Abstract
The cardiac hormone, B-type natriuretic peptide (BNP), is one of human natriuretic peptides which possesses cardiorenal protective actions and is used as a therapeutic and a biomarker for heart failure (HF). Its prohormone, proBNP1-108, is processed by the proNPs convertases, corin or furin, to inactive NT-proBNP1-76 and active BNP1-32. Paradoxically, circulating NT-proBNP and BNP are elevated in HF leading to the use of BNP as a sensitive and predictive marker of HF. This paradox may be explained by the “non-specific” nature of conventional assays and/or a relative deficiency state of “active BNP” as characterized by an increase in inactive proBNP1-108 and a decrease in active BNP1-32. Therefore, understanding the regulation of proBNP1-108 processing and the role of the convertase corin may be important in understanding the physiology of HF. Corin is expressed in heart and kidney and may play an important role in regulating blood pressure and remodeling of the heart. The processing of proBNP1-108 by corin may be controlled by O-linked glycosylation of proBNP1-108. A potential impairment of proBNP1-108 processing in HF may be linked to dysregulation of the convertase corin, which may offer therapeutic opportunities to control proBNP1-108 processing and its activation in HF.
Keywords: B-type natriuretic peptide, heart failure, corin, processing, glycosylation
2. Introduction
Natriuretic peptides (NPs) are a family of vasodilating, natriuretic, and diuretic peptides involved in maintaining cardiorenal homeostasis. These peptides are produced as pre-prohormones which are subsequently processed into prohormones by cleavage of an N-terminal signal peptide. Human pre-proBNP, a 134-amino acid (AA) peptide, is cleaved to 108-AA proBNP [1]. ProBNP is stored in secretory granules in atrial cardiomyocytes and cleaved to form BNP1-32 upon secretion. A role for both corin and furin has been implicated in processing of BNP [2, 3]. Both proBNP1-108 and BNP1-32 peptide are released from the myocardium in response to various physiologic and pathophysiologic stimuli, such as myocardial wall stretch.
Today, BNP is widely used worldwide as a biomarker for HF. Using state of the art Fourier transform ion cyclotron resonance (FT-ICR) mass spectrometry on human HF samples, much of plasma BNP immunoreactivity measured by commonly used assays was determined to be due to altered circulating molecular forms of BNP with reduced cyclic guanosine monophosphate (cGMP)-activating properties [4]. We now know that BNP circulates in various forms – its precursor proBNP1-108, mature BNP 1-32, N-terminal peptide proBNP1-76, and BNP 3-32. Importantly in vitro analysis reported that only BNP 1-32 and BNP 3-32 could stimulate cGMP production in human cardiac fibroblasts and cardiomyocytes [5]. Thus, patients with HF have low circulating “functional” BNP 1-32 levels while other non-functional BNPs, including proBNP1-108, are higher than normal subjects [4, 6]. This functional deficiency state of active BNP may affect the progression of HF and the remodeling process. The utility of BNP (especially proBNP 1-108, mature BNP 1-32 and NT-proBNP) continues to grow with its use now as a prognostic biomarker for future adverse cardiovascular outcomes [7], and as a guide to therapy in HF [8]. Now we realized the importance of knowing how proBNP processing is controlled, especially by the cardiac proNP convertase corin. Corin was first identified in human heart by Yan and colleagues in 1997 [9]. Here we will discuss BNP molecular forms in HF and corin physiology and pathophysiology in HF. While corin may process both pro-atrial natriuretic peptide (ANP) and proBNP, our focus on BNP is driven by its more conventional use in the US as a biomarker and therapeutic agent.
3. BNP molecular forms: implications for biomarker and therapeutic use in cardiovascular disease
3.1 Background
In 1981, deBold and colleagues reported the discovery of atrial granules in the atrial myocardium but not in the ventricular myocardium [1]. These granules have been well established as the site of proANP and proBNP synthesis and processing, with release of mature active ANP1-28 and BNP1-32. Subsequently, it was shown that proBNP1-108 may also be released as a prohormone and processed in the circulation [10-12].
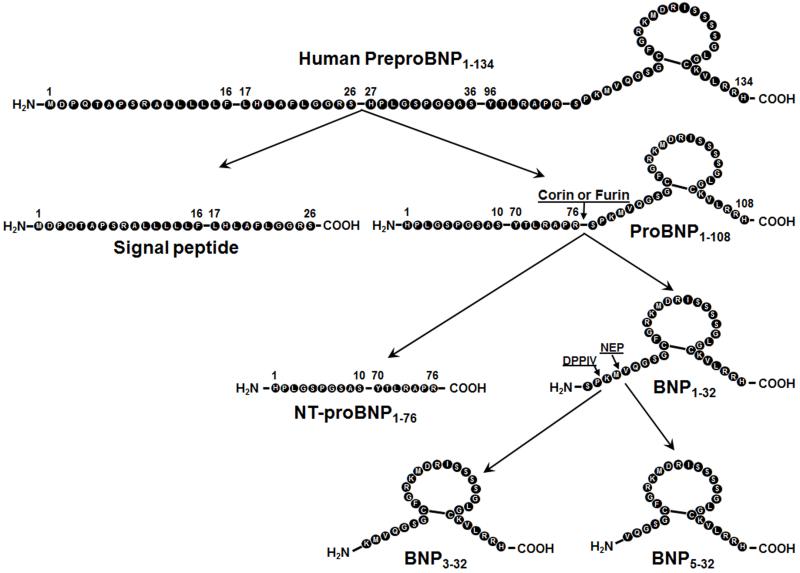
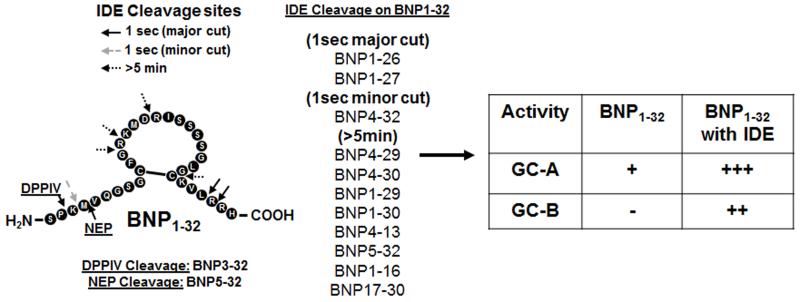
The human BNP gene encodes for a pre-proBNP molecule of 134 amino acids, including a signal peptide of 26 AA. The pre-proBNP1-134 is cleaved to proBNP1-108, and then proBNP1-108 is processed by proNPs convertases, corin or furin (9, 13), to inactive NT-proBNP1-76 and active BNP1-32. The active BNP1-32 can then be degraded to BNP3-32 by Dipeptidyl peptidase-4 (DPPIV) (Figure 1) [14, 15], to BNP5-32 by neutral endopeptidase (=neprilysin, NEP) [16], to BNP8-32 by meprin [16, 17], and/or to smaller degradation peptides by Insulin degrading enzyme (IDE) [18-20] (Figure 2). It should be noted that evidence suggests that meprin may not be present in humans [21].
Figure 1. PreproBNP1-134 amino acid sequence with sites of processing and degradation.
Figure 2. BNP1-32 degradation.
BNP1-32 is degraded by DPPIV, NEP or IDE. After IDE degradation, BNP may be a dual activator of GC-A/-B [15, 18, 40].
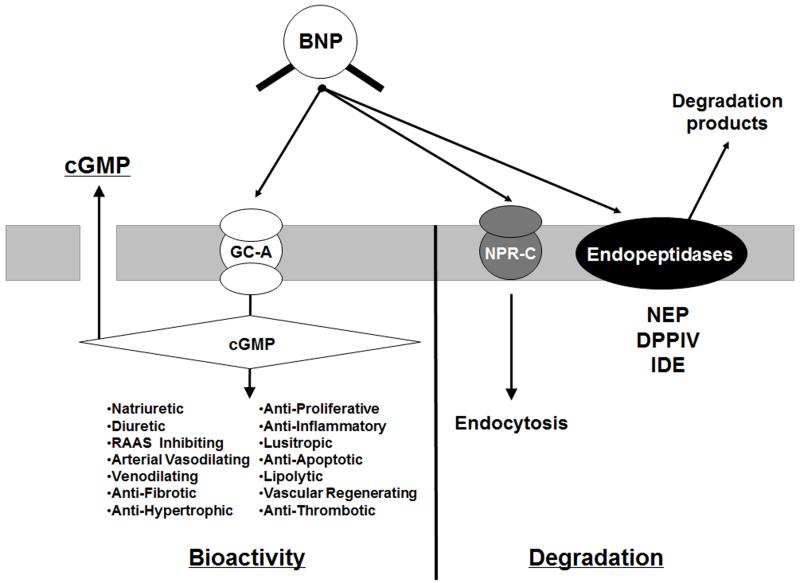
Not all processed and degraded forms of BNP are active. Our in vitro work suggests that mature BNP1-32 is the most active, with the order of prominent BNP forms: BNP1-32>BNP3-32>BNP8-32>>proBNP1-108 as tested in human cell lines [5, 17, 22]. The BNP response is generated through binding of BNP forms to the particulate guanylyl cyclase (GC) receptor–A (GC-A; =natriuretic peptide receptor (NPR)-A). Upon binding, GC-A activates cGMP, resulting in natriuresis, vasorelaxation, diuresis, inhibition of the renin-angiotensin-aldosterone system, enhanced myocardial relaxation, inhibition of fibrosis and hypertrophy, promotion of cell survival, and inhibition of inflammation (Figure 3) [23, 24]. BNP molecular forms, except NT-proBNP1-76 and proBNP1-108, can also activate NPR-C, once thought to be a clearance receptor, but which may also have anti-fibrotic properties [25]. Ralat and colleagues reported that after degradation by IDE, the smaller BNP forms produced could activate the GC-B receptor (Figure 2) [18], inducing bone growth, controlling vascular tone, and inhibiting cell proliferation and inflammation [26], however more conformational studies are needed.
Figure 3. BNP bioactivity via GC-A and cGMP activation and clearance through NPR-C and degradation pathways.
