Abstract
In almost all patients, malignant glioma recurs following initial treatment with maximal safe resection, conformal radiotherapy, and temozolomide. This review describes the many options for treatment of recurrent malignant gliomas, including reoperation, alternating electric field therapy, chemotherapy, stereotactic radiotherapy or radiosurgery, or some combination of these modalities, presenting the evidence for each approach. No standard of care has been established, though the antiangiogenic agent, bevacizumab; stereotactic radiotherapy or radiosurgery; and, perhaps, combined treatment with these 2 modalities appear to offer modest benefits over other approaches. Clearly, randomized trials of these options would be advantageous, and novel, more efficacious approaches are urgently needed.
Introduction
Malignant gliomas almost inevitably recur following initial treatment. For patients with glioblastoma (GBM) treated with the current standard of care (maximal safe resection, fractionated external beam radiotherapy, and concurrent and adjuvant temozolomide) in the European Organisation for Research and Treatment of Cancer–National Cancer Institute of Canada randomized trial,1 2- and 5-year progression-free survivals (PFSs) of only 11% and 4%, respectively, were observed with less than 10% of patients surviving more than 5 years from diagnosis.
Today, most patients with malignant glioma and the clinicians caring for them face the challenge of managing recurrent disease following multimodality treatment. A variety of approaches for treatment of recurrent disease exists, and this article describes these options, the evidence supporting their use, and their relative risks, efficacy, and logistics.
Diagnosis of Recurrence
Historically, the predominant site of initial recurrence following radiotherapy alone has been within a few centimeters of the tumor bed and resection site.2–5 Despite the addition of temozolomide to radiotherapy for GBM, local failure remains the most common site of initial recurrence.6–9 Nonetheless, it is essential to remember that malignant gliomas are infiltrative in nature, as the brain offers minimal barriers to spread within its confines, and that distant failures (in the brain) are likely to occur.
Immediately following primary concurrent chemoradiation, many patients with GBM develop pseudoprogression, that is, the false radiographic appearance of progressive disease. This phenomenon has been estimated to occur in approximately 20% of patients with recurrent malignant glioma10 and typically appears within 6 months of completion of radiotherapy. Conversely, the use of antiangiogenic therapies (vide infra) can produce “pseudoresponses,” in which the disease is disproportionately less apparent radiographically though the change in tumor burden may be minimal. Although a great deal of progress has been made in establishing the radiographic criteria for disease progression in treated malignant glioma,11–13 the interpretation of magnetic resonance (MR) imaging studies is complicated by radiotherapeutic effects and concomitant biochemotherapies. Although a variety of other imaging modalities, including single photon emission computed tomography and positron emission tomography with various biomarkers, exist, no method has emerged as providing an unambiguous method of ruling in recurrence or progression and ruling out purely radiation-induced changes.14
The gold standard for diagnosis of recurrent disease is, of course, a definitive histologic confirmation. However, before performing a biopsy to establish or deny gross recurrence, it is essential to ask whether the value of making the diagnosis outweighs the risk of the procedure. Inherent in this judgment is the upfront probability that an apparent lesion represents recurrent disease. During the first 6 months following treatment of the primary disease with radiotherapy, there is a substantial probability that radiographic changes represent pseudoprogression and many practitioners may elect to follow up the patient with closely spaced MR imaging examinations in the absence of clinically significant new symptoms. At longer times, the probability that there is recurrent disease, often in admixture with local radiotherapeutic effects, is very high. In addition, biopsy can be complicated by impaired wound healing from previous radiation therapy or ongoing chemotherapy, particularly bevacizumab (BVZ).15 Thus, the appearance of a new, distinct lesion on MR images may be sufficient to initiate further interventions without histologic confirmation of recurrence, especially when the lesion is outside the high-dose area of initial radiotherapy or appears more than 6–12 months after completion of radiotherapy or both.
Surgery
Surgical resection of recurrent lesions has the advantage of being potentially diagnostic and therapeutic. In particular, surgery tends to be most beneficial when there is a well-demarcated lesion involving noneloquent brain, producing a symptomatic mass effect on normal brain structures. However, reoperation may be complicated by several factors. First, the site of recurrence is at or near the resection bed, and this volume has typically received a full dose of radiation during the initial course of treatment, potentially impairing wound healing. Second, the goal of the initial glioma surgery is to achieve maximal safe resection and, consequently, surgical margins may often abut eloquent areas. Thus, for recurrences near the resection cavity, the extent of reoperation may be severely constrained. Third, the use of salvage chemotherapy, particularly antiangiogenic agents, can also increase the rate and severity of wound-healing complications.15
Notwithstanding these potential limitations, reoperation can often be safely performed by an experienced neurosurgeon, as described in several recent reports.16–18 However, this is not equivalent to stating that reoperation should be performed on most patients.19 Studies on reoperation of recurrent glioma,17,18,20–28 summarized in Table 1, do not show a consistent benefit to surgical resection as compared with no reoperation, particularly when the typically more favorable attributes of surgical candidates are considered. In reviewing these reports, higher Karnofsky performance status, lower age, and smaller, more readily resectable recurrent tumors tend to be associated with more favorable outcomes. In addition, several small studies19,23,26 suggest that superior survival may be associated with a combination of resection (an effective “local” therapy) and systemic adjuvant therapy (ie, “global” brain therapy).
Table 1.
