Abstract
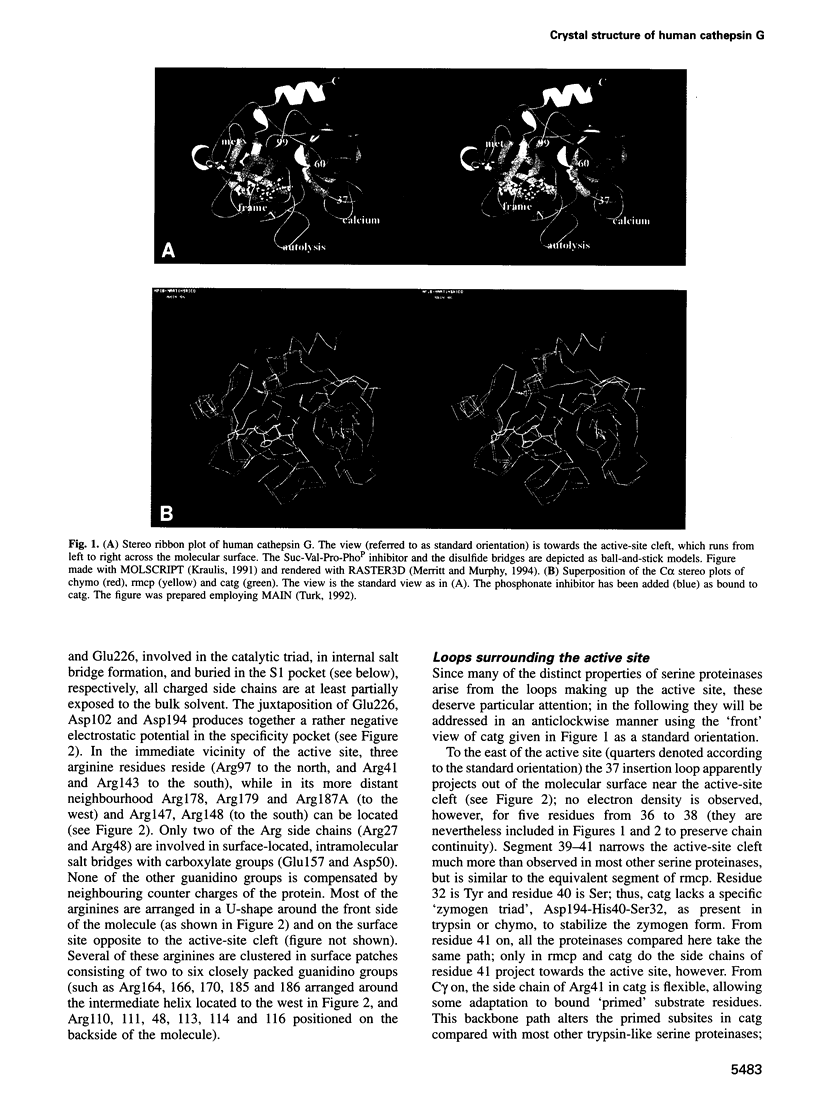
The crystal structure of human neutrophil cathepsin G, complexed with the peptidyl phosphonate inhibitor Suc-Val-Pro-PheP-(OPh)2, has been determined to a resolution of 1.8 A using Patterson search techniques. The cathepsin G structure shows the polypeptide fold characteristic of trypsin-like serine proteinases and is especially similar to rat mast cell proteinase II. Unique to cathepsin G, however, is the presence of Glu226 (chymotrypsinogen numbering), which is situated at the bottom of the S1 specificity pocket, dividing it into two compartments. For this reason, the benzyl side chain of the inhibitor PheP residue does not fully occupy the pocket but is, instead, located at its entrance. Its positively charged equatorial edge is involved in a favourable electrostatic interaction with the negatively charged carboxylate group of Glu226. Arrangement of this Glu226 carboxylate would also allow accommodation of a Lys side chain in this S1 pocket, in agreement with the recently observed cathepsin G preference for Lys and Phe at P1. The cathepsin G complex with the covalently bound phosphonate inhibitor mimics a tetrahedral substrate intermediate. A comparison of the Arg surface distributions of cathepsin G, leukocyte elastase and rat mast cell protease II shows no simple common recognition pattern for a mannose-6-phosphate receptor-independent targeting mechanism for sorting of these granular proteinases.
