of the abstract due to inadequate content or not meeting inclusion criteria (Figure 1). Thirty manuscripts were reviewed in full by two authors (DCB, LB). Of these, 15 manuscripts were included in the final review along with three additional studies which were identified from the study bibliographies. Thus, 18 manuscripts met inclusion criteria for inclusion in this review: 16 retrospective cohort studies and 2 retrospective case-control studies (representing 14 separate institutions and consortia) (Figure 1).
Figure 1.
Flow chart showing the progress of selecting manuscripts
Question 1: What is the risk of CNS tumors following radiation to the cranium for a pediatric cancer as compared with the general population?
Fourteen retrospective cohort studies8, 10, 22–33 evaluated risk of subsequent CNS tumors following treatment for a childhood or young adult malignancy (Table 2). These cohort studies included more than 150,000 survivors of childhood or young adult cancer distributed over 7 decades (1940 – 2005). Among these cancer survivors, there were 959 subsequent CNS tumors.
Table 2.
Subsequent Neoplasms of the Central Nervous System among Survivors of Pediatric or Young Adult Cancer
| Study, Year (Reference); Location | STROBE Criteria Met, n/nt |
Primary Cancer Diagnosis: |
Era | Patients, N |
Exposure to Cranial Radiation, % |
Patients With Brain Tumors, n |
Follow-up (years) |
SIR, by Age at Primary Cancer Diagnosis |
AER**, by Age at Primary Cancer Diagnosis |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age Range (years) |
SIR (95% CI) | Interval Since Primary Diagnosis, (years)** |
AER** | ||||||||
| Single Institution | |||||||||||
| Baker KS et al(21), 2003; United States (University of Minnesota) | 26/33 | Bone Marrow Transplant Recipients | 1974 – 2001 | 3372 | 78% | Glioma: 2 Other: 2 |
5 | 1 – 67 | 9.5 (2.5 – 21.1) | All | 3.4 |
| Hijiya N et al(22), 2007; United States (St. Jude) | 25/33 | ALL | 1962 – 1998 | 2169 | NR | Glioma: 21 Meningioma: 24 Other: 3 |
18.7 | 0 – 21 | 31.8 (19.7 – 47.6) | NR | NR |
| Cardous-Ubbink MC(23), 2007; The Netherlands (Amsterdam) | 31/33 | Childhood Cancer | 1966 – 2002 | 2603 | NR | Brain: 4 Meningioma:12 |
16.8 | 0 – 18 | Brain: 10.8 (2.93–27.6) Meningioma: 41.2 (21.3–71.9) |
NR | Brain: 2.1 Meningioma: 6.9 |
| Multiple Institutions | |||||||||||
| Neglia JP et al(24), 1991; Children’s Cancer Study Group | 25/32 | ALL | 1972 – 1988 | 9720 | NR | Glioma: 14 Menginioma: 2 Other 8 |
4.7 | 0 – 21 | 21.7$ | NR | NR |
| Rosso P et al(25), 1994; Italy (36 institutions) | 27/32 | Childhood Cancer | 1960 – 1986 | 3310 | 75.5%* | Glioma: 8 Other:1 |
5.8 | 0 – 16 | 52.3 (22.5 – 103.0)# | 0 – 4 5 – 9 10 – 14 > 15 All |
17.3 902 0 0 72.8 |
| Löning, L et al(26), 2000 Germany (BFM consortium) |
24/33 | ALL | 1979 – 1995 | 5006 | 77.2% | Glioma: 8 Meningioma: 2 Other: 3 |
5.7 | 0 – 18 | 18.6 (9.8 – 29.4) | NR | NR |
| Bhatia S et al(8), 2002; United States and Canada (Children’s Cancer Group) | 27/32 | ALL | 1983 – 1995 | 8831 | 38% | Glioma: 13 Meningioma: 2 Other: 4 |
5.5 | 0 – 21 | 10.1 (5.9 – 16.2) | All | 2.8 |
| Armstrong GT et al(31), 2009 United States (Childhood Cancer Survivor Study) |
31/33 | CNS Tumors | 1970 – 1986 | 2821 | 60.5 | Glioma: 15 Meningioma: 63 Other: 1 |
15.4 | 0 – 21 | Glioma: 24.3 (13.6 – 40.1) Meningioma: 714.7(192.3–1829.7) All: 25.3 (15.5 to 39.1) |
NR | NR |
| Friedman DL et al(32), 2010; United States (Childhood Cancer Survivor Study) | 31/33 | Childhood Cancer | 1970 – 1986 | 14359 | 59.4% | Glioma: 43 Other: 37 Meningioma: 170 |
22.7 | 0 – 21 | Glioma: 8.9 (6.8 – 11.7) Meningioma: 87.8 (26.5 – 291.4) All: 10.4 (8.3 – 13.1) |
NR | NR |
| Taylor AJ et al(10), 2010; United Kingdom (British Childhood Cancer Survivor Study) | 30/33 | Childhood Cancer | 1940 – 1991 | 17980 | 51.3% | Glioma: 73 Meningioma: 137 Other: 37 |
17.3 | 0 – 4 5 – 9 10 – 14 15 – 19 20 – 29 ≥ 30 0 – 15 |
20.6 (13.6 – 30.1) 7.5 (3.4 – 14.3) 11.0 (5.5 – 19.7) 12.5 (6.2 – 22.3) 7.2 (3.5 – 13.3) 5.0 (1.6 – 11.7) 10.8 (8.5 – 13.6)& Glioma: 10.8 (8.5 – 13.6) |
0 – 4 5 – 9 10 – 14 15 – 19 20 – 29 ≥ 30 |
3.0& 1.0& 1.8& 2.6& 2.1& 2.6& |
| Population Based | |||||||||||
| Jenkinson H and Hawkins M(27), 1999; United Kingdom | 14/32 | ALL | 1962 – 1987 | 3961 | 87% | Glioma: 8 Other: 2 |