Surgery for Recurrent Malignant Gliomas
| Institution | Number of Patients GBM/Total |
Reoperation Period |
Median OS for GBM After Reoperation (mo) |
Factors Associated With Improved OS |
Factors Not Associated With Improved OS |
|---|---|---|---|---|---|
| Memorial Sloan-Kettering20 | 38/55 | 1972–1983 | 8.3 | KPS ≥ 70, gross total resection, and AA | |
| Miami25 | 12/33 | 1986–1992 | 8 | Younger age and higher KPS | |
| Munich28 | 38/38 | 1993–1998 | 5.3 | Age < 50 y, KPS ≥ 90, and gross total resection | |
| VU26 | 32/32* | 1999–2005 | 3 (S only), 7 (CT or SRS), and 8 (S + CT or SRS) | S + CT or SRS | |
| NIH27 | 34/34 | Not stated | 7.4 | Noneloquent site, KPS < 80, and tumor volume <50 mL | – |
| North American Brain Tumor Consortium22 | 593/593 | 1998–2008 | 7.3 (S) and 6.4 (no S) | – | S |
| North American Brain Tumor Consortium21 | 224/333† | 1995–2002 | 7.0 (All) | Younger age, higher KPS, non-GBM histology, no CS, and frontal lobe location | S |
| EORTC24 | 300/300‡ | 1999–2010 | 6.2 | Higher KPS, 1 lesion and tumor diameter <42 mm | Age, sex, and S |
| Catholic University (Rome)23 | 76/76 | 2002–2008 | 7 | S + AT and KPS ≥ 70 | S and gross total resection |
| Mayo (Rochester)18 | 62§/131 | 1995–2010 | 12§ | – | – |
| Johns Hopkins17 | 224/224‖ | 1997–2007 | Not stated | Increased number of reoperations |
Abbreviations: AA, anaplastic astrocytoma; AT, adjuvant therapy; CS, corticosteroid use; CT, chemotherapy; EORTC, European Organisation for Research and Treatment of Cancer; KPS, Karnofsky performance status; NIH, National Institutes of Health; S, surgery at reoperation.
9 Patients with S only at recurrence, 11 with S + CT/SRS, and 12 with CT/SRS only.
181 Patients underwent S at recurrence.
130 Patients enrolled on an S protocol.
46 Patients with primary and 16 patients with secondary WHO grade IV tumors who underwent one or more reoperations.
Overall, 168, 41, and 15 patients with GBM underwent 1, 2, or 3 reoperations, respectively.
Alternating Electric Field Therapy
In preclinical studies, low voltage, intermediate-frequency alternating electric fields (AEFs) have been shown to kill a variety of tumor cells.29,30 Purportedly, the application of an AEF kills rapidly growing cells by preventing the mitotic spindle from properly aligning during cell division.30 A phase I study of AEFs in recurrent GBM29 showed that the treatment was well tolerated with a median time to progression of 26 weeks and a median overall survival (OS) of 62 weeks.
A subsequent phase III trial in 337 adult patients with recurrent GBM randomized these patients to the use of a portable AEF device alone vs “active” chemotherapy.31 In this trial, chemotherapy was chosen at the discretion of the physician, with 31%, 31%, 25%, 15%, 11%, or 5% of the regimens containing BVZ, irinotecan, nitrosoureas, carboplatin, temozolomide, or other agents, respectively. Although all patients received radiation therapy during their initial treatment, it is not clear how frequently salvage radiotherapy was attempted. The portable AEF device was applied to the bare scalp essentially continuously for several months, though brief breaks were permitted for 1–2 h/d for hygiene and 2–3 d/mo. In this trial, 78% of the 116 patients starting AEF therapy completed at least 1 month of therapy.
No significant difference in median OS was observed (6.6 months for the AEF group vs 6.0months for the chemotherapy arm, hazard ratio for death 0.86 in favor of AEF, P = 0.27). Median PFS was similar in the 2 groups (2.2 vs 2.1 months). While systemic side effects were more common in the chemotherapy arm, central nervous system toxicity appeared similar in both arms. Serious adverse events were less AEF (6% vs 16%), with the chief non–central nervous system concern in the AEF arm being mild-to-moderate scalp dermatitis. Sufficient quality-of-life (QoL) data were available for analysis in 27%of patients. The QoL results in the domain of cognitive and emotional functioning and role functioning appear to favor AEF, physical functioning seemed better in the chemotherapy arm, and no differences in global health or social functioning were apparent. Treatment-related symptoms, pain, and fatigue were judged worse in the chemotherapy group. The statistical significance of these QoL differences is unclear.
In April 2011, the Food and Drug Administration subsequently granted approval to market a portable AEF device (NovoTTF-100A, Haifa, Israel), indicated for the treatment of recurrent GBM. Preclinical studies suggest that AEFs may enhance the efficacy of chemotherapy,32 and a trial of this device and temozolomide in GBM is underway.31 To assess the efficacy of AEF therapy vs biochemotherapy, radiotherapy or both, several questions must be addressed. The phase III trial did not test AEF vs necessarily the best therapy, “only” the physician's choice of chemotherapy. It would be interesting to evaluate AEFs (± chemotherapy?) against anti-angiogenic agents, radiosurgery, and combination regimens, discussed later.
Chemotherapy
Given the infiltrative nature of gliomas, it seems logical to use agents that treat the entire neuraxis (ie, “global” brain treatments) to address gross and diffuse disease. By contrast, surgery and focal radiotherapy (discussed later) represent “local” treatments and by design do not directly address subclinical disease. A broad range of chemotherapy and biochemotherapy agents have been and are being evaluated for the treatment of recurrent gliomas, and a detailed discussion of the many trials is beyond the scope of this article. However, the major developments and obstacles are presented later. It is important to note that since 2005, virtually all patients with recurrent malignant glioma would have undergone initial treatment with radiotherapy and concurrent or adjuvant temozolomide, most following at least subtotal resection.
Table 2 summarizes selected studies33–57 of chemotherapeutic agents in the treatment of recurrent glioma. The initial studies on cytotoxic chemotherapeutic agents showed short OS and PFS following recurrence, approximately 3–4 and 6–7 months, respectively. For example, Wong performed a meta-analysis of 8 consecutive phase II chemotherapy trials in recurrent malignant glioma conducted at M.D. Anderson Cancer Center between 1986 and 1995. For all histologies, the median PFS and OS were 2.4 and 7.0 months, respectively, with 1-year OS of 47% and 21% in anaplastic astrocytoma and GBM, respectively. Similarly, Gorlia analyzed the outcome of 8 phase I–II trails in 300 patients with recurrent GBM performed through the European Organisation for Research and Treatment of Cancer between 1999 and 2010. The median PFS and OS were 1.8 and 6.2 months, respectively, and the 1-year OS was 22%. Although better performance status and unifocal and smaller lesions were associated with improved survival, there was no significant difference in outcome in the patients who had received temozolomide and radiotherapy vs radiotherapy alone at initial presentation.
Table 2.