Full text
PDF



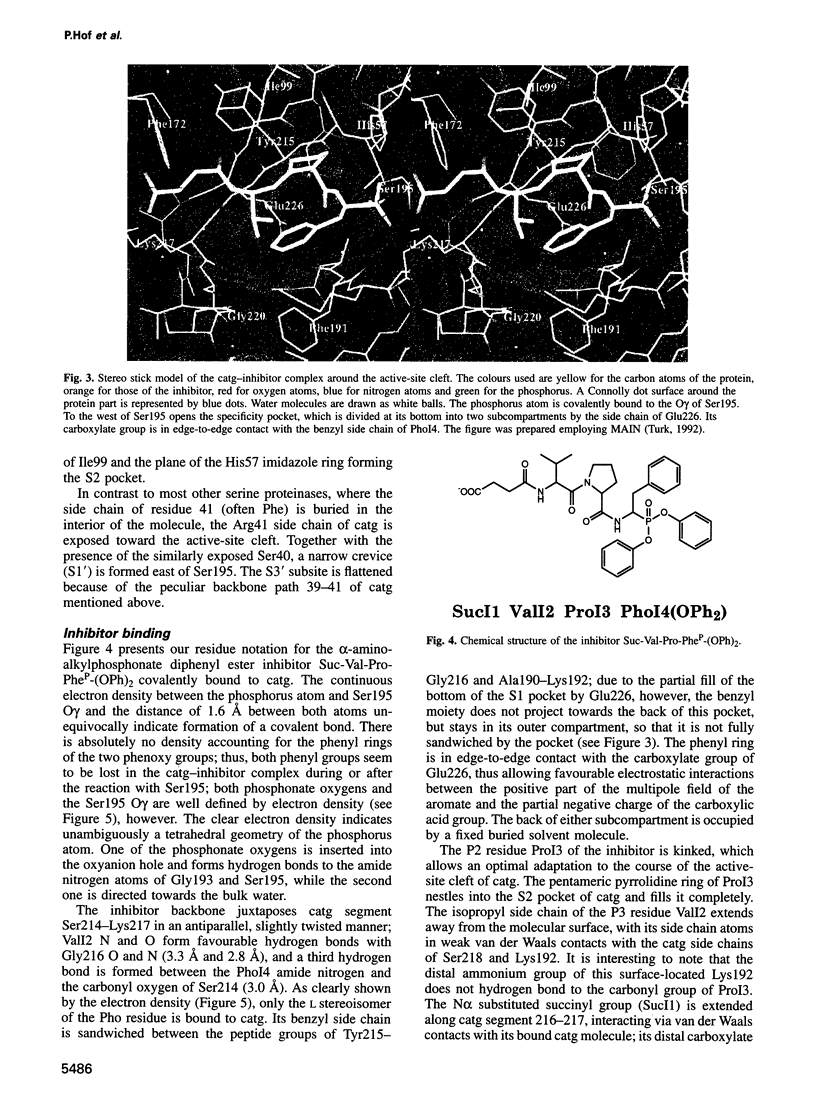
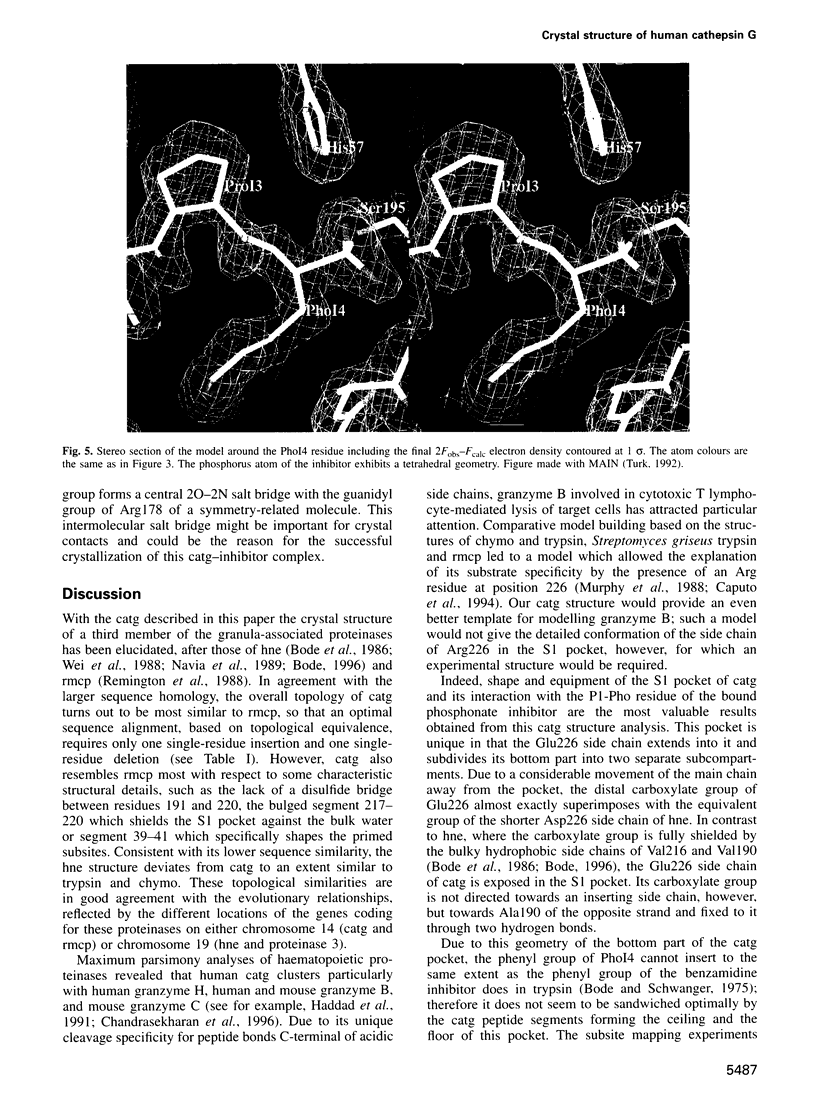




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. H., Tracy P. B. Human coagulation factor V is activated to the functional cofactor by elastase and cathepsin G expressed at the monocyte surface. J Biol Chem. 1995 Jan 20;270(3):1408–1415. doi: 10.1074/jbc.270.3.1408. [DOI] [PubMed] [Google Scholar]
- Avril L. E., Di Martino-Ferrer M., Pignede G., Séman M., Gauthier F. Identification of the U-937 membrane-associated proteinase interacting with the V3 loop of HIV-1 gp120 as cathepsin G. FEBS Lett. 1994 May 23;345(1):81–86. doi: 10.1016/0014-5793(94)00410-2. [DOI] [PubMed] [Google Scholar]
- Avril L. E., di Martino-Ferrer M., Brillard-Bourdet M., Gauthier F. Inhibition of U-937 membrane-associated cathepsin G by GP120 (IIIB) and V3 loop-derived peptides from several strains of HIV-1. FEBS Lett. 1995 Jul 3;367(3):251–256. doi: 10.1016/0014-5793(95)00571-p. [DOI] [PubMed] [Google Scholar]
- Baggiolini M., Schnyder J., Bretz U., Dewald B., Ruch W. Cellular mechanisms of proteinase release from inflammatory cells and the degradation of extracellular proteins. Ciba Found Symp. 1979;(75):105–121. doi: 10.1002/9780470720585.ch7. [DOI] [PubMed] [Google Scholar]
- Bangalore N., Travis J. Comparison of properties of membrane bound versus soluble forms of human leukocytic elastase and cathepsin G. Biol Chem Hoppe Seyler. 1994 Oct;375(10):659–666. doi: 10.1515/bchm3.1994.375.10.659. [DOI] [PubMed] [Google Scholar]
- Bangalore N., Travis J., Onunka V. C., Pohl J., Shafer W. M. Identification of the primary antimicrobial domains in human neutrophil cathepsin G. J Biol Chem. 1990 Aug 15;265(23):13584–13588. [PubMed] [Google Scholar]
- Barton G. J. ALSCRIPT: a tool to format multiple sequence alignments. Protein Eng. 1993 Jan;6(1):37–40. doi: 10.1093/protein/6.1.37. [DOI] [PubMed] [Google Scholar]
- Baugh R. J., Travis J. Human leukocyte granule elastase: rapid isolation and characterization. Biochemistry. 1976 Feb 24;15(4):836–841. doi: 10.1021/bi00649a017. [DOI] [PubMed] [Google Scholar]
- Beatty K., Bieth J., Travis J. Kinetics of association of serine proteinases with native and oxidized alpha-1-proteinase inhibitor and alpha-1-antichymotrypsin. J Biol Chem. 1980 May 10;255(9):3931–3934. [PubMed] [Google Scholar]
- Bertrand J. A., Oleksyszyn J., Kam C. M., Boduszek B., Presnell S., Plaskon R. R., Suddath F. L., Powers J. C., Williams L. D. Inhibition of trypsin and thrombin by amino(4-amidinophenyl)methanephosphonate diphenyl ester derivatives: X-ray structures and molecular models. Biochemistry. 1996 Mar 12;35(10):3147–3155. doi: 10.1021/bi9520996. [DOI] [PubMed] [Google Scholar]