BNP stimulates cGMP activity through binding of GC-A. NPs also bind to the non-GC linked NP clearance receptor (NPR-C). The NPs are degraded by peptidases such as neutral endopeptidase 24.11 (NEP), dipeptidyl peptidase IV (DPP4), and insulin depredating enzyme (IDE).
The various circulating BNP molecular forms are detected by specific or non-specific immunoassays, and are now used in clinical practice as a biomarker to diagnose HF, especially assays for proBNP1-108, NT-proBNP1-76, and BNP1-32 forms.
3.2 BNP signal peptide
Historically, the BNP signal peptide was thought to play a role in organelle transport of the peptide, and not to play a role in either BNP function or as a biomarker of cardiac function. Recently however, Siriwardena and colleagues developed a signal peptide specific assay for BNP and found that a fragment from the signal peptide of BNP is present not only in the extracts of human hearts, but also in the circulation in the plasma [27]. Interestingly, the BNP signal peptide was significantly increased in patients with acute ST-elevation myocardial infarction (STEMI), peaking before any other biomarker of myocardial injury, such as myoglobin, creatine phosphokinase MB isozyme (CK-MB) and Troponin-I. However, the BNP signal peptide did not increase in HF patients, so further studies are required to clarify whether the BNP signal peptide will be useful as a biomarker or for understanding the mechanism of BNP signal peptide release.
3.3 NT-proBNP1-76 and BNP1-32
Currently, circulating BNP1-32 or NT-proBNP1-76 levels are widely used as sensitive biomarkers and predictors of prognosis. Maisel and colleagues elegantly reported in 2002 that measuring BNP levels in patients who came to the emergency department with dyspnea was useful in the diagnosis of HF [28]. NT-proBNP1-76 is also used to diagnose HF in the clinic. In 2006, Januzzi and colleagues reported in a multi-center trial of 1256 emergency department patients, that NT-proBNP1-76 levels were significantly higher in patients with acute HF and that the levels of NT-proBNP1-76 correlated with HF symptom severity [29]. McKie and colleagues examined plasma NT-proBNP1-76 levels in stage A/B HF patients looking at 10 year cardiovascular events. Kaplan-Meier curves of unadjusted cumulative incidence of death, heart failure, cerebrovascular accident, and myocardial infarction (MI) in stage A/B HF divided into two groups, above and below the age- and sex-specific 80th percentile plasma NT-proBNP1-76 levels, showed a significantly higher cumulative incidence for each outcome among subjects with plasma NT-proBNP1-76 levels above the 80th percentile [7]. These results suggested NT-proBNP1-76 is also a useful marker for predicting mortality and cardiac events in stage A/B HF patients.
It is important to understand that the commercially available assays for BNP1-32 and NT-proBNP1-76 used in clinical investigations and in clinical practice are not specific and detect other non-active forms of BNP. Ordonez-llanos and colleagues reviewed the reactivity of different BNP molecular forms with or without glycosylation in BNP1-32 and NT-proBNP1-76 assays [30]. BNP1-32 assays detected BNP1-32, BNP3-32, and proBNP1-108, but not NT-proBNP1-76. Roche’s NT-proBNP1-76 assay detected non-glycosylated proBNP1-108 and non-glycosylated NT-proBNP1-76, but not BNP1-32, glycosylated proBNP1-108, or glycosylated NT-proBNP1-76. The cross-reactivity of commercial assays can therefore affect the sensitivity and specificity of diagnosing HF with BNP1-32 and NT-proBNP1-76 assays. To determine which assay is best for diagnosing HF in the clinical setting, Masson and colleagues directly compared BNP1-32 and NT-proBNP1-76 levels in a large population of patients with HF [31]. In 3916 patients, NT-proBNP1-76 and BNP1-32 had similar relationships with age, cardiac and renal function. Either peptide ranked as the first independent predictor of outcomes in HF, however, NT-proBNP1-76 was superior to BNP1-32 for predicting mortality and morbidity or hospitalization for HF.
Although BNP1-32 is inferior to NT-proBNP1-76 as a biomarker, the BNP1-32 form has another important role as GC-A agonist for HF. Recombinant BNP1-32, called nesiritide, is a strong agonist for the GC-A receptor and has been studied in clinical trials for the treatment of acute HF in the US. From in vivo data and a single center trial of patients with HF, BNP1-32 promoted natriuresis and diuresis with reduced cardiac filling pressure, increased cardiac output, and with suppression of renin-angiotensin-aldosterone system [32, 33]. Nesiritide was approved by the US FDA in 2001, but controversial results from the ASCEND-HF trial lead to questions about the effectiveness and safety of Nesiritide. O’Connor finally reported that nesiritide administration (bolus and continuous infusion less than 1 day) improved acute symptoms with no serious adverse events, however death or re-hospitalization did not improve according to a randomized multicenter placebo controlled study of 7141 patients [34]. Hypotension occurred more often in patients who received nesiritide than in patients with placebo groups, which may have affect the outcomes in the nesiritide group.
Our group has focused on the anti-remodeling effect of chronic BNP1-32 administration for chronic stable HF. In 2006, Chen compared the effects acute injection versus chronic injection of BNP1-32 for 10 days in experimental HF [35, 36]. Chronic subcutaneous (SQ) administration of BNP1-32 showed no development of tolerance, with plasma cGMP levels and urinary cGMP excretion similar on day 10 to day 1 acute administration. Chen et al also reported on a randomized placebo-controlled double blinded proof of concept clinical trial [37]. In the treatment group, patients were given a 10 ug/kg SQ injection of BNP1-32 bid per day for 8 weeks. Chronic SQ BNP1-32 resulted in a reduction of left ventricular (LV) systolic and diastolic volume index and LV mass index, improved diastolic function and decreased plasma renin activity, suggesting BNP1-32 has anti-remodeling properties. Taken together, BNP1-32 therapy does not improve outcomes in acute decompensated HF which suggests we need new NPs which have fewer side effects for acute HF therapy. However, it may be useful as a chronic therapy for stable HF patients to reverse cardiac remodeling.
3.4 Degradation products: BNP3-32, BNP5-32 and smaller peptides
Seminov and colleagues reported that recombinant proBNP1-108 is cleaved to BNP1-32 by furin, while corin cleaves proBNP1-108 to BNP4-32 [38], however, other investigators reported that corin cleaves BNP1-108 to BNP1-32, which we will discuss in later sections. Peptides smaller than BNP1-32 are also made by degrading enzymes, DPPIV, NEP, IDE, and meprin. Figure 1 illustrates cleavage sites by those enzymes on human BNP. DPPIV cleaves BNP1-32 to BNP3-32 [39], NEP cleaves BNP1-32 to BNP5-32 [16,40], IDE cleaves several degradation products from BNP1-32 [18], and meprin cleaves BNP1-32 to BNP8-32 in canine [17]. These smaller BNP cleavage products were reported by Miller and colleagues to be present in the circulation of HF patients by mass spectrometry, including BNP1-32, BNP3-32, and BNP5-32 [41].
Are all processed and degraded forms of BNP able to bind the receptor and show activity? There are only a few studies to clarify these questions. In vitro, Ralat and colleagues reported cGMP activity after IDE degradation of BNP1-32 [18]. After IDE treatment for 5 min, BNP1-32 activated 4 fold greater cGMP in human GC-A expressing HEK293 cells compared to BNP1-32 without IDE treatment. Interestingly, BNP1-32 with IDE treatment also activated 7 fold greater cGMP in human GC-B expressing human embryonic kidney (HEK) 293 cells compared to without IDE treatment, suggesting the BNP forms generated by IDE degradation may be dual GC agonists (Figure 2). Boerrigter and colleagues compared BNP1-32 and BNP3-32 produced by DPPIV cleavage [15] and BNP8-32 generated by meprin in an in vivo canine model [17]. BNP1-32, BNP3-32, and BNP8-32 were administered to normal anesthetized canines and hemodynamics, renal function and circulating and cGMP excretion were examined. Compared to BNP1-32, BNP3-32 showed reduced natriuresis and diuresis and lacked vasodilating actions with reduced circulating cGMP levels and urinary cGMP excretion. BNP8-32 showed similar vasodilating actions as BNP1-32, however reduced diuretic and natriuretic actions with reduced circulating cGMP levels and urinary cGMP excretion. However, BNP8-32 may not be produced in humans because evidence suggests that meprin may not be present and not cleave BNP1-32 to BNP8-32 in the humans [21].
3.5 ProBNP1-108
The BNP1-32 precursor, proBNP1-108 has been thought to be an inactive peptide. Our group reported that BNP1-32 activated cGMP in human cardiac fibroblasts, but proBNP1-108 and NT-proBNP1-76 did not [5]. Dickey and colleagues however, reported in 2011 that proBNP1-108 could significantly activate cGMP in GC-A expressing HEK293 cells, although much more weakly than BNP1-32 [22]. Additional studies are necessary to confirm proBNP1-108 as an inactive or weakly active BNP peptide.
In recent years, a specific assay for proBNP1-108 was produced. Giuliani and colleagues developed the proBNP1-108 assay using a direct antibody against the hinge region, which is present only in the intact proBNP1-108 molecule [42]. Using this assay, Macheret and colleagues from our laboratory reported circulating proBNP1-108 levels in the general community. This population based study included a cohort of 1939 adults from Olmsted County, Minnesota [43]. ProBNP1-108 was detected in normal humans and was a sensitive biomarker for the detection of systolic dysfunction. We also reported proBNP1-108 presence in the plasma of normal healthy humans subjects [12]. Miller and colleagues also reported circulating proBNP1-108 was observed in HF patients [41].
Is proBNP1-108 a sensitive marker for HF? Macheret and colleagues reported that the proBNP1-108/NT-proBNP1-76 ratio could be a marker for HF, with a decrease in the ratio correlating with heart failure stage in the general community [43]. Also, in a cohort of 187 New York Heart Association (NYHA) class III-IV HF patients, Miller and colleagues reported that combined elevation of proBNP1-108 with Troponin T was a significant predictor of death or need for cardiac transplantation [44]. Dries and colleagues examined plasma proBNP1-108 and BNP1-32 (Architect BNP immunoassay) in 756 systolic HF patients [45]. Higher levels of proBNP1-108 were associated with an increased risk of all-cause death or cardiac transplantation (Hazard Ratio 4.9). Therefore, proBNP1-108 could be a good marker to predict outcomes of HF, and may play an important role in HF.