Selected Studies of Chemotherapy or Biochemotherapy Agents in Recurrent Malignant Gliomas
| Institution | Agent | Number of Patients GBM/ Total |
Median OS After Recurrence (mo) |
Median 6-mo PFS After Recurrence (%) |
Comments |
|---|---|---|---|---|---|
| M.D. Anderson55 | Multiple* | 225/375 | 4.9 (GBM) | 15 (GBM) | |
| EORTC24 | Multiple† | 300/300 | 6.2 | 14.7 | None showed clinically relevant activity |
| NIH41 | BVZ | 48/48 | 7.2 | 29 | Phase II |
| Duke54 | BVZ + IRT | 23/23 | 9.6 | 30 | Phase II |
| Rigshospitalet46 | BVZ + IRT | 32/85 | 7.9 (GBM) | 29 | Phase II |
| GEINO38 | BVZ + IRT | 92/130 | 8.8 (GBM) | 42 | Retrospective |
| UCLA50 | BVZ + IRT | 44/44 | 9 | 41 | Retrospective |
| ASMO34 | BVZ + IRT | 92/115 | 8 (GBM) | 46 (All) | Retrospective |
| Henry Ford57 | BVZ + IRT | 37/51 | 11.5 (GBM) | 64 (GBM) | Retrospective |
| Multileft36 | BVZ ± IRT | 177/177 | 9.2 (BVZ alone) and 8.7 (BVZ + IRT) | 43 (BVZ alone) and 50(BVZ + IRT) | Phase II |
| University of Washington47 | BVZ + CBP | 14/19 | 10 (GBM) | 40 (GBM) | |
| Duke52 | BVZ + CBP + IRT | 40/40 | 8.3 | 47 | |
| North Central Cancer Group37 | BVZ + SOR | 54/54 | 5.6 | 20 | |
| Rigshospitalet44 | BVZ + TEM | 13/13 | 3.5 | Not stated | Judged not effective |
| EORTC35 | CCNU + DAS | 26/26 | 6.4 | 8 | |
| UCLA49 | ERL + SIR | 14/19 | Not stated | Median PFS 1 mo | |
| Cedars-Sinai39 | Gimatecan | 29/29 | Not stated | 12 | Judged not effective |
| Dana Farber53 | LAP + PAZ | 41/41 | Not stated | 0 (Biomarker +) vs 15 (biomarker −) | Judged not effective |
| Aristotle40 | LAP + TMZ | 14/16 | 5 | ~20 | Judged not effective |
| M.D. Anderson56 | LON + TMZ | 36/36 | 14.9‡ | 42‡ | |
| Rigshospitalet48 | Nintedanib | 25/25 | 6 | 4 | Judged not effective |
| North American Brain Tumor Consortium45 | SOR + TEM | 31/31 | Not stated | 0 | Judged not effective |
| NIH43 | Sunitinib | 63/63 | 9.4 | 0 (BVZ resistant) 10 (BVZ naïve) | |
| Columbia33 | TMZ | 68/120 | 8.8 | 35 | Only TMZ-naïve pts |
| MSK51 | TMZ | 32/47 | 7 | 19 | 49% Had failed BVZ |
| NIH42 | Vandetanib | 32/64 | 6.3 | 7 | Judged not effective |
Abbreviations: ASMO, Anatolian Society of Medical Oncology; CBP, carboplatin; CCNU, lomustine; DAS, dasatinib; EORTC, European Organisation for Research and Treatment of Cancer; ERL, erlotinib; GEINO, El Grupo Español de Investigación en Neurooncología; IRT, irinotecan; LAP, lapatinib; LON, lonafarnib; MSK, Memorial Sloan-Kettering; NIH, National Institutes of Health; PAZ, pazopanib; PFS, progression-free survival; SIR, sirolimus; SOR, sorafenib; TEM, temsirolimus; TMZ, temozolomide; UCLA, University of California, Los Angeles.
Pooled analysis of 8 phase II trials (IFNβ, IFNβ + cis-retinoic acid, menogaril, CBP, CBP + 5FU + procarbazine, and difluoromethylomithine) conducted between 1986 and 1995.
Pooled analysis of 2 phase I (LON and enzastaurin) and 6 phase II (DACA, glufosfamide, imatinib, erlotinib, and sagopilone) trials conducted between 1999 and 2010.
Disease-specific survival (not OS) and 6-month PFS for 26 patients receiving maximum-tolerated dose of LON.
Human malignant gliomas highly express vascular endothelial growth factor (VEGF), and anti-VEGF agents exhibit activity against GBM in preclinical models.58 BVZ, a recombinant humanized antibody against VEGF, appears to offer improved survival in recurrent gliomas when compared with that by other agents alone.34,36,38,41,46,50,54,57–60 In a trial of 35 patients with recurrent GBM treated with BVZ and irinotecan, Vredenburgh et al54 found 6-month PFS and OS of 46% and 77%, respectively. In a separate study, Kreisl et al41 administered BVZ to 48 patients with recurrent GBM and obtained 6-month PFS and OS of 29% and 57%, respectively. A multi-institutional trial36 of 177 patients with recurrent GBM revealed a median OS in patients treated with BVZ alone vs BVZ plus irinotecan of 9.2 vs 8.7 months, respectively (difference not significant).