- Blevins R. A., Tulinsky A. The refinement and the structure of the dimer of alpha-chymotrypsin at 1.67-A resolution. J Biol Chem. 1985 Apr 10;260(7):4264–4275. doi: 10.2210/pdb5cha/pdb. [DOI] [PubMed] [Google Scholar]
- Bode W., Schwager P. The refined crystal structure of bovine beta-trypsin at 1.8 A resolution. II. Crystallographic refinement, calcium binding site, benzamidine binding site and active site at pH 7.0. J Mol Biol. 1975 Nov 15;98(4):693–717. doi: 10.1016/s0022-2836(75)80005-2. [DOI] [PubMed] [Google Scholar]
- Bode W., Wei A. Z., Huber R., Meyer E., Travis J., Neumann S. X-ray crystal structure of the complex of human leukocyte elastase (PMN elastase) and the third domain of the turkey ovomucoid inhibitor. EMBO J. 1986 Oct;5(10):2453–2458. doi: 10.1002/j.1460-2075.1986.tb04521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandstetter H., Bauer M., Huber R., Lollar P., Bode W. X-ray structure of clotting factor IXa: active site and module structure related to Xase activity and hemophilia B. Proc Natl Acad Sci U S A. 1995 Oct 10;92(21):9796–9800. doi: 10.1073/pnas.92.21.9796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann T., Schnierer S., Tschesche H. Recombinant aprotinin homologue with new inhibitory specificity for cathepsin G. Eur J Biochem. 1991 Nov 15;202(1):95–99. doi: 10.1111/j.1432-1033.1991.tb16348.x. [DOI] [PubMed] [Google Scholar]
- Campbell E. J., Silverman E. K., Campbell M. A. Elastase and cathepsin G of human monocytes. Quantification of cellular content, release in response to stimuli, and heterogeneity in elastase-mediated proteolytic activity. J Immunol. 1989 Nov 1;143(9):2961–2968. [PubMed] [Google Scholar]
- Caputo A., James M. N., Powers J. C., Hudig D., Bleackley R. C. Conversion of the substrate specificity of mouse proteinase granzyme B. Nat Struct Biol. 1994 Jun;1(6):364–367. doi: 10.1038/nsb0694-364. [DOI] [PubMed] [Google Scholar]
- Caughey G. H. Serine proteinases of mast cell and leukocyte granules. A league of their own. Am J Respir Crit Care Med. 1994 Dec;150(6 Pt 2):S138–S142. doi: 10.1164/ajrccm/150.6_Pt_2.S138. [DOI] [PubMed] [Google Scholar]
- Chandrasekharan U. M., Sanker S., Glynias M. J., Karnik S. S., Husain A. Angiotensin II-forming activity in a reconstructed ancestral chymase. Science. 1996 Jan 26;271(5248):502–505. doi: 10.1126/science.271.5248.502. [DOI] [PubMed] [Google Scholar]