While ProBNP1-108 was found in the human circulation of both normals and HF patients, the more important question may be whether it is processed into active forms or not in the circulation. We will discuss proBNP1-108 processing later in this review but first we address the “BNP paradox” in HF.
4. BNP paradox in heart failure
There is a paradox in HF in that BNP is elevated, yet the kidney is vasoconstricted and sodium retaining. The biological significance of this elevation of BNP is now emerging. We have reported that despite an elevation in BNP, as measured by point-of-care testing in subjects with HF and NYHA Class IV symptoms, an absence of BNP1-32 was demonstrated by quantitative mass spectral analysis [4]. Using mass spec analysis, Hawkridge and colleagues examined BNP1-32 levels in 4 severe NYHA class IV HF patients who had high BNP levels by clinical BNP assay. Three of these 4 patients showed no BNP 1-32 by Mass spec, while one patient showed no BNP 1-32, but did express a higher molecular form of BNP which may be proBNP1-108. Niederkofler and colleagues also reported BNP molecular forms detected by Mass spec in patients with HF [46]. They found rapid degradation of BNP1-32 and several degraded forms in HF plasma. Both BNP1-32 and BNP degraded products were detected at low levels by mass spec whereas BNP levels were very high as determined by the Triage Biosite BNP assay in the same samples from patients with HF, suggesting BNP degradation is accelerated in HF. Further, Seferian and colleagues reported that proBNP1-108 was the major immunoreactive form of BNP in patients with HF [47]. Seferian designed assays for proBNP1-108, NT-proBNP1-76, and BNP1-32 using specific monoclonal antibodies for each. In patient plasma, the molar concentration of NT-proBNP1-76 was almost 10 times that of proBNP1-108, and the mean proBNP1-108:BNP1-32 ratio was 6.3, suggesting proBNP1-108 was circulating in much higher concentrations than BNP1-32, and is the major BNP form circulating in HF patients. Why does the concentration of proBNP1-108 remain high in HF? Reasons may include: 1) Excessive proBNP1-108 production or accelerated degradation of BNP1-32; 2) Impaired processing of proBNP1-108; and/or 3) Low activity/expression of proBNP1-108 convertases. Indeed some investigators reported that the pro-peptide convertases corin and furin may play important roles in disease states because they can convert proBNP1-108 to active BNP forms. In light of the importance of this issue, we will briefly summarize some key points related to corin and furin in subsequent sections.
5. BNP processing and pro-peptide convertases
It has been reported proBNP1-108 is cleaved to BNP1-32 by the proNPs convertases, corin and furin. Corin is a transmembrane cardiac serine protease that converts proANP1-126 to active ANP1-28 and proBNP1-108 to active BNP1-32 [2]. Furin is an intracellular endoprotease which is enriched in the Golgi apparatus and functions to cleave several pro-proteins, including proBNP1-108 and proCNP1-103, to their mature and active forms [3, 48]. Using mass spec, Seminov and colleagues reported on the processing of proBNP1-108 by furin and corin in HEK293 cells [13]. Their studies suggest furin-mediated cleavage of proBNP1-108 results in BNP1-32 and corin-mediated cleavage leads to the production of BNP4-32. As both proNPs convertases corin and furin process proBNP1-108 to biologically active forms of BNP, they each may play an important role of the physiology of normal and cardiovascular disease state.
In the next section, we will focus on corin, one of the proBNP convertases, and its important role in the heart in mediating the conversion of the proNP hormones into mature biologically active peptides and how this affects cardio-renal physiology in humans. The other convertase furin may also play important role for NP biology, however, very few studies have been done about BNP and furin biology, which are warranted in further studies.
6. Cardiac serine protease, Corin
6.1 Expression and function
6.1.1 General information under physiologic conditions
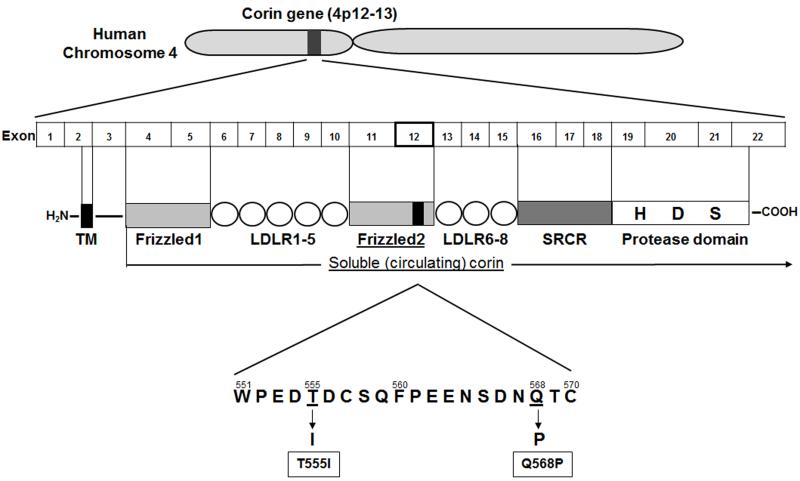
In 1999, Yan and colleagues were the first to report corin, a transmembrane serine protease that is highly expressed in the human heart [9]. Corin contains two frizzled-like cysteine-rich motifs, seven low density lipoprotein receptor (LDLR) repeats, a macrophage scavenger receptor-like domain, and a trypsin-like protease domain in the extracellular resign (Figure 4). The human corin gene localizes to chromosome 4 (4p12-13), and its mRNA is highly expressed only in the heart, whereas mouse corin was detected in the heart and weakly in the testis and kidney. Interestingly, human corin mRNA was found in several cell lines derived from uterine tumors and osteosarcomas. In adult mouse heart, corin mRNA was found in the cardiac myocytes of the both the atrium and the ventricle by in situ hybridization. Interestingly, corin mRNA was detected at higher levels in cardiac myocytes of embryonic hearts than adult ones. Corin mRNA expression was also observed in other organs, including developing kidney medulla, and vertebra of embryonic mice. These findings suggest that corin may play an important role in the development of the heart, kidney and bone, and in control of adult heart physiology.
Figure 4. A schematic structure of human corin gene, the domain structure of corin protein, and corin single nucleotide polymorphisms (SNPs).
Hooper and colleagues reported human corin protein localization using polyclonal antibody antibodies they produced against human corin [49]. Corin immunoreactivity of approximately 125-135 KD was seen in lysates from human heart tissue by Western immunoblot (WB). Using immunohistochemistry, corin protein was found in cardiac myocytes, but not in skeletal or smooth muscle.
Currently, the most important known role for corin is in the cleavage of proANP1-126 or proBNP1-108 to their mature forms. Yan and colleagues were the first to report that corin cleaves proBNP1-108 [2]. After transfection of corin into HEK 293 cells, corin protein was observed in cell lysates and in membrane fractions as a 150 KD protein, larger than the predicted 116kD. This larger than predicted protein size was explained by the fact that corin contains 19 potential N-linked glycosylation sites, and therefore is detected as a higher molecular weight glycosylated form. Co-transfection of proBNP1-108 and corin resulted in processing of proBNP1-108 to BNP1-32, suggesting that corin is a proBNP1-108 convertase.
Peng and colleagues confirmed that corin cleaved proBNP at multiple sites [50]. ProBNP1-108 T71A glycosylation defective mutant co-transfected with furin or corin was processed and secreted to BNP1-32 in both HEK293cells and HL cells. Then they produced double or triple mutations of R73A, R76A, and K79A which may result in BNP1-35, BNP1-32, and BNP4-32, respectively if the proBNP is processed. Using HEK293 cells which express furin but not corin, R73A and R76A were important for furin mediated proBNP processing but not K79A. Interestingly, in HL cells which expressed furin and corin, when all three residues were mutated, proBNP processing was prevented. According to these results, corin may process proBNP to not only BNP1-32, but also BNP4-32.
Corin contains an integral transmembrane domain near its amino terminus, with a large portion of the peptide existing extracellularly. The next question became what part of the corin peptide is important for proNP cleavage, and is anchorage of the peptide to the cell necessary for activity? To address these questions, Knappe and colleagues produced two different corin peptide constructs: One was a soluble corin which does not contain the transmembrane domain, and the other was a short form corin containing only the catalytic domain [51]. Soluble corin was able to process proANP1-126 to ANP1-28, however, the shorter catalytic domain corin could not. The soluble corin activity was inhibited by some trypsin-like serine protease inhibitors, including benzamidine, phenylmethanesulfonylfluoride (PMSF), leupeptin, but not ethylenediaminetetraacetic acid (EDTA) or pepstatin. To further assess domains important for corin activity, mutants lacking the frizzled 1 domain or the LDLR repeats were produced, and demonstrated that both of these regions are important for recognizing and cleaving proANP1-126.
Recently, circulating (=soluble) corin in human plasma has been reported. Following this discovery, Jiang et al examined whether soluble corin is shed from cardiomyocytes [52]. Using two corin transfected cell lines, HEK293 cells and a cardiomyocyte cell line, HL-1, conditioned medium was assessed for soluble corin. Three molecular weight soluble corin proteins were identified, ~180KD, ~160KD, and ~100KD. Using several protease inhibitors, they determined that the 180 KD protein is shed by cleavage by a disintegrin and metalloproteinase domain-containing protein (ADAM)10 C-terminal of the transmembrane domain, the160 KD protein is auto-cleaved by corin within the frizzled-1 domain, and the 100 KD protein is also auto-cleaved by corin within the LDLR5 region. They further examined those three corin fragments to verify proANP1-126 cleavage by each. The 180 KD soluble corin cleaved proANP1-126, but the 160 KD and the 100 KD corin did not. They concluded that ADAM-mediated shedding and corin auto-cleavage are important in the regulation of circulating corin.
Our laboratory has also reported on corin expression and circulating corin levels in normal humans [12]. We observed corin protein expression in normal heart and kidney by Immunohistochemistry and corin protein expression in LV by WB. Using a corin enzyme-linked immunosorbent assay (ELISA), we examined circulating corin levels in 55 subjects and found a large distribution of soluble corin (median 889.4, 25th quartile 587.0 – 75th quartile 1477.2 ng/L), with significantly higher soluble corin in males than in females. We also reported a weak positive correlation of soluble corin with age, which we will discuss later.