NR | 0 – 18 | 23 (11 – 43)^ | NR | NR |
| Inskip PD and Curtis RE(28), 2007; United States (SEER Registry) | 30/33 | Childhood Cancer | 1973 – 2002 | 25965 | NR | All CNS: 51 | 8.9 | 0 – 18 | 7.9$ | All | 1.9 |
| Maule M et al(29), 2007; (13 population-based cancer registries)@ | 27/31 | Leukemia, Lymphoma, Hodgkin’s Disease | 1943 – 2000 | 16540 | NR | All CNS: 19 | 6.5 | < 1 1 – 4 5 – 9 10 – 14 15 – 19 ≥20 All |
6.91 (1.43 – 20.2) 5.65 (2.07 – 12.3) 15.5 (7.72 – 27.7) 9.31 (2.54 – 23.8) 3.78 (0.09 – 21.1) 3.48 (0.09 – 19.4) 8.52 (5.13 – 13.3) |
< 1 1 – 4 5 – 9 10 – 14 15 – 19 ≥20 All |
5.1 1.6 5.7 5.5 4.1 5.3 1.9 |
| Olsen JH et al(30), 2009; (5 Nordic countries)@ | 26/32 | Childhood Cancer | 1943 – 2005 | 47697 | NR | All CNS: 262 | 10.0 | 0 – 19 | 8.1 (7.1 – 9.1) | All | 22.9 |
SIR = Standardized Incidence Ratio; NR = Not Reported
The percentage of patients treated with radiation therapy to the head was not reported separately
Excludes single case of brain lymphoma as a subsequent brain tumor following leukemia therapy
Relative Risk, not SIR
Ratio of observed vs. expected, not SIR
Glioma only, does not include meningiomas
A portion of the patients from Nordic countries are also included in the international registry
Absolute Excess Risk, excess cases per 10,000 person years
The study populations, objectives, and design varied. Most studies examined cohorts of childhood cancer, ALL or primary CNS tumor survivors. Although not reported in all studies, the reported rates of treatment with cranial radiation therapy in the cohorts ranged from 38% to 78%.8, 10, 22, 26–28, 32, 33 The precision and generalizability of risk estimates of several of the cohort studies were limited by lack of detailed information about CNS radiation therapy,23–25, 29–31 relatively small sample sizes or numbers of CNS tumor cases,22, 26 included less than 10 years of follow-up,8, 22, 25–27, 29, 30 or were single institution cohorts.22–24 Many studies examined only malignant tumors and excluded most or all meningiomas because they were considered to be benign tumors.8, 22, 26, 28–31
Despite these limitations, each study reported an increased incidence of subsequent tumors of the CNS. Among the studies that reported rates of cranial radiation therapy,8, 22, 26–28 the standardized incidence ratios (SIRs) for a subsequent CNS tumor (including both gliomas and meningiomas) ranged from 8·1 – 52·3, and the absolute excess risk of a subsequent CNS tumor among childhood cancer survivors (many of whom were exposed to cranial radiation) compared to the general population ranged from 1·9 – 72·8 per 10,000 person years. Although only calculated in three manuscripts, the SIRs for subsequent gliomas ranged from 8·9 to 24·3 and SIRs for subsequent meningiomas ranged from 41·2 – 714·7.2, 8, 12 Most importantly, the increased rates of subsequent CNS tumors did not appear to plateau over time.23, 30, 34
Two large cohort studies have demonstrated that the cumulative dose of radiation exposure correlates with rates of subsequent CNS tumors.7, 10 Taylor and colleagues’ report from the British Childhood Cancer Survivor Study (BCCSS) demonstrated a linear correlation between dose of radiation therapy and relative risk of both subsequent high-grade gliomas/PNETs and meningiomas.10 In their report, the significantly increased relative risk of subsequent meningiomas first appeared at exposure doses of 20 Gy and increased to a relative risk of > 479·1 (95% confidence intervals: 25·0 – < 657·2; p = < 0·001) at exposure doses of at least 40 Gy. Neglia and colleagues’ study from the North American Childhood Cancer Survivor Study (CCSS) demonstrated a linear correlation between doses of radiation exposure and rates of both high-grade gliomas and meningiomas.7 Odds ratios for subsequent glioma increased with dose of radiation exposure and peaked at 21-fold for exposures of 30 – 44·9 Gy (p < 0·001). Odds ratios for meningiomas also increased with dose of radiation exposure and peaked at 96·3-fold at exposures doses of 30 – 44·9 Gy (p < 0·001). The excess relative risk was 0·33 per Gy (95% confidence intervals = 0·07 – 1·71) for high-grade gliomas and 1·06 per Gy (95% confidence intervals = 0·21 – 8·15) for meningiomas.