On May 5, 2009, the Food and Drug Administration approved a single-agent BVZ for the treatment of recurrent GBM after the “standard” therapy. It is important to recognize that BVZ may be associated with severe, potentially life-threatening side effects, including gastrointestinal perforation, wound-healing complications, hemorrhage, and blood clots. Given the conflicting preliminary results from phase III studies on the efficacy of BVZ in the initial treatment of GBM, BVZ should be employed judiciously in the recurrent setting. In addition, patients receiving BVZ for treatment of recurrent disease have been observed to exhibit fulminant progression, whereas discontinuation of BVZ at the progression of disease may be associated with adverse outcomes. It is unclear whether BVZ is unique among other antiangiogenic agents in its role in the treatment of recurrent malignant gliomas, as well as the optimum combination of BVZ and agents having other mechanisms of action.37,42–45,47,52 Finally, the combination of BVZ and radiosurgery may afford some benefits, as described later, though this remains an area of active investigation and controversy.61
Radiation Therapy
Monomodality Therapy
Essentially all recurrent primary malignant gliomas would have been treated with partial-brain irradiation to a dose of approximately 60 Gy in total, in 1.8–12.0 Gy fractions, as discussed elsewhere in this issue. Transformed or secondary malignant gliomas should be considered for radiation using conventional regimens if not previously irradiated. In the setting of previous partial-brain irradiation and recurrence within the volume of the brain receiving an initial high dose of radiation (the most common scenario), it is difficult to administer another “conventional” course of irradiation to the recurrent lesion and margin without risking adverse toxicity. Thus, hypofractionated stereotactic radiotherapy (HFSRT) or stereotactic radiosurgery (SRS) to a limited volume is often employed, as discussed later. Alternatively, Marples et al have proposed a novel approach to improve the therapeutic ratio and permit effective re-treatment of large volumes, based on the principle of low-dose hypersensitivity and the application of pulsed irradiation.62–64
Conceptually, SRS and HFSRT have some attractive features. First, the area requiring re-treatment is close to or within the area that has been manipulated during the initial surgery and treated to a high dose of irradiation. Radiation Therapy Oncology Group (RTOG) 9005 demonstrated that SRS of recurrent primary brain tumors could be performed with minimal morbidity and established the maximum-tolerated dose for single-fraction SRS in this setting.65 Second, a short course of radiation has obvious logistic advantages over the much longer courses of radiation typically employed in primary treatment. Third, application of a high dose of radiation over a short period may evoke different mechanisms of tumor response and enhance tumor control,66,67 though this last point is controversial.68
However, the concept of SRS or HFSRT in the treatment of recurrent malignant gliomas also presents logical inconsistencies. First, as most patients with recurrent disease initially received a high-dose radiation to the area of recurrence, it is not clear why a second treatment should be any more effective than the first. At the very least, one would expect a “narrower” therapeutic window due to the effects of the initial irradiation. Second, an SRS boost as part of the initial treatment of malignant gliomas showed no benefit over conventional radiotherapy alone in RTOG 9305.69 Third, as malignant gliomas have a diffuse, broadly infiltrative component in addition to discrete nodular disease, it is unclear why a strictly “local” treatment of the nodules alone should substantially alter the outcome.
Despite these apparent limitations, SRS, SRT, and even conventionally fractionated SRT appear to provide reasonable OS in comparison with that by chemotherapy alone for the treatment of recurrent glioma, with median OS ranging from 5–13 months (typically 8–10 months) as shown in Table 3. It is noteworthy that several of the early studies involving single-fraction SRS reported fairly high rates of late complications (20%–40%) requiring reoperation and that this problem is often viewed as a substantial limitation of SRS.70–73 The use of HFSRT appears to mitigate the rate of adverse radiation events, as shown in the study by Fogh et al,74 who reported worsened symptoms in only 1 patient (of 147 patients) at 6 weeks follow-up.
Table 3.
Studies of Stereotactic Radiosurgery (SRS) and Conventionally and Hypofractionated Stereotactic Radiotherapy (FSRT and HFSRT, Respectively) for Recurrent Malignant Gliomas
| Institution | Technique, Median Dose Regimen(s) |
Number of Patients GBM/Total |
BVZ at SRS |
Median OS After Recurrence, GBM (mo) |
Toxicity |
|---|---|---|---|---|---|
| Minnesota71 | SRS, 20 Gy × 1 | 26/35 | No | 8 | 31% With reoperation |
| Harvard73 | SRS, 13 Gy × 1 | 86/86 | No | 10 | 48% Risk of reoperation at 2 y |
| Minnesota70 | FSRT, 2.5 Gy × 15 | 15/25 | No | 7.1 | 30% vs 8% late complications with SRS vs FSRT |
| SRS, 17 Gy × 1 | 27/46 | ||||
| Heidelberg92 | FSRT, 2 Gy × 18 | 59/172 | No | 8 | RN in 1 pt |
| Heidelberg93 | SRS, 15 Gy × 1 | 32/32 | No | 10 | No RN |
| Rochester94 | SRS, 15 Gy × 1 | 18/18 | No | 5.3 | RN in 1 pt |
| Jefferson74 | HFSRT, 3.5 Gy × 10 | 105/147 | Yes (2%) | 10 | 1 Gr 3 toxicity |
| Sungkyunkwan72 | SRS, 16 Gy × 1 | 65/114 | No | 13 | RN in 22% |
| Henry Ford88 | SRS, 18 Gy × 1 or | 26/26 | No concurrent | 8.4 | 2 And 1 pts in SRS and FSRT groups with RN |
| HFSRT, 6 Gy × 6 | 10/10 | 7.5 | |||
| Gulhane95 | SRS, 16 Gy × 1 | 19/19 | No | 9.3 | No Gr ≥ 3 toxicity |
| NYU96 | SRS, 15 Gy × 1 | 16/26 | No | 12.9 | RN in 2 pts |
| Haukeland97 | SRS, 12.2 Gy × 1 | 51/51 | No | 12 | 9.8% Complication rate vs 25.2% in reoperation |
| Hoag98 | HFSRT, 5 Gy × 5 | 4/8 | No | 7.6 (All pts) | RN in 1 pt |
| Staten Island99 | SRS, 6 Gy × 4 | 88/88 | No* | 7.0 | 12% With reoperation |
| UCSF100 | SRS, 15 Gy × 1 | 14/26 | No† | 8.8 | Not stated |
| Sant'Andrea101 | HFSRT, 6 Gy × 5 | 38/54 | No‡ | 12.4 (All) | Gr 3 CNS toxicity in 7% |
| MSK83 | HFSRT, 6 Gy × 5 | 20 | Yes (all) | 13 | Gr 3 CNS toxicity in 3 pts |
| Duke82 (retrospective) | SRS, 18 Gy × 1 or 5 Gy × 5 | 49/63 | No (16) | 4 | Crude rate of RN 19% vs 5% (− BVZ vs + BVZ) |
| Yes (33) | 11 | ||||
| Henry Ford86 | SRS, 18 Gy × 1 (7) or HFSRT, 6 Gy × 6 | 18/23 | Yes | 7 RT + BVZ vs 3.3 BVZ alone | Not stated |
| Ludwig-Maximilians89 | FSRT, 2 Gy × 18 | 22/30 | No (10) | 5.8 (All) | One each Gr 3 and 4 in BVZ group |
| Yes (20) | Not reached (all) | ||||
| Pittsburgh85 | SRS, 16 Gy × 1 | 11/11 | No (44§) | 12 | 9% vs 46% ARE (+ BVZ vs −BVZ) |
| Yes (11) | 18 | ||||
| St. Gallen84 | FSRT, 2.67 Gy × 15 | 8/14 | No (4) | 9 (All) | RN in 1 non-BVZ patient |
| Yes (10) | |||||
| Cincinnati90 | HFSRT, 6 Gy × 5 | 30/35 | Yes (30) | 8.6 (All) | RN in 3 pts (all non-BVZ) |
| Duke (prospective)81 | SRS, 18 Gy × 1 | 8/15 | Yes (all) | 14.4 (All) | 1 Grade 3 (headache) |
Abbreviations: ARE, adverse radiation event; CNS, central nervous system; Gr, grade; MSK, Memorial Sloan-Kettering; RN, radionecrosis; RT, radiation therapy; UCSF, University of California, San Francisco.