6.1.2 Animal models
Animal models of cardiac disease and corin deficient animal models have been produced to aid in the investigation of corin. Langenickel and colleagues examined a rat HF model [53]. First, rat corin cDNA was cloned and sequenced, and found to be 86% homologeous to human corin. Rat corin mRNA was detected in the heart, especially the atrium, by Northern blot analysis, and in kidney, aorta, brain and testis by RT-PCR. HF was induced in the rat model by infrarenal aortocaval shunt for 4 weeks, resulting in increased total heart weight and each chamber weights, impaired dP/dt max, elevated LV-end diastolic pressure and central venous pressure. Sham and HF hearts were examined for ANP release by left atrial (LA) stretch. ANP secretion increased in sham hearts, but was blunted in HF hearts compared to sham and corin mRNA expression was decreased in the atria of HF compared to sham atria.
However, other investigators have reported that corin expression is increased in animal HF models. Tran and colleagues reported that both corin and ANP mRNA increased in phenylephrine-stimulated rat neonatal cardiomyocytes and also in non-infarcted LV myocardium of 8 week MI rats [54]. Calderone and colleagues reported ANP, BNP and corin mRNA expression increased in the non-infarcted and scar myocardium LV one week after MI in rats [55]. Jiang and colleagues induced acute myocardial necrosis in rats and examined corin mRNA expression, circulating BNP levels, and the BNP degrading enzyme, NEP, activity [56]. Here rats were given subcutaneous injection of isoproterenol (ISO) twice per day with or without premedication of omapatrilat (OMA), a dual NEP and angiotensin converting enzyme inhibitor. ISO induced myocardium necrosis and impaired cardiac function with increased circulating BNP levels, and NEP treatment protected the necrosis and impairment. In the ISO group, both corin and BNP mRNA expression were up-regulated in myocardium and NEP activity was down-regulated, however, ISO+OMA alleviated these changes. Importantly, Chan and colleagues reported corin-deficient mice (Cor−/−) [57] and corin knockout (KO) mice have elevated proANP1-126 levels and undetectable mature ANP1-28. In addition, the corin KO mice had spontaneous hypertension as compared with wild type mice and exhibited cardiac hypertrophy resulting in a mild decline in cardiac function later in life. Based on these animal model studies, corin expression is observed in cardiac disease models, however the changes in corin expression were dependent on disease state/animal model used/atrium or ventricle.
6.1.3 Human corin single nucleotide polymorphism (SNP)
Some investigators have reported a single nucleotide polymorphism (SNP) in human corin. In 2005, Dries and colleagues first reported corin SNPs Q568P and T555I [58]. The 2 nonsynonymous, nonconservative (type I) SNPs in highly conserved amino acids were found in exon 12, the cysteine-rich frizzled-like domain of corin (Figure 4). These 2 SNPs were called T555I (threonine to isoleucine at amino acid position 555), and Q568P (glutamic acid to proline at amino acid position 568). In the Dallas Heart Study involving patients 65 years of age or older with 50% Black participants, approximately 12% were heterozygous for the SNPs (carrying 1 copy of the I555 or P568; corin+/−), and only 0.4 % were homozygous (carrying both copies of the I555 and P568; corin−/−). The SNPs were associated with higher blood pressure and an increased risk for prevalent hypertension. In addition, Rame and colleagues examined the relationship between the SNPs and systolic blood pressure and indexed left ventricular mass derived from cardiac magnetic resonance imaging (MRI) in the Dallas Heart Study. They found that subjects expressing the corin SNPs who had higher Systolic blood pressure (>130 mmHg) had more LV hypertrophy compared to non-carriers [59]. Further, they demonstrated that expression of the corin alleles caused impairment in proBNP1-108 processing. Finally, they examined the corin allele in subjects from African American Heart Failure Trial Genetic Risk Assessment in Heart Failure sub-study. They divided subjects into two groups, with or without treatment with isosorbide-dinitrate and hydralazine, retrospectively. In subjects without treatment, survival free from death or first heart failure hospitalization was significantly worse in carriers of the corin allele compared to non-carriers. There was no significant difference in survival between carriers and non-carriers of the corin allele in the groups that received medication, which may suggest that treatment is more favorable in hypertensive-corin allele patients. Interestingly but logically, the corin allele was significantly associated with lower circulating BNP values. Both proBNP1-108 and BNP1-32 levels were measured to exam the ratio of proBNP1-108 to BNP1-32, as a higher ratio would indicate greater impairment in BNP1-108 processing. The proBNP1-108/BNP1-32 ratio was significantly higher in the corin allele group, suggesting lower circulating BNP levels are the result of impaired proBNP1-108 processing in corin allele carriers [60].
Wang and colleagues examined the effect of corin variant T555I/Q568P on corin function in vitro [61]. First, they found that lacking the corin frizzeled-like domain 2 resulted in a 70% loss of proANP1-126 to ANP1-28 processing. Homozygote variants carrying both T555I and Q568P had reduced pro-ANP1-126 and pro-BNP1-108 processing activity compared to wild type in HEK293 cells because of impaired corin zymogen activation and the absence of an activated protease domain fragment whereas heterozygote variants carrying either T555I or Q568P did not show the effect. This presents a discrepancy between the heterozygote clinical data and in vitro data, which was explained by the suggestion that carrying only one SNP, either T555I or Q568P, is sufficient to alter the corin frizzled-like domain structure in mice.
When considered together, these studies suggest that corin may play a role in controlling hypertension and cardiac hypertrophy, specifically through proBNP1-108 processing, however we need further studies to understand corin effects on blood pressure and LV remodeling through NP processing.
6.1.4 Human disease states and gender differences
As mentioned in section 6.1.1., circulating (=soluble) corin is present in human plasma which has activity to process proNPs. The first report regarding plasma corin levels in human HF was performed by Dong and colleagues. In normal subjects (n=198) plasma corin levels (mean 690, SD 260 pg/ml) are widely distributed with gender (male>female) differences, but not age. In 291 patients with HF, the corin level (mean 365, SD 259 pg/ml) was significantly associated in gender, but not age [62], and also the reduction of plasma corin levels correlated with the severity of HF. Corin levels in AMI were also assessed, and plasma corin levels in AMI were similar to that of control subjects. In 2010, Shrestha and colleagues reported on the relationship between circulating corin levels and cardiac function and clinical events [63]. In 126 patients with chronic systolic HF (<35% ejection fraction (EF)), plasma corin and NPs levels were measured (ANP1-28, NT-proANP, BNP1-32, and NT-proBNP1-76) and cardiac structure and clinical outcomes (all-cause mortality, cardiac transplantation, or HF hospitalization) were assessed. Plasma corin levels in HF (median 1220, 25th quartile 886.0 – 75th quartile 1396 pg/mL) differed with gender (male>female) and decreased with increasing NYHA class. There was no correlation between corin levels and age, systolic blood pressure, or glomerular filtration rate. Plasma corin levels modestly correlated with LV hypertrophy, including LV mass index and LV wall thickness. There was no correlation between corin levels and echo parameters, such as cardiac function, whereas all plasma NP or NT-proNP levels had strong correlations. In Cox proportional hazards analysis, higher plasma corin levels did not reduce the risk of adverse clinical events in the population. Ibenuogu and colleagues reported plasma corin levels in 14 severe HF patients (NYHA class III-IV, median EF 18% and median BNP 1940 pg/ml), where plasma corin levels were 7.6 fold lower than in 16 normal controls [64]. They also measured NT-proANP and proANP1-28, which were high. Using a novel immunoassay, the level of uncleaved proANP was determined and was significantly higher in HF, suggesting proANP1-126 processing was impaired. This study addresses the interesting question of whether lower corin levels in HF is associated with NP processing impairment in clinical setting, although the sample number was small and they did not examined proBNP1-108 processing.
Interestingly, most studies have observed gender differences in circulating corin levels. Dong et al elegantly summarized these levels in their review as well [65], plasma corin levels in healthy males is higher than those in healthy females according to 3 different population (corin levels had a mean ± SD: of 798 ± 285 in males (n=104) and 551 ± 224 in females (n=94) [62]; 842 ± 283 in males (n=182) and 569 ± 192 in females (n=166) [66] with corin levels having a median (25th – 75th quartiles) of 1623 (1187-1827) in males and 810 (509-982) in females [12]). How about NP levels? ANP1-28, NT-proANP, BNP1-32, and NT-proBNP1-76 are higher in females than in males in both the general population [67-69] and in HF [70]. Much remains to be elucidated on the role of gender specific hormones in regulating the NP signaling system, but corin may clearly be affected by gender. As we have recently observed that proBNP1-108 is higher in females than males in the general population [43], it is tempting to speculate that higher corin in males accelerates proBNP1-108 processing to mature BNP1-32, but due to either insufficient receptor concentration or to greater proteolytic degradation, is not available for cardio-protection. Indeed, studies have documented that DPPIV, which degrades mature BNP1-32 to less biologically active BNP3-32, is also higher in males than females, supporting this speculation [15, 71]. Further studies are required to clarify this issue.
How does corin expression in the heart tissue change in human HF? Chen and colleagues reported corin expression in explanted hearts of dilated cardiomyopathy [72]. In normal hearts, corin mRNA and protein expressions were higher in atrium than in ventricle. In failing heart, corin protein expression was significantly higher in right ventricle (RV) and LV compared to normal heart, but there was no significant difference in right atrium (RA) and LA. Peltonen and colleagues examined mRNA expression of NP system genes, including corin, by RT-PCR in tissue from the aortic valve [73]. They compared the tissue in Aortic valve regurgitation (AR), AR+fibrosis, Aortic valve stenosis (AS), and normals. In AS aortic valves, CNP, GC-A, GC-B, and furin mRNA expressions were significantly lower than in normal valves, however, all other mRNA expressions, including corin, in AS valve and in AR or AR fibrosis valves, were not significantly different than normal valves. These results suggest corin plays important roles in heart tissue, and raises an important question as to why circulating corin decreases in HF while corin expression in LV or RV increases in HF. We summarize the current findings of corin in Table 1.