Question 2: What are the outcomes in children with subsequent neoplasms of the CNS who have been treated with cranial radiation for a pediatric cancer?
Four studies which included 141 patients examined the outcomes of subsequent high-grade gliomas among childhood cancer survivors (Table 3).7, 13, 35, 36 Three of the studies were cohort studies and one was a single institution report. Two studies included the median age at first cancer diagnosis: 2·5 years and 4·3 years.35, 36 The interval between first cancer diagnosis and subsequent high-grade glioma was 8 – 20 years.7, 13, 35, 36 The two most common primary childhood cancer diagnoses were ALL and CNS tumors.10 Nearly all (95 – 100%) patients had received CNS directed radiation therapy as treatment of their primary malignancy. In the examined manuscripts, nearly all subsequent gliomas were described as high-grade (WHO grades III and IV) gliomas, with the majority being described as glioblastoma.7, 13, 35, 36 The 5 year survival rates were reported in 3 studies13, 35, 36 and ranged from 0 – 19·5%.
Table 3.
Outcomes of Subsequent High-Grade Gliomas among Childhood Cancer Survivors
| Characteristic | General Population | Childhood Cancer Survivors with Subsequent Gliomas | |||
|---|---|---|---|---|---|
| Study | Central Brain Tumor Registry of the United States (CBTRUS) 2012 Statistical Report | Carret AS Radiotherapy and Oncology 2006;81:33–38(34) | Walter AW Journal of Clinical Oncology 1998;16:3761–3767(35) | Taylor AJ Journal of Clinical Oncology 2009; 27:5781–5787(13) | Neglia JP J Natl Cancer Inst 2006;98:1528 – 37(7) |
| Year of Publication | 2012 | 2006 | 1998 | 2009/2010 | 2010 |
| Patients with High-Grade Gliomas | 2227 | 18 | 10 | 73 | 40 |
| STROBE Criteria(17) met, n/n | N/A | 19/30 | 25/30 | 30/33 | 31/32 |
| Study design | N/A | Cohort | Cohort | Population-based Cohort | Cohort |
| Median age at first cancer diagnosis (range) | --- | 4·25 years (0·1 – 9 years) | 2·5 years (1·9 – 15·3 years) | n/a | n/a |
| Exposure to cranial radiation, % | --- | 18 (100%) | 10 (100%) | n/a | 38 (95%) |
| Median interval to subsequent high-grade gliomas (range) | --- | 8 years (6 – 14 years) | 9·1 years | 20 years (mean) | 9 years |
| Median age at diagnosis of subsequent high-grade gliomas (range) | 20 – 44 years | 14 years (7 – 19 years) | 9·4 years (7·6 – 13·2 years) | n/a | 15 years |
| Survival of patients with high-grade gliomas, % | N/A | 1 (6%) | 1 (10%) | 11 (15%) | n/a |
| 5 year survival rate | 16·6% | 0% | 10% | 19·5% (95% CI: 9·8% – 33·7%) | n/a |
Seven studies which included 256 patients examined the outcomes of subsequent meningiomas among childhood cancer survivors (Table 4).7, 13, 23, 34, 36–38 These studies included case series, review of the literature,37 institutional reports,23, 34, 36, 38 and cohort studies.7, 13 Median or mean ages at diagnosis of the primary cancer, described in 4 studies, ranged from 2·5 – 7·6 years.34, 36–38 The mean or median intervals between primary cancer diagnosis and subsequent meningioma diagnosis were 10·7 – 23·1 years.7, 13, 23, 34, 36–38 The two most common primary cancer diagnoses included ALL and brain tumors, and nearly all (90 – 100%) patients had received radiation therapy as treatment for their primary malignancy. Survival of childhood cancer survivors with subsequent meningiomas, reported in four studies, ranged from 73 – 100%.13, 23, 36, 37
Table 4.