All patients received concurrent paclitaxel.
All patients received marimastat after SRS.
All patients received concurrent low-dose temozolomide.
Matched case-controls undergoing SRS but not receiving BVZ.
Combined Modality Therapy With BVZ
Apart from the potential, direct antitumor effect of BVZ in the treatment of gliomas, as discussed earlier, it may offer some specific additional benefits when used in combination with radiotherapy. As described by Moeller et al,75–77 a paradoxical effect of radiotherapy is the upregulation of hypoxia factor–mediated angiogenesis, an unwanted effect that could be potentially blocked by antiangiogenic agents. In addition, adverse radiation events following SRS appear to be substantially reduced by the use of BVZ.78–80
The combination of BVZ and SRS or HFSRT may provide superior outcomes when compared with that by either modality alone.81–86 A prospective trial of HFSRT and BVZ from Memorial Sloan-Kettering Cancer Center83 showed that the combination was well tolerated and demonstrated an OS of 12 months after HFSRT. In a follow-up study, the predominant pattern of failure continued to be at or near the site of HFSRT.87 Cabrera et al81 assessed the toxicity of concurrent BVZ and SRS in a cohort of 15 patients with recurrent malignant gliomas. Only 1 grade 3 and no grade 4–5 toxicities were observed, and QoL and neurocognition were well preserved following SRS; the median OS was 14 months in this group of 8 grade IV and 7 grade III gliomas.
In a retrospective study from Duke University,82 OS in heavily pretreated patients with recurrent GBM who received BVZ at approximately the time of SRS was significantly higher than in those who did not receive it (11 vs 4 months, P = 0.014 on univariate analysis, respectively). On multivariate analysis, survival after SRS was statistically more favorable for patients who received BVZ, had a Karnofsky performance status >70, or were younger than 50 years. It is noteworthy that most of these patients received a variety of chemotherapies after SRS, and the authors of the Duke study emphasize that ongoing, continual chemotherapy is an integral component of their approach to recurrent gliomas. A case-control study from the University of Pittsburgh85 revealed a significantly higher OS (18 vs 12 months, P = 0.005) in patients who were treated with SRS and BVZ vs controls who were receiving SRS alone.
Although the median OS with SRS and BVZ in Table 3 appears higher than that of BVZ alone (Table 2), this is not a direct comparison of the 2 approaches. A small retrospective analysis from Henry Ford Hospital88 found a substantially higher median OS in patients treated with SRS or HFSRT and BVZ than that in those receiving only BVZ (7 vs 3 months, respectively). Several studies have reported low rates of radionecrosis and adverse radiation events in patients treated with SRS or HFSRT and BVZ. For example, in the retrospective study from Duke University,82 4 of 21 (19%) patients treated with SRS alone exhibited symptomatic radionecrosis vs only 2 of 42 (5%) patients receiving both SRS and BVZ. Similarly, in the studies from Memorial Sloan-Kettering Cancer Center,83 Ludwig Maximilian University of Munich,89 and the University of Cincinnati,90 0%, 7%, and 9% rates of radionecrosis were observed in patients receiving SRS and BVZ, respectively.
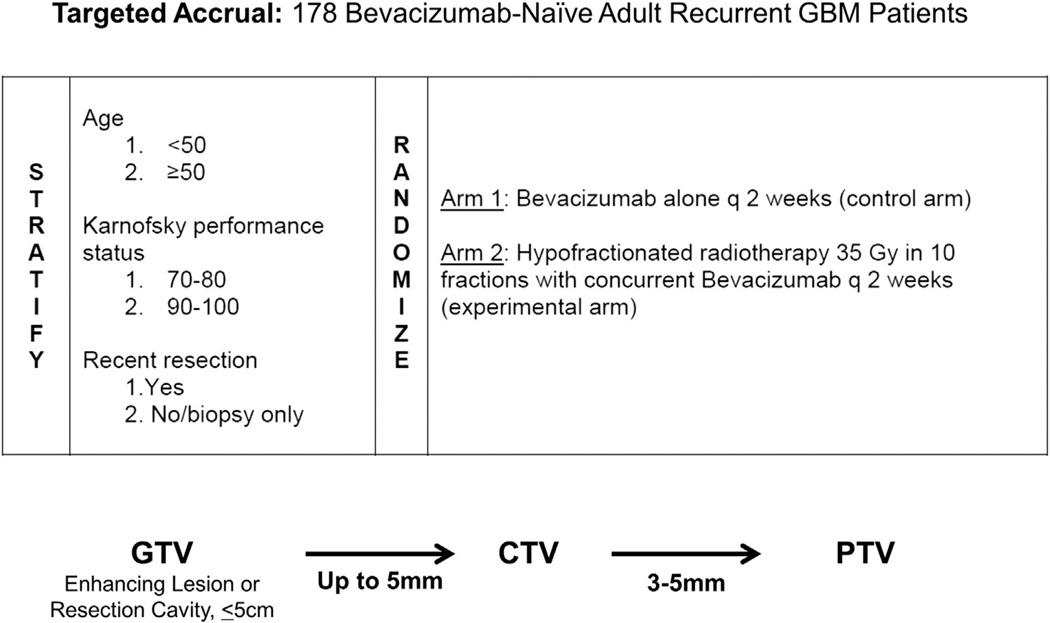
Selection bias undoubtedly influences the outcome, but the magnitude and direction of that influence in recurrent glioma therapy are unclear. For example, the fact that focal radiotherapy requires a discrete target would exclude those patients with diffuse brain disease from treatment with radiosurgery but not necessarily from treatment with systemic chemotherapy. RTOG 1205 is currently enrolling BVZ-naïve patients with recurrent glioma for a randomized trial of BVZ alone vs HFSRT and BVZ (Fig.). The targeted accrual for this study is 178 patients with a primary end point of OS.
Figure.