Table 1. Summary of corin findings.
| Corin | References | |
|---|---|---|
| Type | Transmembrane serine protease | 9 |
| Role | Process proBNP1-108 and proANP1-126 | 2 |
| Existence in Human | Heart and kidney | 9,12,49, |
| Knockout mice | HT and LVH | 57 |
| Animal model | ||
| Heart Failure | Rat mRNA (atrium) - decrease | 53 |
| Rat mRNA (ventricle)- increase | 56 | |
| Myocardial Infarction | Rat mRNA (ventricle) - increase | 54, 55 |
| Human SNPs | HT and LVH, impaired BNP processing | 58-61 |
| Human disease state | ||
| Heart Failure | decrease (plasma) | 63,64 |
| increase (ventricle) | 72 | |
| Valvular Disease | no change (aortic valve) | 73 |
SNPs, single nucleotide polymorphisms; BNP, b-type natriuretic peptide; ANP, atrial natriuretic peptide; HT, hypertension; LVH, left ventricular hypertrophy.
6.2 Other topics
6.2.1 BNP processing in the circulation
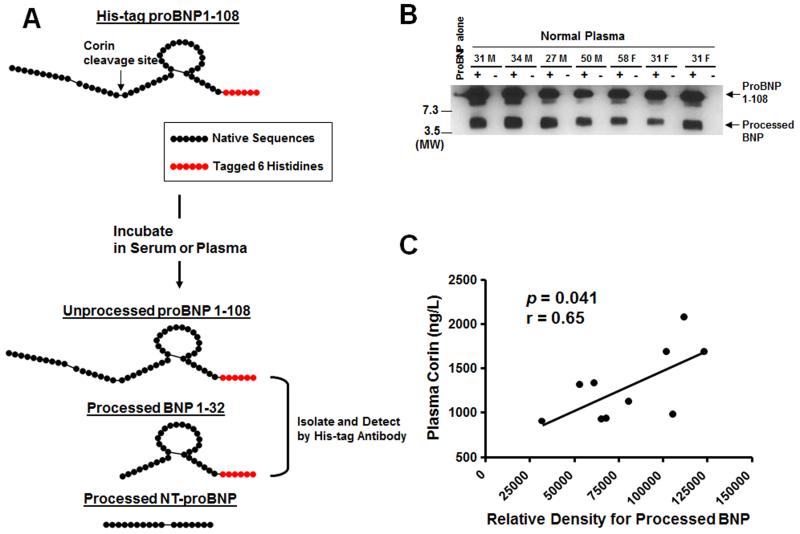
We have already discussed the “BNP paradox’ and the possibility that an impairment of processing from proBNP1-108 to active BNP1-32 may play a key role in HF, as has been suggested by Rame and colleagues to explain the increase in circulating proBNP1-108 in HF plasma [60]. We now know that proBNP1-108 is indeed a circulating hormone under physiological conditions, which led us to examine the question of whether this physiologic proBNP1-108 can be processed in the circulation. We examined whether proBNP1-108 is processed in normal blood ex vivo [12]. Fresh serum or EDTA plasma was obtained from normal volunteers. Samples were incubated with or without exogenous proBNP1-108 tagged with 6 Histidines in the C-terminal end to facilitate isolation of unprocessed proBNP1-108 (predicted molecular weight (MW) = 12.8 KD) or processed BNP1-32 (predicted MW = 4.3 KD). The processed peptides were isolated by immunoprecipitation, detected by WB, and sequenced (Figure 5A). ProBNP1-108 (approx. 12 KD) was processed into a smaller molecular form (approx. 4 KD) in fresh or stored serum from normal human subjects which was confirmed to be BNP1-32 and BNP3-32 (Figure 5B). The relative density of processed BNP by WB positively correlated to plasma corin levels (Figure 5C).
Figure 5. ProBNP processing in normal human circulation.
[12] A: The schema of His-tag proBNP1-108 processing. Native sequences are shown as black-closed circle, and tagged Histidines are shown as red-closed circle. B: Representative WB for His-tag protein in healthy volunteers. Number and M or F; Age and Male or Female, +; with His-tag proBNP1-108, −; without His-tag proBNP1-108, MW; Molecular weight. C: The relationship between plasma corin levels and processed BNP.
In the in vivo setting, Seminov and colleagues reported that human proBNP1-108 was processed in the circulation of normal rats [13]. They studied the processing of exogenous glycosylated or non-glycosylated proBNP1-108, as well as clearance rate of proBNP1-108 together with BNP1-32 and NT-proBNP1-76 using immunoassays, gel filtration, and mass spectrometry techniques. After glycosylated proBNP1-108 injection, BNP1-32 was not shown over 12 min, however, non-glycosylated proBNP was processed into BNP1-32. By mass spec, BNP1-32, BNP3-32, BNP4-32, BNP5-32, and BNP5-31 were observed in rat circulation after non-glycosylated proBNP injection. Next they calculated the clearance curves after injection of glycosylated or non-glycosylated proBNP1-08, BNP1-32 or NT-proBNP1-76. In rat, the half-life of BNP1-32 was 6.5 min, and the half-life of non-glycosylated proBNP1-108, glycosylated proBNP1-108, non-glycosylated NT-proBNP1-76 and glycosylated NT-proBNP1-76 were 8.7min, 9.0 min, 15.5 min, and 15.7 min, respectively. Interestingly, there was no difference of half-life between non-glycosylated and glycosylated proBNP1-108 or between non-glycosylated and glycosylated NT-proBNP1-76 in vivo in the rat whereas glycosylated proBNP1-108 protein was not processed to BNP1-32 in other in vitro studies. It should also be noted that proBNP1-108 has a longer half-life than BNP1-32 while NT-proBNP1-76 has the longest half-life which underscores its robustness as a biomarker.
6.2.2 Role of glycosylation on BNP molecular forms and processing
In a previous section, we discussed whether the glycosylation state of proBNP1-108 may affect its half-life. In this section, we will discuss glycosylation of BNP molecular forms and its effects on proBNP1-108 processing. Schellenberger and colleagues were the first to report on O-linked glycosylation of proBNP1-108 [74]. Using Chinese hamster ovary cells, recombinant proBNP1-108 was produced. The recombinant proBNP1-108 was treated with or without a de-glycosylation cocktail containing neuraminidase, beta-galactosidase, N-actyl glucosaminidase and end-O-glycosidase. The de-glycosylation cocktail treated proBNP1-108 had a lower molecular weight (11 KD) than non-treated proBNP1-108 (13 KD). They found the 13 KD proBNP1-108 had five complete O-linked glycosylation and two partial O-linked glycosylation sites which were identified through the characteristic carbohydrate marker ions after digestion with trypsin followed by mass spec (Figure 6). They also found glycosylated proBNP1-108 in the plasma of HF patients using immunoprecipitation and WB.
Figure 6. ProBNP1-108 O-linked glycosylation sites.
[74]
In 2009, Semenov and colleagues reported that proBNP1-108 processing was suppressed by T71 glycosylation close to the corin cleavage site. The author produced several mutants at each O-glycosylation site, and found that a T71A proBNP1-108 mutant was processed when transfected in HEK293 cells, suggesting T71 O-glycosylation may play a key role in inhibition of proBNP1-108 processing by proNP convertases [75]. Another group, Jiang and colleagues, reported that O-glycosylation affected proBNP1-108 stability [76]. Using two different cell lines, HEK293 cells and HL cells (cardiomyocytes) and Ben-gal, an O-glycosylation inhibitor, they showed that transfected proBNP1-108 had a lower molecular weight in both cell lines, indicating Ben-gal inhibited O-glycosylation of the peptide. Looking at secreted proBNP1-108 from cells treated with or without Ben-gal showed that the half-life of the secreted proBNP1-108 from HEK293 cells was 3 times longer with Ben-gal than without Ben-gal, and was five times longer with Ben-gal in HL cells. The authors concluded O-glycosylated proBNP1-108 was much more stable than partially glycosylated proBNP1-108. Based on these findings, Semenov and colleagues further examined whether corin and furin could process glycosylated proBNP1-108 to BNP1-32. In furin transfected LoVo furin deficient cells, non-glycosylated proBNP1-108 was processed to BNP1-32 and proBNP1-108 T71A glycosylation defective mutant was also processed, however WT glycosylated proBNP1-108 was not processed. In corin transfected HEK cells, non-glycosylated proBNP1-108 was processed to BNP4-32 and proBNP1-108 T71A glycosylation defective mutant was also processed, but WT glycosylated proBNP1-108 was not. So, the authors proposed that O-glycosylation inhibits proBNP1-108 processing by corin to BNP4-32 [13]. This is the first report that corin processes proBNP into BNP4-32 in vivo, but Peng and colleagues confirmed that corin cleaved proBNP at multiple sites which we already discussed in a previous section [50]. Peng and colleagues also reported on proBNP1-108 glycosylation. ProBNP1-108 T71A glycosylation defective mutant co-transfected with furin or corin was processed and secreted to BNP1-32 in both HEK293 cells and HL cells.
O-linked glycosylated proBNP1-108 is not processed by corin or furin, but which proBNP1-108 is produced in cells and secreted into the circulation, glycosylated or non-glycosylated? Tonne and colleagues expressed pre-proBNP1-134 in cardiomyocytes to determine the dominant intracellular and extracellular forms of proBNP1-108 and found that the predominant intracellular form of BNP was non-glycosylated proBNP1-108 rather than BNP1-32 [77]. Glycosylated proBNP1-108 but not non-glycosylated proBNP1-108 was detected in cultured supernatant of preproBNP-expressing cardiomyocytes. Next they transfected cardiomyocytes with a T71A mutant of pre-proBNP1-134 without glycosylated on T71, which reduced glycosylated proBNP1-108 and increased BNP1-32 in the medium. Glycosylated proBNP1-108 is resistant to processing by corin, and the major circulating form of proBNP1-108 may be glycosylated proBNP1-108, suggesting glycosylation status may be a key point to consider in the BNP paradox and future HF therapies.