Outcomes of Subsequent Meningiomas among Childhood Cancer Survivors
| Characteristic | General Population | Childhood Cancer Survivors with Subsequent Meningiomas | ||||||
|---|---|---|---|---|---|---|---|---|
| Study | National Cancer Data Base McCarthy BJ Journal of Neursurgery 1998; 88:831–839(20) | Ghim TT Cancer 1993; 71:4091 – 4095(36) | Goshen Y Pediatric Blood and Cancer 2007;49:294(37) | Hijiya N JAMA 2007;297:1207(22) | Walter AW Journal of Clinical Oncology 1998;16:3761–3767(35) | Taylor AJ Journal of Clinical Oncology 2009; 27:5781–5787(13) | Neglia JP J Natl Cancer Inst 2006;98:1528 – 37(7) | Vinchon M Child’s Nervous System 2011;27:445–453(33) |
| Year of Publication | 1998 | 1993 | 2007 | 2007 | 1998 | 2009 | 2010 | 2011 |
| Patients with Meningiomas | Benign: 1428 Atypical: 49 Malignant: 133 |
15 | 16 | 16 | 11 | 137 | 40 | 17 |
| STROBE Criteria(17) met, n/n | N/A | 11/31 | 19/30 | 25/31 | 25/30 | 30/33 | 31/32 | 20/32 |
| Study design | N/A | Cohort | Cohort | Cohort | Cohort | Cohort | Cohort | Cohort |
| Median age at first cancer diagnosis (range) | --- | 2·5 years (mean) (0·2 – 9 years) | 7·6 years (2 – 14 years) | n/a | 5 years (2·1 – 11·9 years) | n/a | n/a | 6·9 years (mean) (4·8 – 9·1 years) |
| Exposure to cranial radiation, % | --- | 100% | 15 (94%) | 16 (100%) | 11 (100%) | 134 (98%) | 60 (90%) | 11 (100%) |
| Median interval to second meningioma (range) | --- | 10·7 years (mean) (5 – 15 years) | 21 years (10 – 21 years) | 20·6 years (12·6 – 31·7 years) | 19·3 years (12·6 – 29 years) | 23·1 years (mean) | 17 years | 18·1 years (5·8 – 28·9 years) |
| Median age at second neoplasm diagnosis of a subsequent meningioma (range) | 0 – 44 years | 13·4 years (mean) (9 – 18 years) | 28·7 years (20 – 39 years) | n/a | 16·8 years | n/a | 25·5 years | n/a |
| Survival of patients with meningiomas, % | N/A | 11 (73%) | n/a | 15 (94%) | 11 (100%) | 95 (69%) | n/a | n/a |
| 5 year survival rate | Benign:89·1% Atypical: 77·9% Malignant:65·1% |
n/a | n/a | n/a | 100% | Low-Grade: 84·3% High-Grade: 57·3% |
n/a | n/a |
Question 3: Are outcomes of subsequent neoplasms different from primary neoplasms of the same histology?
The reported 5 year survival rates for patients with subsequent high-grade gliomas ranged from 0 – 19·5%13, 35, 36 and is similar to the 5 year survival rate of 16·6% in adults aged 20 – 44 years with glioblastoma in the CBTRUS.19 Survival of childhood cancer survivors with subsequent meningiomas ranged from 73 – 100%.13, 23, 36, 37 One of these studies13 described 5 year survival rates of subsequent low-grade and high-grade meningiomas of 84·3% and 57·3%, respectively. These survival rates are similar to the 5 year survival rates of people aged 0 – 44 years with benign and malignant meningiomas from the National Cancer Database from the United States of 89·1% and 65·1%, respectively.21
The amount of detail in these studies varies with regards to age at diagnosis of first cancer and diagnosis of a second cancer, primary cancer treatment (including radiation dosimetry), the treatment of subsequent cancers, era of treatment and survival rates. Keeping these limitations in mind, available evidence suggests that the outcomes for childhood cancer survivors diagnosed with both subsequent high-grade gliomas and subsequent meningioma are similar to young adults in the general population.
DISCUSSION
Evidence from multiple retrospective cohort studies demonstrates that survivors of childhood cancer have an 8·1 – 52·3 times higher incidence of developing subsequent CNS neoplasms compared with the general population. High-grade gliomas and meningiomas are the two most common subsequent CNS neoplasms, although medulloblastoma/primitive neuro-ectodermal tumors (PNETs), schwannomas, and low-grade gliomas have also been reported at much lower rates in some studies (Table 2).10, 32 Studies identified higher SIRs for meningiomas than for gliomas. Potential explanations for this observation include a higher dose-sensitivity relationship with radiation exposure for meningioma than gliomas.7, 10 Alternatively, increased SIRs for meningiomas may be explained as a result of surveillance bias due to an increased number of subsequent meningiomas being detected among childhood cancer survivors exposed to radiation and decreased number of primary meningiomas being diagnosed in the general young adult population. Early studies suggested that high-grade gliomas occur in the first decade after primary cancer diagnosis, but more recent studies with longer follow-up have demonstrated that they also occur in the second decade after primary cancer therapy.13, 35, 36 Recent studies have also emphasized that the incidence of subsequent meningiomas does not plateau.23, 30, 34, 38 For example, a manuscript by Armstrong and colleagues from the CCSS reported that survivors of brain tumors who had not developed meningiomas at 20 years after diagnosis of their original cancer still had an 5·3% incidence of meningiomas in the subsequent decade.32
Antecedent radiation to the CNS appears to play a necessary although perhaps not sufficient role in the cause of subsequent CNS tumors among childhood cancer survivors. With rare exceptions, nearly all of the childhood, adolescent and young adult cancer survivors described in the reports who subsequently developed CNS tumors had received treatment with cranial radiation. Cohort studies from both the CCSS and BCCSS have demonstrated that the dose of radiation exposure to the cranium has a linear relationship with the risk of both subsequent high-grade gliomas and meningiomas.7, 10 Furthermore, younger age at exposure to radiation therapy was associated with an increased risk of subsequent CNS tumors.7, 10 Finally, the study from the BCCSS identified the cumulative dose of intrathecal methotrexate, but not other chemotherapeutic agents, as being associated with the development of subsequent meningiomas.10 However, childhood leukemia survivors exposed to intrathecal methotrexate as an alternative to cranial radiation do not appear to be at high risk of subsequent CNS tumors.8