- Ermolieff J., Boudier C., Laine A., Meyer B., Bieth J. G. Heparin protects cathepsin G against inhibition by protein proteinase inhibitors. J Biol Chem. 1994 Nov 25;269(47):29502–29508. [PubMed] [Google Scholar]
- Faber J. P., Poller W., Olek K., Baumann U., Carlson J., Lindmark B., Eriksson S. The molecular basis of alpha 1-antichymotrypsin deficiency in a heterozygote with liver and lung disease. J Hepatol. 1993 Jul;18(3):313–321. doi: 10.1016/s0168-8278(05)80275-2. [DOI] [PubMed] [Google Scholar]
- Fioretti E., Angeletti M., Coletta M., Ascenzi P., Bolognesi M., Menegatti E., Rizzi M., Ascoli F. Binding of bovine basic pancreatic trypsin inhibitor (Kunitz) as well as bovine and porcine pancreatic secretory trypsin inhibitor (Kazal) to human cathepsin G: a kinetic and thermodynamic study. J Enzyme Inhib. 1993;7(1):57–64. doi: 10.3109/14756369309020189. [DOI] [PubMed] [Google Scholar]
- Frommherz K. J., Faller B., Bieth J. G. Heparin strongly decreases the rate of inhibition of neutrophil elastase by alpha 1-proteinase inhibitor. J Biol Chem. 1991 Aug 15;266(23):15356–15362. [PubMed] [Google Scholar]
- Gabay J. E. Antimicrobial proteins with homology to serine proteases. Ciba Found Symp. 1994;186:237–249. doi: 10.1002/9780470514658.ch14. [DOI] [PubMed] [Google Scholar]
- Garwicz D., Lindmark A., Gullberg U. Human cathepsin G lacking functional glycosylation site is proteolytically processed and targeted for storage in granules after transfection to the rat basophilic/mast cell line RBL or the murine myeloid cell line 32D. J Biol Chem. 1995 Nov 24;270(47):28413–28418. doi: 10.1074/jbc.270.47.28413. [DOI] [PubMed] [Google Scholar]
- Griffiths G. M., Isaaz S. Granzymes A and B are targeted to the lytic granules of lymphocytes by the mannose-6-phosphate receptor. J Cell Biol. 1993 Feb;120(4):885–896. doi: 10.1083/jcb.120.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullberg U., Lindmark A., Nilsson E., Persson A. M., Olsson I. Processing of human cathepsin G after transfection to the rat basophilic/mast cell tumor line RBL. J Biol Chem. 1994 Oct 7;269(40):25219–25225. [PubMed] [Google Scholar]
- Haddad P., Jenne D., Tschopp J., Clément M. V., Mathieu-Mahul D., Sasportes M. Structure and evolutionary origin of the human granzyme H gene. Int Immunol. 1991 Jan;3(1):57–66. doi: 10.1093/intimm/3.1.57. [DOI] [PubMed] [Google Scholar]
- Hase-Yamazaki T., Aoki Y. Stimulation of human lymphocytes by cathepsin G. Cell Immunol. 1995 Jan;160(1):24–32. doi: 10.1016/0008-8749(95)80005-4. [DOI] [PubMed] [Google Scholar]