Another area of concern for BNP glycosylation involves NT-proBNP1-76. In Figure 6, we can see that all of the O-glycosylation sites are located in the NT-proBNP1-76 portion of proBNP1-108, which may interfere with detection of NT-proBNP1-76 by NT-proBNP1-76 assays. Seferian and colleagues assessed the affect of glycosylation on NT-proBNP1-76 detection using monoclonal antibodies against several regions of NT-proBNP1-76 [78]. Treatment of endogenous NT-proBNP1-76 with glycosidase resulted in significant improvement of NT-proBNP1-76 detection, especially in the mid-region of NT-proBNP1-76 (AA28-56), suggesting the assays using antibodies against the mid-region could underestimate the concentration of NT-proBNP1-76. Similarly, Nishikimi and colleagues examined the effect of glycosylation on NT-proBNP1-76 assays in HF plasma [79]. Plasma samples from 186 patients with HF were examined. Samples were incubated with or without de-glycosylating enzymes and NT-proBNP1-76 levels were measured by Roche Elecsys proBNP I, which utilizes antibodies to AA1-21 and 39-50. After treatment with de-glycosylating enzymes, NT-proBNP1-76 levels were about six times higher than without treatment, suggesting the Elecsys ProBNP I assay measures only 20% of the total NT-proBNP1-76. Non-glycosylated NT-proBNP1-76 levels, glycosylated NT-proBNP1-76 levels, and BNP1-32 levels have a strong correlation to LVEF, fractional shortening, and LV mass index, so it does not affect the power to predict cardiac function or prognosis, but it is still important to recognize that some assays underestimate the real NT-proBNP1-76 levels.
In summary, O-linked glycosylation of proBNP1-108 reduces its processing by proNPs convertases and may cause underestimation of circulating NT-proBNP1-76 or proBNP1-108 levels.
7. Clinical implication and future directions
We now know that a major molecular form of BNP that circulates in human HF and possesses reduced biological function is proBNP1-108. The mechanism of a potential defect in proBNP1-108 processing may be linked to dysregulation of the processing enzyme corin, which may provide a therapeutic opportunity. Is exogenous corin protein a potential drug for cardiovascular diseases? Maybe because a corin like drug may improve the impairment of endogenous proBNP processing into BNP1-32 which would result in the increase in physiological BNP1-32 concentrations with less hypotensive effects than administration of recombinant BNP1-32 which rapidly reaches pharmacologic concentration. Before we can make this assessment, we need in vivo data to observe whether exogenous corin can process proBNP1-108 under physiologic conditions and to determine other potential yet unknown reactions of corin in the body. Currently most of drugs related to enzyme reactions are enzyme inhibitors, such as Angiotensin converting enzyme inhibitor for hypertension and HF and HMG-CoA reductase inhibitor (=statin) for hyperlipidemia. To date, enzyme replacement therapy is targeted for lysosomal storage diseases, such as Fabry disease, which completely lacks certain enzymes. The enzyme is given to patients by intravenous injection [80]. However, we don’t know if it is a reduction in corin presence or activity that causes a reduction in processing in HF. In addition, plasma corin levels in both normal and HF samples showed huge concentration variations which require further analysis to determine “normal ranges”. Much remains to be elucidated, including: 1) can corin process other proproteins or does it have other functions? 2) can proBNP1-108 be processed by enzymes other than corin or furin and if so, what are these enzymes? For the moment, it may be most effective to supplement HF patients with GC-A activators such as ANP1-28 (Carperitide) or a dual GC-A/-B activator such as CD-NP (Cenderitide) which have less hypotensive effects than nesiritide.
In conclusion, many areas of BNP molecular form function and the role of corin in BNP processing remain unclear, but promise to give us useful knowledge on the physiology and pathophysiology of cardiovascular diseases and the heart as an endocrine organ.
Acknowledgments
None.
Souse of Funding
This work was supported by grants from the National Institute of Health (RO1 HL36634 and PO1 HL76611) awarded to Dr. John C. Burnett Jr., American Heart Association Post-Doctoral Fellowship (10POST3600045) and Scientist Development Grant (12SDG11460017) awarded to Dr. Tomoko Ichiki, and the Mayo Foundation.
Abbreviations
- BNP
B-type natriuretic peptide
- HF
heart failure
- NPs
natriuretic peptides
- AA
amino acids
- FI-ICR
fourier transform ion cyclotron resonance
- cGMP
cyclic guanosine monophosphate
- ANP
atrial natriuretic peptide
- DPPIV
dipeptidyl peptidase-4
- NEP
neutral endopeptidase
- GC
guanylyl cyclase receptor
- NPR
natriuretic peptide receptor
- STEMI
ST-elevation myocardial infarction
- CK-MB
creatin phosphokinase MB isozyme
- SQ
subcutaneous
- LV
left ventricle/ventricular
- HEK
human embryonic kidney
- NYHA
New York Heart Association
- LDLR
low density lipoprotein receptor
- PMSF
phenylmethanesulfonylfluoride
- EDTA
ethylenediaminetetraacetic acid
- ADAM
a disintegrin and metalloproteinase domain containing protein
- WB
Western immunoblot
- ELISA
enzyme-linked immunosorbent assay
- LA
left atrium
- MI
myocardial infarction
- ISO
isoproterenol
- KO
knockout
- SNP
single nucleotide polymorphism
- MRI
magnetic resonance imaging
- AMI
acute myocardial infarction
- EF
ejection fraction
- RV
right ventricle
- RA
right atrium
- AR
aortic valve regurgitation
- AS
aortic valve stenosis
- MW
molecular weight
References
- [1].de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life. Sci. 1981;28:89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- [2].Yan W, Wu F, Morser J, Wu Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc.Natl. Acad. Sci. U. S. A. 2000;97:8525–8529. doi: 10.1073/pnas.150149097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sawada Y, Suda M, Yokoyama H, Kanda T, Sakamaki T, Tanaka S, et al. Stretch-induced hypertrophic growth of cardiocytes and processing of brain-type natriuretic peptide are controlled by proprotein-processing endoprotease furin. J. Biol. Chem. 1997;33:20545–20554. doi: 10.1074/jbc.272.33.20545. [DOI] [PubMed] [Google Scholar]
- [4].Hawkridge AM, Heublein DM, Bergen HR, 3rd, Cataliotti A, Burnett JC, Jr., Muddiman DC. Quantitative mass spectral evidence for the absence of circulating brain natriuretic peptide (BNP-32) in severe human heart failure. Proc. Natl. Acad. Sci. U. S. A. 2005;102:17442–17447. doi: 10.1073/pnas.0508782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Heublein DM, Huntley BK, Boerrigter G, Cataliotti A, Sandberg SM, Redfield MM, et al. Immunoreactivity and guanosine 3′,5′-cyclic monophosphate activating actions of various molecular forms of human B-type natriuretic peptide. Hypertension. 2007;49:1114–1119. doi: 10.1161/HYPERTENSIONAHA.106.081083. [DOI] [PubMed] [Google Scholar]
- [6].Liang F, O’Rear J, Schellenberger U, Tai L, Lasecki M, Schreiner GF, et al. Evidence for functional heterogeneity of circulating B-type natriuretic peptide. J. Am. Coll. Cardiol. 2007;49:1071–1078. doi: 10.1016/j.jacc.2006.10.063. [DOI] [PubMed] [Google Scholar]
- [7].McKie PM, Cataliotti A, Lahr BD, Martin FL, Redfield MM, Bailey KR, et al. The prognostic value of N-terminal pro-B-type natriuretic peptide for death and cardiovascular events in healthy normal and stage A/B heart failure subjects. J. Am. Coll. Cardiol. 2010;55:2140–2147. doi: 10.1016/j.jacc.2010.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lainchbury JG, Troughton RW, Strangman KM, Frampton CM, Pilbrow A, Yandle TG, et al. N-terminal pro-B-type natriuretic peptide-guided treatment for chronic heart failure: results from the BATTLESCARRED (NT-proBNP-Assisted Treatment To Lessen Serial Cardiac Readmissions and Death) trial. J. Am. Coll. Cardiol. 2009;55:53–60. doi: 10.1016/j.jacc.2009.02.095. [DOI] [PubMed] [Google Scholar]
- [9].Yan W, Sheng N, Seto M, Morser J, Wu Q. Corin, a mosaic transmembrane serine protease encoded by a novel cDNA from human heart. J. Biol. Chem. 1999;274:14926–14935. doi: 10.1074/jbc.274.21.14926. [DOI] [PubMed] [Google Scholar]
- [10].Lisy O, Redfield MM, Schirger JA, Burnett JC., Jr Atrial BNP endocrine function during chronic unloading of the normal canine heart. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288:R158–162. doi: 10.1152/ajpregu.00444.2004. [DOI] [PubMed] [Google Scholar]
- [11].Lisy O, Redfield MM, Jovanovic S, Jougasaki M, Jovanovic A, Leskinen H, et al. Mechanical unloading versus neurohumoral stimulation on myocardial structure and endocrine function In vivo. Circulation. 2000;102:338–43. doi: 10.1161/01.cir.102.3.338. [DOI] [PubMed] [Google Scholar]
- [12].Ichiki T, Huntley BK, Heublein DM, Sandberg SM, McKie PM, Martin FL, et al. Corin is present in the normal human heart, kidney, and blood, with pro-B-type natriuretic peptide processing in the circulation. Clin Chem. 2011;57:40–47. doi: 10.1373/clinchem.2010.153908. [DOI] [PubMed] [Google Scholar]