Individual studies have identified additional genetic risks factors for the development of subsequent high-grade gliomas, such as polymorphisms in the thiopurine methyltransferase gene39 or suggested inherited predispositions to cancer, such as Li Fraumeni Syndrome and neurofibromatosis type-1.10, 35, 40 In contrast, a report by Taylor and colleagues did not identify CNS tumor survivors with germline RB gene mutations as being at higher risk for subsequent CNS tumors.10
The outcome for childhood cancer survivors who develop subsequent high-grade gliomas is poor. The 5 year survival rates of patients ranged from 0 – 19·5% and overall survival rates ranged from 6 – 15%.13, 35, 36 In contrast, the outcome for childhood cancer survivors diagnosed with subsequent meningiomas is relatively good. The 5 year survival rates of affected patients ranged from 73 – 100% and overall survival rates ranged from 69 – 100%.13, 23, 36, 37 Outcomes for subsequent high-grade gliomas and meningiomas among childhood cancer survivors are similar to young adult patients diagnosed with primary gliomas and meningiomas. The 5 year survival rate of 2227 young adults aged 20 – 44 years with primary glioblastoma from the CBTRUS registry from the years 2004 – 2008 database was 16·6%, which was similar to the 5 year survival rates reported among childhood cancer survivors.13, 19, 35, 36 It would be assumed that patients with subsequent high-grade gliomas would have received therapy similar to contemporary therapy for primary high-grade gliomas,41, 42 although we acknowledge that the treatment of subsequent high-grade gliomas is challenged and potentially limited by patients’ prior exposure to radiation therapy for their primary childhood malignancy.35
The 5 year survival rates for 1,610 young patients aged 0 – 44 years with meningiomas from the National Cancer Data Base were 89·1% for benign meningiomas, 77·9% for atypical meningiomas and 65·1% for malignant meningiomas, which is similar to overall survival rates of 69 – 100% and 5 year survival rates (low-grade meningiomas = 84·3% and high-grade meningiomas = 57·3%) from the literature.13, 21, 23, 36–38 None of the manuscripts included treatment of the subsequent meningiomas; this would likely consist of surgical resection which is the treatment of choice for primary meningiomas.43 Fortunately, prior treatment exposures would be expected to have little impact upon successful treatment of the subsequent meningioma by complete surgical resection of the tumor.38
Determining the optimal frequency of screening for subsequent CNS tumors among childhood cancer survivors requires additional formal study. Single institution case series that have examined screening neuro-imaging have reported high rates of subsequent meningiomas among childhood cancer survivors exposed to cranial radiation therapy.6, 38, 44 These studies screened a total of 152 patients and found a rate of subsequent meningiomas of 18% (Table 5); the investigators recommend routine screening of childhood cancer survivors treated with radiation therapy for meningiomas in order to facilitate easier resection and reduce mortality and morbidity among survivors with small tumors. However, the value of routine screening for subsequent CNS tumors and the impact of screening upon outcomes has not yet been validated.
Table 5.
Studies Describing Asymptomatic Meningiomas Detected by Screening MRI of Childhood Cancer Survivors
| Study | Initial Diagnosis | Total Number of Survivors | Number of Survivors Screened | Number of Meningiomas Identified | Interval from Initial Cancer Diagnosis to Meningioma Diagnosis |
|---|---|---|---|---|---|
| Goshen Y(37) | ALL | 88 | 76 | 16* (21%) | 21 years (median) |
| Banerjee J(6) | ALL | 60 | 49 | 11 (22%) | 25 years (mean) |
| Pääkkö E(43) | Non-CNS tumor survivors | 44 | 27 | 2 (7%) | 16 years (median) |
| Total | 192 | 152 | 28 (18%) |
included 1 symptomatic meningioma.
Successful screening methodologies for late effects should consider several factors, including the prevalence and severity of the condition, the sensitivity, specificity, predictive value and costs of the screening measures. Other factors include the number of survivors necessary to be screened for a given duration to prevent one adverse event, the potential harms and benefits of screening to individuals, inventions available and the potential reduction in morbidity and mortality associated with early detection of the health condition.45, 46 Recommendations for screening for subsequent CNS tumors by neuro-imaging will need to take into account or comment upon optimal intervals for screening neuro-imaging, costs of neuro-imaging and whether earlier detection of the subsequent neoplasm makes an impact upon effectiveness of treatment upon morbidity or mortality. For example, the outcome of patients with high-grade gliomas, including subsequent high-grade gliomas, is very poor. There is no evidence that survival would be improved by early detection before developing symptoms. Likewise, the outcome for patients with subsequent meningiomas is relatively good following surgical resection and there is no conclusive evidence demonstrating that delayed diagnosis would worsen survival or tumor-related morbidity.