Radiation Therapy Oncology Group (RTOG) protocol 1205, a randomized trial of bevacizum (BVZ) vs radiotherapy + BVZ in BVZ-naïve patients with recurrent glioma. In this trial, 3D conformal radiotherapy, intensity-modulated radiotherapy, proton therapy, and stereotactic radiosurgery (SRS) techniques are permitted. However, SRS is not required.
Radiotherapy Technique
A variety of stereotactic systems, target volumes, and dosing schemes have been used in the treatment of recurrent malignant gliomas. The optimum technique has not been established, and the following regimens are presented to illustrate potential advantages and disadvantages of each system.
In the Memorial Sloan-Kettering approach,83 the target volume is the contrast-enhancing lesion on T1-weighted MR images uniformly expanded by 5 mm. The regimen is delivered as five 6 Gy daily fractions.
In the Duke approach,81,82 the planning target volume (PTV) is the contrast-enhancing lesion on T1-weighted fine-cut MR images uniformly expanded by 1 mm. For PTV < 2 cm or ≥2 and <3 cm in maximum dimension, doses of 20–24 or 18 Gy are delivered in a single fraction. PTV 3–5 cm in greatest dimension is treated with five 5 Gy daily fractions (25 Gy total).
In the Jefferson approach,74 the PTV is the contrast-enhancing lesion on T1-weighted MR images (no additional expansion). Treatment consists of ten 3.5 Gy daily fractions (35 Gy total).
In the RTOG 1205 protocol, the PTV is the contrast-enhancing lesion or resection cavity on T1-weighted MR images, expanded by 5–10 mm at the discretion of the treating physician. Treatment consists of ten 3.5 Gy daily fractions (35 Gy total).
In these studies, the PTV is smallest in the Duke and the Jefferson approaches and largest in the RTOG protocol. Assuming an alpha-beta ratio of 10 Gy and disregarding repopulation effects, the biological equivalent dose91 is lowest in the 5 Gy × 5 regimen (37.5 Gy10); intermediate in the RTOG, Jefferson, Memorial Sloan-Kettering, and 18-Gy single-fraction approaches (47.5–50.4 Gy10); and highest in the single-fraction treatments of 20–24 Gy (60–81.6 Gy10). Logistically, the single-fraction regimen is obviously advantaged, though it is important to factor in the time required for planning when comparing the overall length of these courses.
Observations on Management
The optimum management for recurrent glioma has not been identified. A variety of treatments—radiation therapy or radiosurgery, surgery, chemotherapy, or some combination of these options— as well as supportive care alone are available. Clearly, decisions on care should be made in the setting of a multidisciplinary team representing radiation oncology, surgery, and neuro-oncology, attentive to the specific patient's situation and wishes. Nevertheless, we offer some general observations on therapy for patients with recurrent disease following initial maximum safe resection, partial-brain irradiation, and concurrent or adjuvant temozolomide.
In general, younger patients with less disease burden who have a better performance status tend to derive the most benefit from salvage therapies. However, survival is highly variable on an individual basis. Surgery may be appropriate in patients with a symptomatic lesion located in a noneloquent site or for which resection would relieve the mass effect. The role of AEF therapy is intriguing but requires further evaluation. Although BVZ may offer survival advantages over other biochemotherapy approaches, the benefit is modest. SRS and HFSRT are associated with median OS of approximately 8–12 months, and the addition of BVZ may improve outcomes and reduce adverse radiation effects. However, the efficacy of this approach is not proven.
Combination therapy with multiple biochemotherapies and SRT may provide superior outcomes than that by monomodality treatment, but improving the current limited efficacy of salvage therapy requires novel approaches and robust enrollment on clinical trials. Ultimately, research in malignant gliomas needs to address prevention of this disease and the development of first-line treatments such that the incidence of recurrence is substantially reduced.
Acknowledgments
Disclosures: Dr Kirkpatrick has received research funding from Genentech and Varian Medical Systems.
References
- 1.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 2.Hess CF, Schaaf JC, Kortmann RD, et al. Malignant glioma: Patterns of failure following individually tailored limited volume irradiation. Radiother Oncol. 1994;30:146–149. doi: 10.1016/0167-8140(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 3.Hochberg FH, Pruitt A. Assumptions in the radiotherapy of glioblastoma. Neurology. 1980;30:907–911. doi: 10.1212/wnl.30.9.907. [DOI] [PubMed] [Google Scholar]
- 4.Lee SW, Fraass BA, Marsh LH, et al. Patterns of failure following high-dose 3-D conformal radiotherapy for high-grade astrocytomas: A quantitative dosimetric study. Int J Radiat Oncol Biol Phys. 1999;43:79–88. doi: 10.1016/s0360-3016(98)00266-1. [DOI] [PubMed] [Google Scholar]
- 5.Wallner KE, Galicich JH, Krol G, et al. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys. 1989;16:1405–1409. doi: 10.1016/0360-3016(89)90941-3. [DOI] [PubMed] [Google Scholar]
- 6.Brandes AA, Tosoni A, Franceschi E, et al. Recurrence pattern after temozolomide concomitant with and adjuvant to radiotherapy in newly diagnosed patients with glioblastoma: Correlation with MGMT promoter methylation status. J Clin Oncol. 2009;27:1275–1279. doi: 10.1200/JCO.2008.19.4969. [DOI] [PubMed] [Google Scholar]
- 7.McDonald MW, Shu HK, Curran WJ, Jr, et al. Pattern of failure after limited margin radiotherapy and temozolomide for glioblastoma. Int J Radiat Oncol Biol Phys. 2011;79:130–136. doi: 10.1016/j.ijrobp.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 8.Milano MT, Okunieff P, Donatello RS, et al. Patterns and timing of recurrence after temozolomide-based chemoradiation for glioblastoma. Int J Radiat Oncol Biol Phys. 2010;78:1147–1155. doi: 10.1016/j.ijrobp.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 9.Pan H, Alksne J, Mundt AJ, et al. Patterns of imaging failures in glioblastoma patients treated with chemoradiation: A retrospective study. Med Oncol. 2012;29:2040–2045. doi: 10.1007/s12032-011-0116-5. [DOI] [PubMed] [Google Scholar]