- Jenne D. E., Masson D., Zimmer M., Haefliger J. A., Li W. H., Tschopp J. Isolation and complete structure of the lymphocyte serine protease granzyme G, a novel member of the granzyme multigene family in murine cytolytic T lymphocytes. Evolutionary origin of lymphocyte proteases. Biochemistry. 1989 Sep 19;28(19):7953–7961. doi: 10.1021/bi00445a060. [DOI] [PubMed] [Google Scholar]
- Maison C. M., Villiers C. L., Colomb M. G. Proteolysis of C3 on U937 cell plasma membranes. Purification of cathepsin G. J Immunol. 1991 Aug 1;147(3):921–926. [PubMed] [Google Scholar]
- McGuire M. J., Lipsky P. E., Thiele D. L. Generation of active myeloid and lymphoid granule serine proteases requires processing by the granule thiol protease dipeptidyl peptidase I. J Biol Chem. 1993 Feb 5;268(4):2458–2467. [PubMed] [Google Scholar]
- Merritt E. A., Murphy M. E. Raster3D Version 2.0. A program for photorealistic molecular graphics. Acta Crystallogr D Biol Crystallogr. 1994 Nov 1;50(Pt 6):869–873. doi: 10.1107/S0907444994006396. [DOI] [PubMed] [Google Scholar]
- Mikes O., Holeysovský V., Tomásek V., Sorm F. Covalent structure of bovine trypsinogen. The position of the remaining amides. Biochem Biophys Res Commun. 1966 Aug 12;24(3):346–352. doi: 10.1016/0006-291x(66)90162-8. [DOI] [PubMed] [Google Scholar]
- Molino M., Blanchard N., Belmonte E., Tarver A. P., Abrams C., Hoxie J. A., Cerletti C., Brass L. F. Proteolysis of the human platelet and endothelial cell thrombin receptor by neutrophil-derived cathepsin G. J Biol Chem. 1995 May 12;270(19):11168–11175. doi: 10.1074/jbc.270.19.11168. [DOI] [PubMed] [Google Scholar]
- Murphy M. E., Moult J., Bleackley R. C., Gershenfeld H., Weissman I. L., James M. N. Comparative molecular model building of two serine proteinases from cytotoxic T lymphocytes. Proteins. 1988;4(3):190–204. doi: 10.1002/prot.340040306. [DOI] [PubMed] [Google Scholar]
- Navia M. A., McKeever B. M., Springer J. P., Lin T. Y., Williams H. R., Fluder E. M., Dorn C. P., Hoogsteen K. Structure of human neutrophil elastase in complex with a peptide chloromethyl ketone inhibitor at 1.84-A resolution. Proc Natl Acad Sci U S A. 1989 Jan;86(1):7–11. doi: 10.1073/pnas.86.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen C. A., Campbell E. J. Neutrophil proteinases and matrix degradation. The cell biology of pericellular proteolysis. Semin Cell Biol. 1995 Dec;6(6):367–376. doi: 10.1016/s1043-4682(05)80007-8. [DOI] [PubMed] [Google Scholar]
- Padmanabhan K., Padmanabhan K. P., Tulinsky A., Park C. H., Bode W., Huber R., Blankenship D. T., Cardin A. D., Kisiel W. Structure of human des(1-45) factor Xa at 2.2 A resolution. J Mol Biol. 1993 Aug 5;232(3):947–966. doi: 10.1006/jmbi.1993.1441. [DOI] [PubMed] [Google Scholar]