- [13].Semenov AG, Tamm NN, Seferian KR, Postnikov AB, Karpova NS, Serebryanaya DV, et al. Processing of pro-B-type natriuretic peptide: furin and corin as candidate convertases. Clinical chemistry. 2010;56:1166–1176. doi: 10.1373/clinchem.2010.143883. [DOI] [PubMed] [Google Scholar]
- [14].Brandt I, Lambeir AM, Ketelslegers JM, Vanderheyden M, Scharpe S, De Meester I. Dipeptidyl-peptidase IV converts intact B-type natriuretic peptide into its des-SerPro form. Clin Chem. 2006;52:82–87. doi: 10.1373/clinchem.2005.057638. [DOI] [PubMed] [Google Scholar]
- [15].Boerrigter G, Costello-Boerrigter LC, Harty GJ, Lapp H, Burnett JC., Jr Des-serine-proline brain natriuretic peptide 3-32 in cardiorenal regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R897–901. doi: 10.1152/ajpregu.00569.2006. [DOI] [PubMed] [Google Scholar]
- [16].Pankow K, Wang Y, Gembardt F, Krause E, Sun X, Krause G, et al. Successive action of meprin A and neprilysin catabolizes B-type natriuretic peptide. Circ. Res. 2007;101:875–882. doi: 10.1161/CIRCRESAHA.107.153585. [DOI] [PubMed] [Google Scholar]
- [17].Boerrigter G, Costello-Boerrigter LC, Harty GJ, Huntley BK, Cataliotti A, Lapp H, et al. B-type natriuretic peptide 8-32, which is produced from mature BNP 1-32 by the metalloprotease meprin A, has reduced bioactivity. Am. J. Physiol. Regul. Integ.r Comp. Physiol. 2009;296:R1744–1750. doi: 10.1152/ajpregu.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ralat LA, Guo Q, Ren M, Funke T, Dickey DM, Potter LR, et al. Insulin-degrading enzyme modulates the natriuretic peptide-mediated signaling response. J. Biol. Chem. 2011;286:4670–4679. doi: 10.1074/jbc.M110.173252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Toll L, Brandt SR, Olsen CM, Judd AK, Almquist RG. Isolation and characterization of a new atrial peptide-degrading enzyme from bovine kidney. Biochem. Biophys. Res. Commun. 1991;175:886–93. doi: 10.1016/0006-291x(91)91648-v. [DOI] [PubMed] [Google Scholar]
- [20].Muller D, Schulze C, Baumeister H, Buck F, Richter D. Rat insulin-degrading enzyme: cleavage pattern of the natriuretic peptide hormones ANP, BNP, and CNP revealed by HPLC and mass spectrometry. Biochemistry. 1992;31:11138–43. doi: 10.1021/bi00160a026. [DOI] [PubMed] [Google Scholar]
- [21].Dickey DM, Potter LR. Human B-type natriuretic peptide is not degraded by meprin A. Biochem. Pharmacol. 2010;80:1007–11. doi: 10.1016/j.bcp.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dickey DM, Potter LR. ProBNP(1-108) is resistant to degradation and activates guanylyl cyclase-A with reduced potency. Clin. Chem. 2011;57:1272–1278. doi: 10.1373/clinchem.2011.169151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr. Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- [24].Garbers DL, Chrisman TD, Wiegn P, Katafuchi T, Albanesi JP, Bielinski V, et al. Membrane guanylyl cyclase receptors: an update. Trends. Endocrinol. Metab. 2006;17:251–8. doi: 10.1016/j.tem.2006.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Huntley BK, Ichiki T, Sangaralingham SJ, Chen HH, Burnett JC., Jr B-type natriuretic peptide and extracellular matrix protein interactions in human cardiac fibroblasts. J. Cell Physiol. 2009;225:251–255. doi: 10.1002/jcp.22253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pagel-Langenickel I, Buttgereit J, Bader M, Langenickel TH. Natriuretic peptide receptor B signaling in the cardiovascular system: protection from cardiac hypertrophy. J. Mol. Med (Berl) 2007;85:797–810. doi: 10.1007/s00109-007-0183-4. [DOI] [PubMed] [Google Scholar]
- [27].Siriwardena M, Kleffmann T, Ruygrok P, Cameron VA, Yandle TG, Nicholls MG, et al. B-type natriuretic peptide signal peptide circulates in human blood: evaluation as a potential biomarker of cardiac ischemia. Circulation. 2010;122:255–64. doi: 10.1161/CIRCULATIONAHA.109.909937. [DOI] [PubMed] [Google Scholar]
- [28].Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N. Engl. J. Med. 2002;347:161–7. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- [29].Januzzi JL, van Kimmenade R, Lainchbury J, Bayes-Genis A, Ordonez-Llanos J, Santalo-Bel M, et al. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT-proBNP Study. Eur. Heart J. 2006;27:330–337. doi: 10.1093/eurheartj/ehi631. [DOI] [PubMed] [Google Scholar]
- [30].Ordonez-Llanos J, Merce-Muntanola J. Natriuretic peptides: Laboratory condiderations. In: Januzzi JL, editor. Cardiac markers in clinical practice. Jones and Bartlett Publications; Sudbury: 2010. pp. pp377–402. [Google Scholar]
- [31].Masson S, Latini R, Anand IS, Vago T, Angelici L, Barlera S, et al. Direct comparison of B-type natriuretic peptide (BNP) and amino-terminal proBNP in a large population of patients with chronic and symptomatic heart failure: the Valsartan Heart Failure (Val-HeFT) data. Clinical chemistry. 2006;52:1528–1538. doi: 10.1373/clinchem.2006.069575. [DOI] [PubMed] [Google Scholar]
- [32].Grantham JA, Borgeson DD, Burnett JC., Jr BNP: pathophysiological and potential therapeutic roles in acute congestive heart failure. The American journal of physiology. 1997;272:R1077–1083. doi: 10.1152/ajpregu.1997.272.4.R1077. [DOI] [PubMed] [Google Scholar]
- [33].Marcus LS, Hart D, Packer M, Yushak M, Medina N, Danziger RS, et al. Hemodynamic and renal excretory effects of human brain natriuretic peptide infusion in patients with congestive heart failure. A double-blind, placebo-controlled, randomized crossover trial. Circulation. 1996;94:3184–3189. doi: 10.1161/01.cir.94.12.3184. [DOI] [PubMed] [Google Scholar]
- [34].O’Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, et al. Effect of nesiritide in patients with acute decompensated heart failure. The New England journal of medicine. 2011;365:32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- [35].Chen HH, Grantham JA, Schirger JA, Jougasaki M, Redfield MM, Burnett JC., Jr Subcutaneous administration of brain natriuretic peptide in experimental heart failure. J. Am. Coll. Cardiol. 2000;36:1706–1712. doi: 10.1016/s0735-1097(00)00911-6. [DOI] [PubMed] [Google Scholar]
- [36].Chen HH, Schirger JA, Cataliotti A, Burnett JC., Jr Intact acute cardiorenal and humoral responsiveness following chronic subcutaneous administration of the cardiac peptide BNP in experimental heart failure. Eur. J. Heart Fail. 2006;8:681–686. doi: 10.1016/j.ejheart.2005.12.005. [DOI] [PubMed] [Google Scholar]
- [37].Chen HH, Glockner JF, Schirger JA, Cataliotti A, Redfield MM, Burnett JC., Jr Novel protein therapeutics for systolic heart failure: Chronic subcutaneous BNP. J. Am. Coll. Cardiol. 2012 doi: 10.1016/j.jacc.2012.07.056. in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Semenov AG, Tamm NN, Seferian KR, Postnikov AB, Karpova NS, Serebryanaya DV, et al. Processing of Pro-B-Type Natriuretic Peptide: Furin and Corin as Candidate Convertases. Clin Chem. 2010;56:1166–1176. doi: 10.1373/clinchem.2010.143883. [DOI] [PubMed] [Google Scholar]
- [39].Vanderheyden M, Bartunek J, Goethals M, Verstreken S, Lambeir AM, De Meester I, et al. Dipeptidyl-peptidase IV and B-type natriuretic peptide. From bench to bedside. Clin. Chem. Lab. Med. 2009;47:248–252. doi: 10.1515/CCLM.2009.065. [DOI] [PubMed] [Google Scholar]
- [40].Potter LR. Natriuretic peptide metabolism, clearance and degradation. Febs. J. 2011;278:1808–1817. doi: 10.1111/j.1742-4658.2011.08082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Miller WL, Phelps MA, Wood CM, Schellenberger U, Van Le A, Perichon R, et al. Comparison of mass spectrometry and clinical assay measurements of circulating fragments of B-type natriuretic peptide in patients with chronic heart failure. Circ. Heart Fail. 2011;4:355–360. doi: 10.1161/CIRCHEARTFAILURE.110.960260. [DOI] [PubMed] [Google Scholar]
- [42].Giuliani I, Rieunier F, Larue C, Delagneau JF, Granier C, Pau B, et al. Assay for measurement of intact B-type natriuretic peptide prohormone in blood. Clinical. chemistry. 2006;52:1054–1061. doi: 10.1373/clinchem.2005.061770. [DOI] [PubMed] [Google Scholar]
- [43].Macheret F, Boerrigter G, McKie PM, Costello-Boerrigte LC, Lahr BD, Heublein DM, et al. Pro-B-type natriuretic peptide1-108 circulates in the general community: Plasma determinants and detection of left ventricular systolic dysfunction. J. Am. Coll. Cardiol. 2010;57:1386–1395. doi: 10.1016/j.jacc.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Miller WL, Grill DE, Jaffe AS. Comparison of novel pro-BNP(1-108) and standard BNP assays in heart failure patients, Clinica chimica acta. international journal of clinical chemistry. 2012;413:920–926. doi: 10.1016/j.cca.2012.02.007. [DOI] [PubMed] [Google Scholar]
- [45].Dries DL, Ky B, Wu AH, Rame JE, Putt ME, Cappola TP. Simultaneous assessment of unprocessed ProBNP1-108 in addition to processed BNP32 improves identification of high-risk ambulatory patients with heart failure. Circulation Heart failure. 2010;3:220–227. doi: 10.1161/CIRCHEARTFAILURE.109.903153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Niederkofler EE, Kiernan UA, O’Rear J, Menon S, Saghir S, Protter AA, et al. Detection of endogenous B-type natriuretic peptide at very low concentrations in patients with heart failure. Circulation Heart failure. 2008;1:258–264. doi: 10.1161/CIRCHEARTFAILURE.108.790774. [DOI] [PubMed] [Google Scholar]