- 10.Kruser TJ, Mehta MP, Robins HI. Pseudoprogression after glioma therapy: A comprehensive review. Expert Rev Neurother. 2013;13:389–403. doi: 10.1586/ern.13.7. [DOI] [PubMed] [Google Scholar]
- 11.Chinot OL, Macdonald DR, Abrey LE, et al. Response assessment criteria for glioblastoma: Practical adaptation and implementation in clinical trials of antiangiogenic therapy. Curr Neurol Neurosci Rep. 2013;13:347. doi: 10.1007/s11910-013-0347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quant EC, Wen PY. Response assessment in neuro-oncology. Curr Oncol Rep. 2011;13:50–56. doi: 10.1007/s11912-010-0143-y. [DOI] [PubMed] [Google Scholar]
- 13.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neurooncology working group. J Clin Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 14.Shah AH, Snelling B, Bregy A, et al. Discriminating radiation necrosis from tumor progression in gliomas: A systematic review what is the best imaging modality? J Neurooncol. 2013;112:141–152. doi: 10.1007/s11060-013-1059-9. [DOI] [PubMed] [Google Scholar]
- 15.Bose D, Meric-Bernstam F, Hofstetter W, et al. Vascular endothelial growth factor targeted therapy in the perioperative setting: Implications for patient care. Lancet Oncol. 2010;11:373–382. doi: 10.1016/S1470-2045(09)70341-9. [DOI] [PubMed] [Google Scholar]
- 16.CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2008 (March 23, 2012 Revision) [Accessed September 1, 2012]; Available at: http://www.cbtrus.org. [Google Scholar]
- 17.Chaichana KL, Zadnik P, Weingart JD, et al. Multiple resections for patients with glioblastoma: Prolonging survival. J Neurosurg. 2013;118:812–820. doi: 10.3171/2012.9.JNS1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoover JM, Nwojo M, Puffer R, et al. Surgical outcomes in recurrent glioma: Clinical article. J Neurosurg. 2013;118:1224–1231. doi: 10.3171/2013.2.JNS121731. [DOI] [PubMed] [Google Scholar]
- 19.Brandes AA, Bartolotti M, Franceschi E. Second surgery for recurrent glioblastoma: Advantages and pitfalls. Expert Rev Anticancer Ther. 2013;13:583–587. doi: 10.1586/era.13.32. [DOI] [PubMed] [Google Scholar]
- 20.Ammirati M, Galicich JH, Arbit E, et al. Reoperation in the treatment of recurrent intracranial malignant gliomas. Neurosurgery. 1987;21:607–614. doi: 10.1227/00006123-198711000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Carson KA, Grossman SA, Fisher JD, et al. Prognostic factors for survival in adult patients with recurrent glioma enrolled onto the new approaches to brain tumor therapy CNS consortium phase I and II clinical trials. J Clin Oncol. 2007;25:2601–2606. doi: 10.1200/JCO.2006.08.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clarke JL, Ennis MM, Yung WK, et al. Is surgery at progression a prognostic marker for improved 6-month progression-free survival or overall survival for patients with recurrent glioblastoma? Neuro Oncol. 2011;13:1118–1124. doi: 10.1093/neuonc/nor110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Bonis P, Fiorentino A, Anile C, et al. The impact of repeated surgery and adjuvant therapy on survival for patients with recurrent glioblastoma. Clin Neurol Neurosurg. 2013;115:883–886. doi: 10.1016/j.clineuro.2012.08.030. [DOI] [PubMed] [Google Scholar]
- 24.Gorlia T, Stupp R, Brandes AA, et al. New prognostic factors and calculators for outcome prediction in patients with recurrent glioblastoma: A pooled analysis of EORTC Brain Tumour Group phase I and II clinical trials. Eur J Cancer. 2012;48:1176–1184. doi: 10.1016/j.ejca.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Landy HJ, Feun L, Schwade JG, et al. Retreatment of intracranial gliomas. South Med J. 1994;87:211–214. doi: 10.1097/00007611-199402000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Mandl ES, Dirven CM, Buis DR, et al. Repeated surgery for glioblastoma multiforme: Only in combination with other salvage therapy. Surg Neurol. 2008;69:506–509. doi: 10.1016/j.surneu.2007.03.043. [discussion 509] [DOI] [PubMed] [Google Scholar]
- 27.Park JK, Hodges T, Arko L, et al. Scale to predict survival after surgery for recurrent glioblastoma multiforme. J Clin Oncol. 2010;28:3838–3843. doi: 10.1200/JCO.2010.30.0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinsker M, Lumenta C. Experiences with reoperation on recurrent glioblastoma multiforme. Zentralbl Neurochir. 2001;62:43–47. doi: 10.1055/s-2002-19477. [DOI] [PubMed] [Google Scholar]
- 29.Kirson ED, Dbaly V, Tovarys F, et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc Natl Acad Sci U S A. 2007;104:10152–10157. doi: 10.1073/pnas.0702916104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirson ED, Gurvich Z, Schneiderman R, et al. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004;64:3288–3295. doi: 10.1158/0008-5472.can-04-0083. [DOI] [PubMed] [Google Scholar]
- 31.Stupp R, Wong ET, Kanner AA, et al. NovoTTF-100A versus physician's choice chemotherapy in recurrent glioblastoma: A randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012;48:2192–2202. doi: 10.1016/j.ejca.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 32.Schneiderman RS, Shmueli E, Kirson ED, et al. TTFields alone and in combination with chemotherapeutic agents effectively reduce the viability of MDR cell sub-lines that over-express ABC transporters. BMC Cancer. 2010;10:229. doi: 10.1186/1471-2407-10-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balmaceda C, Peereboom D, Pannullo S, et al. Multi-institutional phase II study of temozolomide administered twice daily in the treatment of recurrent high-grade gliomas. Cancer. 2008;112:1139–1146. doi: 10.1002/cncr.23167. [DOI] [PubMed] [Google Scholar]