- Padrines M., Wolf M., Walz A., Baggiolini M. Interleukin-8 processing by neutrophil elastase, cathepsin G and proteinase-3. FEBS Lett. 1994 Sep 26;352(2):231–235. doi: 10.1016/0014-5793(94)00952-x. [DOI] [PubMed] [Google Scholar]
- Powers J. C., Kam C. M. Isocoumarin inhibitors of serine peptidases. Methods Enzymol. 1994;244:442–457. doi: 10.1016/0076-6879(94)44033-6. [DOI] [PubMed] [Google Scholar]
- Powers J. C., Kam C. M., Narasimhan L., Oleksyszyn J., Hernandez M. A., Ueda T. Mechanism-based isocoumarin inhibitors for serine proteases: use of active site structure and substrate specificity in inhibitor design. J Cell Biochem. 1989 Jan;39(1):33–46. doi: 10.1002/jcb.240390105. [DOI] [PubMed] [Google Scholar]
- Powers J. C., Tanaka T., Harper J. W., Minematsu Y., Barker L., Lincoln D., Crumley K. V., Fraki J. E., Schechter N. M., Lazarus G. G. Mammalian chymotrypsin-like enzymes. Comparative reactivities of rat mast cell proteases, human and dog skin chymases, and human cathepsin G with peptide 4-nitroanilide substrates and with peptide chloromethyl ketone and sulfonyl fluoride inhibitors. Biochemistry. 1985 Apr 9;24(8):2048–2058. doi: 10.1021/bi00329a037. [DOI] [PubMed] [Google Scholar]
- Reilly C. F., Tewksbury D. A., Schechter N. M., Travis J. Rapid conversion of angiotensin I to angiotensin II by neutrophil and mast cell proteinases. J Biol Chem. 1982 Aug 10;257(15):8619–8622. [PubMed] [Google Scholar]
- Remington S. J., Woodbury R. G., Reynolds R. A., Matthews B. W., Neurath H. The structure of rat mast cell protease II at 1.9-A resolution. Biochemistry. 1988 Oct 18;27(21):8097–8105. doi: 10.1021/bi00421a019. [DOI] [PubMed] [Google Scholar]
- Salvesen G., Enghild J. J. An unusual specificity in the activation of neutrophil serine proteinase zymogens. Biochemistry. 1990 Jun 5;29(22):5304–5308. doi: 10.1021/bi00474a013. [DOI] [PubMed] [Google Scholar]
- Salvesen G., Farley D., Shuman J., Przybyla A., Reilly C., Travis J. Molecular cloning of human cathepsin G: structural similarity to mast cell and cytotoxic T lymphocyte proteinases. Biochemistry. 1987 Apr 21;26(8):2289–2293. doi: 10.1021/bi00382a032. [DOI] [PubMed] [Google Scholar]
- Savage M. J., Iqbal M., Loh T., Trusko S. P., Scott R., Siman R. Cathepsin G: localization in human cerebral cortex and generation of amyloidogenic fragments from the beta-amyloid precursor protein. Neuroscience. 1994 Jun;60(3):607–619. doi: 10.1016/0306-4522(94)90490-1. [DOI] [PubMed] [Google Scholar]