- [47].Seferian KR, Tamm NN, Semenov AG, Mukharyamova KS, Tolstaya AA, Koshkina EV, et al. The brain natriuretic peptide (BNP) precursor is the major immunoreactive form of BNP in patients with heart failure. Clinical chemistry. 2007;53:866–873. doi: 10.1373/clinchem.2006.076141. [DOI] [PubMed] [Google Scholar]
- [48].Wu C, Wu F, Pan J, Morser J, Wu Q. Furin-mediated processing of Pro-C-type natriuretic peptide. J. Biol. Chem. 2003;278:25847–25852. doi: 10.1074/jbc.M301223200. [DOI] [PubMed] [Google Scholar]
- [49].Hooper JD, Scarman AL, Clarke BE, Normyle JF, Antalis TM. Localization of the mosaic transmembrane serine protease corin to heart myocytes. Eur. J. Biochem. 2000;267:6931–6937. doi: 10.1046/j.1432-1033.2000.01806.x. [DOI] [PubMed] [Google Scholar]
- [50].Peng J, Jiang J, Wang W, Qi X, Sun XL, Wu Q. Glycosylation and processing of pro-B-type natriuretic peptide in cardiomyocytes. Biochem. Biophys. Res. Commun. 2011;411:593–598. doi: 10.1016/j.bbrc.2011.06.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Knappe S, Wu F, Madlansacay MR, Wu Q. Identification of domain structures in the propeptide of corin essential for the processing of proatrial natriuretic peptide. J. Biol. Chem. 2004;279:34464–34471. doi: 10.1074/jbc.M405041200. [DOI] [PubMed] [Google Scholar]
- [52].Jiang J, Wu S, Wang W, Chen S, Peng J, Zhang X, et al. Ectodomain shedding and autocleavage of the cardiac membrane protease corin. J. Biol. Chem. 2011;286:10066–10072. doi: 10.1074/jbc.M110.185082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Langenickel TH, Pagel I, Buttgereit J, Tenner K, Lindner M, Dietz R, et al. Rat corin gene: molecular cloning and reduced expression in experimental heart failure. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H1516–21. doi: 10.1152/ajpheart.00947.2003. [DOI] [PubMed] [Google Scholar]
- [54].Tran KL, Lu X, Lei M, Feng Q, Wu Q. Upregulation of corin gene expression in hypertrophic cardiomyocytes and failing myocardium. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H1625–31. doi: 10.1152/ajpheart.00298.2004. [DOI] [PubMed] [Google Scholar]
- [55].Calderone A, Bel-Hadj S, Drapeau J, El-Helou V, Gosselin H, Clement R, et al. Scar myofibroblasts of the infarcted rat heart express natriuretic peptides. J. Cell Physiol. 2006;207:165–73. doi: 10.1002/jcp.20548. [DOI] [PubMed] [Google Scholar]
- [56].Jiang W, Cai DY, Pan CS, Qi YF, Jiang HF, Geng B, et al. Changes in production and metabolism of brain natriuretic peptide in rats with myocardial necrosis. Eur. J. Pharmacol. 2005;507:153–162. doi: 10.1016/j.ejphar.2004.11.023. [DOI] [PubMed] [Google Scholar]
- [57].Chan JC, Knudson O, Wu F, Morser J, Dole WP, Wu Q. Hypertension in mice lacking the proatrial natriuretic peptide convertase corin. Proc. Natl. Acad. Sci. U S A. 2005;102:785–790. doi: 10.1073/pnas.0407234102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Dries DL, Victor RG, Rame JE, Cooper RS, Wu X, Zhu X, et al. Corin gene minor allele defined by 2 missense mutations is common in blacks and associated with high blood pressure and hypertension. Circulation. 2005;112:2403–2410. doi: 10.1161/CIRCULATIONAHA.105.568881. [DOI] [PubMed] [Google Scholar]
- [59].Rame JE, Drazner MH, Post W, Peshock R, Lima J, Cooper RS, et al. Corin I555(P568) allele is associated with enhanced cardiac hypertrophic response to increased systemic afterload. Hypertension. 2007;49:857–864. doi: 10.1161/01.HYP.0000258566.95867.9e. [DOI] [PubMed] [Google Scholar]
- [60].Rame JE, Tam SW, McNamara D, Worcel M, Sabolinski ML, Wu AH, et al. Dysfunctional corin i555(p568) allele is associated with impaired brain natriuretic peptide processing and adverse outcomes in blacks with systolic heart failure: results from the Genetic Risk Assessment in Heart Failure substudy. Circ. Heart Fail. 2009;2:541–548. doi: 10.1161/CIRCHEARTFAILURE.109.866822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wang W, Liao X, Fukuda K, Knappe S, Wu F, Dries DL, et al. Corin variant associated with hypertension and cardiac hypertrophy exhibits impaired zymogen activation and natriuretic peptide processing activity. Circ. Res. 2008;103:502–508. doi: 10.1161/CIRCRESAHA.108.177352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Dong N, Chen S, Yang J, He L, Liu P, Zheng D, et al. Plasma soluble corin in patients with heart failure. Circ. Heart Fail. 2010;3:207–211. doi: 10.1161/CIRCHEARTFAILURE.109.903849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Shrestha K, Troughton RW, Borowski AG, Yandle TG, Richards AM, Klein AL, et al. Plasma Corin Levels Provide Minimal Prognostic Utility Incremental to Natriuretic Peptide in Chronic Systolic Heart Failure. Journal of Cardiac Failure. 2010;16:621–627. doi: 10.1016/j.cardfail.2010.03.010. [DOI] [PubMed] [Google Scholar]
- [64].Ibebuogu UN, Gladysheva IP, Houng AK, Reed GL. Decompensated heart failure is associated with reduced corin levels and decreased cleavage of pro-atrial natriuretic peptide. Circ. Heart Fail. 2011;4:114–120. doi: 10.1161/CIRCHEARTFAILURE.109.895581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Dong N, Chen S, Wang W, Zhou Y, Wu Q. Corin in clinical laboratory diagnostics, Clinica chimica acta. international journal of clinical chemistry. 2012;413:378–383. doi: 10.1016/j.cca.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Dong N, Dong J, LiuP P, Xu L, Shi S, Wu Q. Effects of anticoagulants on human plasma soluble corin levels measured by ELISA. Clin. Chim. Acta. 2010;411:1998–2003. doi: 10.1016/j.cca.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC., Jr Plasma brain natriuretic peptide concentration: impact of age and gender. J. Am. Coll. Cardiol. 2002;40:976–982. doi: 10.1016/s0735-1097(02)02059-4. [DOI] [PubMed] [Google Scholar]
- [68].Clerico A, Del Ry S, Maffei S, Prontera C, Emdin M, Giannessi D. The circulating levels of cardiac natriuretic hormones in healthy adults: effects of age and sex. Clin. Chem. Lab. Med. 2002;40:371–377. doi: 10.1515/CCLM.2002.060. [DOI] [PubMed] [Google Scholar]
- [69].Wang TJ, Larson MG, Levy D, Leip EP, Benjamin EJ, Wilson PW, et al. Impact of age and sex on plasma natriuretic peptide levels in healthy adults. Am. J. Cardiol. 2002;90:254–258. doi: 10.1016/s0002-9149(02)02464-5. [DOI] [PubMed] [Google Scholar]
- [70].Hogenhuis J, Voors AA, Jaarsma T, Hillege HL, Boomsma F, van Veldhuisen DJ. Influence of age on natriuretic peptides in patients with chronic heart failure: a comparison between ANP/NT-ANP and BNP/NT-proBNP. Eur. J. Heart Fail. 2005;7:81–86. doi: 10.1016/j.ejheart.2004.03.014. [DOI] [PubMed] [Google Scholar]
- [71].Durinx C, Neels H, Van der Auwera JC, Naelaerts K, Scharpe S, De Meester I. Reference values for plasma dipeptidyl-peptidase IV activity and their association with other laboratory parameters. Clin. Chem. Lab. Med. 2001;39:155–159. doi: 10.1515/CCLM.2001.026. [DOI] [PubMed] [Google Scholar]
- [72].Chen S, Sen S, Young D, Wang W, Moravec CS, Wu W. Protease corin expression and activity in failing hearts. Am. J. Physiol. Heart Circ. Physiol. 2010;299:H1687–92. doi: 10.1152/ajpheart.00399.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Peltonen TO, Taskinen P, Soini Y, Rysa J, Ronkainen J, Ohtonen P, et al. Distinct downregulation of C-type natriuretic peptide system in human aortic valve stenosis. Circulation. 2007;116:1283–9. doi: 10.1161/CIRCULATIONAHA.106.685743. [DOI] [PubMed] [Google Scholar]
- [74].Schellenberger U, O’Rear J, Guzzetta A, Jue RA, Protter AA, Pollitt NS. The precursor to B-type natriuretic peptide is an O-linked glycoprotein. Arch. Biochem. Biophys. 2006;451:160–6. doi: 10.1016/j.abb.2006.03.028. [DOI] [PubMed] [Google Scholar]
- [75].Semenov AG, Postnikov AB, Tamm NN, Seferian KR, Karpova NS, Bloshchitsyna MN, et al. Processing of pro-brain natriuretic peptide is suppressed by O-glycosylation in the region close to the cleavage site. Clinical chemistry. 2009;55:489–498. doi: 10.1373/clinchem.2008.113373. [DOI] [PubMed] [Google Scholar]
- [76].Jiang J, Pristera N, Wang W, Zhang X, Wu Q. Effect of sialylated O-glycans in pro-brain natriuretic peptide stability. Clin. Chem. 2010;56:959–966. doi: 10.1373/clinchem.2009.140558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Tonne JM, Campbell JM, Cataliotti A, Ohmine S, Thatava T, Sakuma T, et al. Secretion of glycosylated pro-B-type natriuretic peptide from normal cardiomyocytes. Clinical chemistry. 2011;57:864–873. doi: 10.1373/clinchem.2010.157438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Seferian KR, Tamm NN, Semenov AG, Tolstaya AA, Koshkina EV, Krasnoselsky MI, et al. Immunodetection of glycosylated NT-proBNP circulating in human blood. Clinical chemistry. 2008;54:866–873. doi: 10.1373/clinchem.2007.100040. [DOI] [PubMed] [Google Scholar]
- [79].Nishikimi T, Ikeda M, Takeda Y, Ishimitsu T, Shibasaki I, Fukuda H, et al. The effect of glycosylation on plasma N-terminal proBNP-76 levels in patients with heart or renal failure. Heart. 2012;98:152–161. doi: 10.1136/heartjnl-2011-300102. [DOI] [PubMed] [Google Scholar]
- [80].Lachmann RH. Enzyme replacement therapy for lysosomal storage diseases. Curr. Opin. Pediatr. 2011;23:588–593. doi: 10.1097/MOP.0b013e32834c20d9. [DOI] [PubMed] [Google Scholar]