At present, the Long-Term Follow-Up Guidelines recommend screening MRI of the brain as clinically indicated for symptomatic patients who have been exposed to radiation to the brain and MRI every other year for patients with neurofibromatosis type-1 beginning two years after radiation exposure.16 Due to the increased risk of subsequent CNS neoplasms among childhood cancer survivors who have been exposed to radiation to the brain, these survivors may benefit from screening for subsequent CNS tumors by MRI, perhaps at five year intervals after completion of treatment. More formal studies will be needed to better define the role of and the most appropriate intervals for screening neuro-imaging for subsequent CNS neoplasms. These studies will need to incorporate multiple variables, including host factors such as inherited predispositions to cancer, radiation repair and drug metabolism and treatment factors, such as radiation dose and chemotherapy exposure. Incorporating such risk factors into screening strategies will identify patients who would be most likely to benefit from screening neuro-imaging.
Footnotes
Conflicts of Interest: The authors state no conflicts of interest.
Authors Contributions: All authors have made substantial contributions to this manuscript, including study design (DCB, PCN, LC, CW, SB, KK, LB), literature search (DCB, PCN, LC, CW, SB, KK, LB), data collection (DCB, KK, LB), data interpretation (DCB, PCN, LC, CW, SB, KK, LB) and writing (DCB, PCN, LC, CW, SB, KK, LB). All authors have approved the manuscript.
References
- 1.American Cancer Society. Cancer Facts & Figures 2010. Atlanta: American Cancer Society; 2010. [Google Scholar]
- 2.Ron E, Modan B, Boice J, et al. Tumors of the brain and nervous system after radiotherapy in childhood. N Engl J Med. 1988;319(16):1033–9. doi: 10.1056/NEJM198810203191601. [DOI] [PubMed] [Google Scholar]
- 3.Yeh H-C, Matanoski GM, Wang N-y, Sandler DP, Comstock GW. Cancer Incidence after Childhood Nasopharyngeal Radium Irradiation: A Follow-up Study in Washington County, Maryland. Am J Epidemiol. 2001;153(8):749–56. doi: 10.1093/aje/153.8.749. [DOI] [PubMed] [Google Scholar]
- 4.Pearce MS, Salotti JA, Little MP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. The Lancet. doi: 10.1016/S0140-6736(12)60815-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Preston DL, Ron E, Yonehara S, et al. Tumors of the Nervous System and Pituitary Gland Associated With Atomic Bomb Radiation Exposure. J Natl Cancer Inst. 2002;94(20):1555–63. doi: 10.1093/jnci/94.20.1555. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee J, Pääkkö E, Harila M, et al. Radiation-induced meningiomas: A shadow in the success story of childhood leukemia. Neuro Oncol. 2009;11(5):543–9. doi: 10.1215/15228517-2008-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neglia J, Robison L, Stovall M, et al. New primary neoplasms of the central nervous system in survivors of childhood cancer: a report From the Childhood Cancer Survivor Study. J Nat Cancer Inst. 2006;98:1528–37. doi: 10.1093/jnci/djj411. [DOI] [PubMed] [Google Scholar]
- 8.Bhatia S, Sather HN, Pabustan OB, Trigg ME, Gaynon PS, Robison LL. Low incidence of second neoplasms among children diagnosed with acute lymphoblastic leukemia after 1983. Blood. 2002;99:4257–64. doi: 10.1182/blood.v99.12.4257. [DOI] [PubMed] [Google Scholar]
- 9.Little MP, Vathaire Fd, Shamsaldin A, et al. Risks of brain tumour following treatment for cancer in childhood: Modification by genetic factors, radiotherapy and chemotherapy. International Journal of Cancer. 1998;78(3):269–75. doi: 10.1002/(SICI)1097-0215(19981029)78:3<269::AID-IJC1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 10.Taylor AJ, Little MP, Winter DL, et al. Population-based risks of CNS tumors in survivors of childhood cancer: the British Childhood Cancer Survivor Study. Journal of Clinical Oncology. 2010;28:5287–93. doi: 10.1200/JCO.2009.27.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moller TR, Garwicz S, Barlow L, et al. Decreasing Late Mortality Among Five-Year Survivors of Cancer in Childhood and Adolescence: A Population-Based Study in the Nordic Countries. J Clin Oncol. 2001;19(13):3173–81. doi: 10.1200/JCO.2001.19.13.3173. [DOI] [PubMed] [Google Scholar]