- Schechter N. M., Wang Z. M., Blacher R. W., Lessin S. R., Lazarus G. S., Rubin H. Determination of the primary structures of human skin chymase and cathepsin G from cutaneous mast cells of urticaria pigmentosa lesions. J Immunol. 1994 Apr 15;152(8):4062–4069. [PubMed] [Google Scholar]
- Schiessler H., Arnhold M., Ohlsson K., Fritz H. Inhibitors of acrosin and granulocyte proteinases from human genital tract secretions. Hoppe Seylers Z Physiol Chem. 1976 Sep;357(9):1251–1260. doi: 10.1515/bchm2.1976.357.2.1251. [DOI] [PubMed] [Google Scholar]
- Selak M. A. Cathepsin G and thrombin: evidence for two different platelet receptors. Biochem J. 1994 Jan 15;297(Pt 2):269–275. doi: 10.1042/bj2970269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Pohl J., Onunka V. C., Bangalore N., Travis J. Human lysosomal cathepsin G and granzyme B share a functionally conserved broad spectrum antibacterial peptide. J Biol Chem. 1991 Jan 5;266(1):112–116. [PubMed] [Google Scholar]
- Sinha S., Watorek W., Karr S., Giles J., Bode W., Travis J. Primary structure of human neutrophil elastase. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2228–2232. doi: 10.1073/pnas.84.8.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder R. A., Kaempfer C. E., Wintroub B. U. Chemistry of a human monocyte-derived cell line (U937): identification of the angiotensin I-converting activity as leukocyte cathepsin G. Blood. 1985 Jan;65(1):176–182. [PubMed] [Google Scholar]
- Tanaka T., Minematsu Y., Reilly C. F., Travis J., Powers J. C. Human leukocyte cathepsin G. Subsite mapping with 4-nitroanilides, chemical modification, and effect of possible cofactors. Biochemistry. 1985 Apr 9;24(8):2040–2047. doi: 10.1021/bi00329a036. [DOI] [PubMed] [Google Scholar]
- Travis J., Garner D., Bowen J. Human alpha-1-antichymotrypsin: purification and properties. Biochemistry. 1978 Dec 26;17(26):5647–5651. doi: 10.1021/bi00619a010. [DOI] [PubMed] [Google Scholar]
- Travis J. Structure, function, and control of neutrophil proteinases. Am J Med. 1988 Jun 24;84(6A):37–42. doi: 10.1016/0002-9343(88)90156-8. [DOI] [PubMed] [Google Scholar]
- Wei A. Z., Mayr I., Bode W. The refined 2.3 A crystal structure of human leukocyte elastase in a complex with a valine chloromethyl ketone inhibitor. FEBS Lett. 1988 Jul 18;234(2):367–373. doi: 10.1016/0014-5793(88)80118-2. [DOI] [PubMed] [Google Scholar]
- Woodbury R. G., Katunuma N., Kobayashi K., Titani K., Neurath H., Anderson W. F., Matthews B. W. Covalent structure of a group-specific protease from rat small intestine. Appendix: crystallographic data for a group specific protease from rat intestine. Biochemistry. 1978 Mar 7;17(5):811–819. doi: 10.1021/bi00598a010. [DOI] [PubMed] [Google Scholar]