- 12.Tukenova M, Diallo I, Hawkins M, et al. Long-term Mortality from Second Malignant Neoplasms in 5-Year Survivors of Solid Childhood Tumors: Temporal Pattern of Risk according to Type of Treatment. Cancer Epidemiology Biomarkers & Prevention. 2010;19(3):707–15. doi: 10.1158/1055-9965.EPI-09-1156. [DOI] [PubMed] [Google Scholar]
- 13.Taylor AJ, Frobisher C, Ellison DW, et al. Survival After Second Primary Neoplasms of the Brain or Spinal Cord in Survivors of Childhood Cancer: Results From the British Childhood Cancer Survivor Study. Journal of Clinical Oncology. 2009;27(34):5781–7. doi: 10.1200/JCO.2009.22.4386. [DOI] [PubMed] [Google Scholar]
- 14.Vasudevan V, Cheung MC, Yang R, et al. Pediatric Solid Tumors and Second Malignancies: Characteristics and Survival Outcomes. Journal of Surgical Research. 2010;160(2):184–9. doi: 10.1016/j.jss.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 15.Morris EB, Gajjar A, Okuma JO, et al. Survival and late mortality in long-term survivors of pediatric CNS tumors. J Clin Oncol. 2007;25:1532–8. doi: 10.1200/JCO.2006.09.8194. [DOI] [PubMed] [Google Scholar]
- 16.Children’s Oncology Group, editor. Long-term follow-up guidelines for survivors of childhood, adolescent and young adult cancers. Arcadia, CA: Children’s Oncology Group; [Date of access: August 27, 2012]. website: www.survivorshipguidelines.com. [Google Scholar]
- 17.Bowers DC, Adkihari S, El-Khashab YM, Gargan L, Oeffinger KC. Survey of long-term follow-up programs in the United States for survivors of childhood brain tumors. Pediatric Blood & Cancer. 2009;53(7):1295–301. doi: 10.1002/pbc.22240. [DOI] [PubMed] [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Bulletin of the World Health Organization. 2007;85(11):867–72. doi: 10.2471/BLT.07.045120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CBTRUS. Central Brain Tumor Registry of the United States; Hinsdale, IL: [Date of access: August 27, 2012]. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2008. website: www.cbtrus.org. [Google Scholar]
- 20.Cahill KS, Claus EB. Treatment and survival of patients with nonmalignant intracranial meningioma: results from the Surveillance, Epidemiology, and End Results Program of the National Cancer Institute. Journal of Neurosurgery. 2011;115(2):259–67. doi: 10.3171/2011.3.JNS101748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCarthy BJ, Davis FG, Freels S, et al. Factors associated with survival in patients with meningioma. Journal of Neurosurgery. 1998;88(5):831–9. doi: 10.3171/jns.1998.88.5.0831. [DOI] [PubMed] [Google Scholar]
- 22.Baker KS, DeFor TE, Burns LJ, Ramsay NKC, Neglia JP, Robison LL. New Malignancies After Blood or Marrow Stem-Cell Transplantation in Children and Adults: Incidence and Risk Factors. Journal of Clinical Oncology. 2003;21(7):1352–8. doi: 10.1200/JCO.2003.05.108. [DOI] [PubMed] [Google Scholar]
- 23.Hijiya N, Hudson MM, Lensing S, et al. Cumulative Incidence of Secondary Neoplasms as a First Event After Childhood Acute Lymphoblastic Leukemia. JAMA: The Journal of the American Medical Association. 2007;297(11):1207–15. doi: 10.1001/jama.297.11.1207. [DOI] [PubMed] [Google Scholar]
- 24.Cardous-Ubbink MC, Heinen RC, Bakker PJM, et al. Risk of second malignancies in long-term survivors of childhood cancer. European Journal of Cancer. 2007;43(2):351–62. doi: 10.1016/j.ejca.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Neglia JP, Meadows AT, Robison LL, et al. Second Neoplasms after Acute Lymphoblastic Leukemia in Childhood. New England Journal of Medicine. 1991;325(19):1330–6. doi: 10.1056/NEJM199111073251902. [DOI] [PubMed] [Google Scholar]
- 26.Rosso P, Terracini B, Fears TR, et al. Second malignant tumors after elective end of therapy for a first cancer in childhood: A multicenter study in Italy. International Journal of Cancer. 1994;59(4):451–6. doi: 10.1002/ijc.2910590402. [DOI] [PubMed] [Google Scholar]
- 27.Löning L, Zimmermann M, Reiter A, et al. Secondary neoplasms subsequent to Berlin-Frankfurt-Münster therapy of acute lymphoblastic leukemia in childhood: significantly lower risk without cranial radiotherapy. Blood. 2000;95(9):2770–5. [PubMed] [Google Scholar]
- 28.Jenkinson H, Hawkins M. Secondary brain tumors in children with ALL. Lancet. 1999;354:1126. doi: 10.1016/S0140-6736(05)76922-1. [DOI] [PubMed] [Google Scholar]
- 29.Inskip PD, Curtis RE. New malignancies following childhood cancer in the United States, 1973–2002. International Journal of Cancer. 2007;121(10):2233–40. doi: 10.1002/ijc.22827. [DOI] [PubMed] [Google Scholar]
- 30.Maule M, Scélo G, Pastore G, et al. Risk of Second Malignant Neoplasms After Childhood Leukemia and Lymphoma: An International Study. Journal of the National Cancer Institute. 2007;99(10):790–800. doi: 10.1093/jnci/djk180. [DOI] [PubMed] [Google Scholar]
- 31.Olsen JH, Möller T, Anderson H, et al. Lifelong Cancer Incidence in 47 697 Patients Treated for Childhood Cancer in the Nordic Countries. Journal of the National Cancer Institute. 2009;101(11):806–13. doi: 10.1093/jnci/djp104. [DOI] [PubMed] [Google Scholar]
- 32.Armstrong GT, Liu Q, Yasui Y, et al. Long-Term Outcomes Among Adult Survivors of Childhood Central Nervous System Malignancies in the Childhood Cancer Survivor Study. Journal of the National Cancer Institute. 2009;101(13):946–58. doi: 10.1093/jnci/djp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedman DL, Whitton J, Leisenring W, et al. Subsequent Neoplasms in 5-Year Survivors of Childhood Cancer: The Childhood Cancer Survivor Study. Journal of the National Cancer Institute. 2010;102(14):1083–95. doi: 10.1093/jnci/djq238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vinchon M, Leblond P, Caron S, Delestret I, Baroncini M, Coche B. Radiation-induced tumors in children irradiated for brain tumor: a longitudinal study. Child’s Nervous System. 2011;27(3):445–53. doi: 10.1007/s00381-011-1390-4. [DOI] [PubMed] [Google Scholar]
- 35.Carret A-S, Tabori U, Crooks B, et al. Outcome of secondary high-grade glioma in children previously treated for a malignant condition: A study of the Canadian Pediatric Brain Tumour Consortium. Radiotherapy and Oncology. 2006;81:33–8. doi: 10.1016/j.radonc.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Walter A, Hancock M, Pui C, et al. Secondary brain tumors in children treated for acute lymphoblastic leukemia at St Jude Children’s Research Hospital. J Clin Oncol. 1998;16(12):3761–7. doi: 10.1200/JCO.1998.16.12.3761. [DOI] [PubMed] [Google Scholar]
- 37.Ghim TT, Seo J-J, O’Brien M, Meacham L, Crocker I, Krawiecki N. Childhood intracranial meningiomas after high-dose irradiation. Cancer. 1993;71(12):4091–5. doi: 10.1002/1097-0142(19930615)71:12<4091::aid-cncr2820711247>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 38.Goshen Y, Stark B, Kornreich L, Michowiz S, Feinmesser M, Yaniv I. High incidence of meningioma in cranial irradiated survivors of childhood acute lymphoblastic leukemia. Pediatric Blood & Cancer. 2007;49(3):294–7. doi: 10.1002/pbc.21153. [DOI] [PubMed] [Google Scholar]
- 39.Relling MV, Rubnitz JE, Rivera GK, et al. High incidence of secondary brain tumours after radiotherapy and antimetabolites. Lancet. 1999;354:34–9. doi: 10.1016/S0140-6736(98)11079-6. [DOI] [PubMed] [Google Scholar]
- 40.Sharif S, Ferner R, Birch JM, et al. Second Primary Tumors in Neurofibromatosis 1 Patients Treated for Optic Glioma: Substantial Risks After Radiotherapy. J Clin Oncol. 2006;24(16):2570–5. doi: 10.1200/JCO.2005.03.8349. [DOI] [PubMed] [Google Scholar]
- 41.Wen PY, Kesari S. Malignant Gliomas in Adults. New England Journal of Medicine. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 42.Reardon DA, Rich JN, Friedman HS, Bigner DD. Recent Advances in the Treatment of Malignant Astrocytoma. Journal of Clinical Oncology. 2006;24(8):1253–65. doi: 10.1200/JCO.2005.04.5302. [DOI] [PubMed] [Google Scholar]
- 43.Kotecha RS, Pascoe EM, Rushing EJ, et al. Meningiomas in children and adolescents: a meta-analysis of individual patient data. The Lancet Oncology. 2011;12(13):1229–39. doi: 10.1016/S1470-2045(11)70275-3. [DOI] [PubMed] [Google Scholar]
- 44.Pääkkö E, Talvensaari K, Pyhtinen J, Lanning M. Late cranial MRI after cranial irradiation in survivors of childhood cancer. Neuroradiology. 1994;36(8):652–5. doi: 10.1007/BF00600433. [DOI] [PubMed] [Google Scholar]
- 45.Hudson MM, Landier W, Ganz PA. Impact of Survivorship-Based Research on Defining Clinical Care Guidelines. Cancer Epidemiology Biomarkers & Prevention. 2011;20(10):2085–92. doi: 10.1158/1055-9965.EPI-11-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rembold CM. Number needed to screen: development of a statistic for disease screening. BMJ. 1998;317(7154):307–12. doi: 10.1136/bmj.317.7154.307. [DOI] [PMC free article] [PubMed] [Google Scholar]