- 34.Demirci U, Tufan G, Aktas B, et al. Bevacizumab plus irinotecan in recurrent or progressive malign glioma: A multicenter study of the Anatolian Society of Medical Oncology (ASMO) J Cancer Res Clin Oncol. 2013;139:829–835. doi: 10.1007/s00432-013-1390-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franceschi E, Stupp R, van den Bent MJ, et al. EORTC 26083 phase I/II trial of dasatinib in combination with CCNU in patients with recurrent glioblastoma. Neuro Oncol. 2012;14:1503–1510. doi: 10.1093/neuonc/nos256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 37.Galanis E, Anderson SK, Lafky JM, et al. Phase II study of bevacizumab in combination with sorafenib in recurrent glioblastoma (N0776): A north central cancer treatment group trial. Clin Cancer Res. 2013;19:4816–4823. doi: 10.1158/1078-0432.CCR-13-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gil MJ, de Las Penas R, Reynes G, et al. Bevacizumab plus irinotecan in recurrent malignant glioma shows high overall survival in a multicenter retrospective pooled series of the Spanish Neuro-Oncology Research Group (GEINO) Anticancer Drugs. 2012;23:659–665. doi: 10.1097/CAD.0b013e3283534d3e. [DOI] [PubMed] [Google Scholar]
- 39.Hu J, Wen PY, Abrey LE, et al. A phase II trial of oral gimatecan for recurrent glioblastoma. J Neurooncol. 2013;111:347–353. doi: 10.1007/s11060-012-1023-0. [DOI] [PubMed] [Google Scholar]
- 40.Karavasilis V, Kotoula V, Pentheroudakis G, et al. A phase I study of temozolomide and lapatinib combination in patients with recurrent high-grade gliomas. J Neurol. 2013;260:1469–1480. doi: 10.1007/s00415-012-6812-z. [DOI] [PubMed] [Google Scholar]
- 41.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kreisl TN, McNeill KA, Sul J, et al. A phase I/II trial of vandetanib for patients with recurrent malignant glioma. Neuro Oncol. 2012;14:1519–1526. doi: 10.1093/neuonc/nos265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kreisl TN, Smith P, Sul J, et al. Continuous daily sunitinib for recurrent glioblastoma. J Neurooncol. 2013;111:41–48. doi: 10.1007/s11060-012-0988-z. [DOI] [PubMed] [Google Scholar]
- 44.Lassen U, Sorensen M, Gaziel TB, et al. Phase II study of bevacizumab and temsirolimus combination therapy for recurrent glioblastoma multiforme. Anticancer Res. 2013;33:1657–1660. [PubMed] [Google Scholar]
- 45.Lee EQ, Kuhn J, Lamborn KR, et al. Phase I/II study of sorafenib in combination with temsirolimus for recurrent glioblastoma or gliosarcoma: North American Brain Tumor Consortium study 05-02. Neuro Oncol. 2012;14:1511–1518. doi: 10.1093/neuonc/nos264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moller S, Grunnet K, Hansen S, et al. A phase II trial with bevacizumab and irinotecan for patients with primary brain tumors and progression after standard therapy. Acta Oncol. 2012;51:797–804. doi: 10.3109/0284186X.2012.681063. [DOI] [PubMed] [Google Scholar]
- 47.Mrugala MM, Crew LK, Fink JR, et al. Carboplatin and bevacizumab for recurrent malignant glioma. Oncol Lett. 2012;4:1082–1086. doi: 10.3892/ol.2012.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muhic A, Poulsen HS, Sorensen M, et al. Phase II open-label study of nintedanib in patients with recurrent glioblastoma multiforme. J Neurooncol. 2013;111:205–212. doi: 10.1007/s11060-012-1009-y. [DOI] [PubMed] [Google Scholar]
- 49.Nghiemphu PL, Lai A, Green RM, et al. A dose escalation trial for the combination of erlotinib and sirolimus for recurrent malignant gliomas. J Neurooncol. 2012;110:245–250. doi: 10.1007/s11060-012-0960-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nghiemphu PL, Liu W, Lee Y, et al. Bevacizumab and chemotherapy for recurrent glioblastoma: A single-institution experience. Neurology. 2009;72:1217–1222. doi: 10.1212/01.wnl.0000345668.03039.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Omuro A, Chan TA, Abrey LE, et al. Phase II trial of continuous low-dose temozolomide for patients with recurrent malignant glioma. Neuro Oncol. 2013;15:242–250. doi: 10.1093/neuonc/nos295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reardon DA, Desjardins A, Peters KB, et al. Phase II study of carboplatin, irinotecan, and bevacizumab for bevacizumab naive, recurrent glioblastoma. J Neurooncol. 2012;107:155–164. doi: 10.1007/s11060-011-0722-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reardon DA, Groves MD, Wen PY, et al. A phase I/II trial of pazopanib in combination with lapatinib in adult patients with relapsed malignant glioma. Clin Cancer Res. 2013;19:900–908. doi: 10.1158/1078-0432.CCR-12-1707. [DOI] [PubMed] [Google Scholar]
- 54.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 55.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 56.Yust-Katz S, Liu D, Yuan Y, et al. Phase 1/1b study of lonafarnib and temozolomide in patients with recurrent or temozolomide refractory glioblastoma. Cancer. 2013;119:2747–2753. doi: 10.1002/cncr.28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zuniga RM, Torcuator R, Jain R, et al. Efficacy, safety and patterns of response and recurrence in patients with recurrent high-grade gliomas treated with bevacizumab plus irinotecan. J Neurooncol. 2009;91:329–336. doi: 10.1007/s11060-008-9718-y. [DOI] [PubMed] [Google Scholar]
- 58.Reardon DA, Turner S, Peters KB, et al. A review of VEGF/VEGFR-targeted therapeutics for recurrent glioblastoma. J Natl Compr Canc Netw. 2011;9:414–427. doi: 10.6004/jnccn.2011.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gil-Gil MJ, Mesia C, Rey M, et al. Bevacizumab for the treatment of glioblastoma. Clin Med Insights Oncol. 2013;7:123–135. doi: 10.4137/CMO.S8503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 61.Cabrera AR, Cuneo KC, Vredenburgh JJ, et al. Stereotactic radiosurgery and bevacizumab for recurrent glioblastoma multiforme. J Natl Compr Canc Netw. 2012;10:695–699. doi: 10.6004/jnccn.2012.0072. [DOI] [PubMed] [Google Scholar]
- 62.Dilworth JT, Krueger SA, Dabjan M, et al. Pulsed low-dose irradiation of orthotopic glioblastoma multiforme (GBM) in a pre-clinical model: Effects on vascularization and tumor control. Radiother Oncol. 2013;108:149–154. doi: 10.1016/j.radonc.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 63.Lee DY, Chunta JL, Park SS, et al. Pulsed versus conventional radiation therapy in combination with temozolomide in a murine orthotopic model of glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2013;86:978–985. doi: 10.1016/j.ijrobp.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 64.Schoenherr D, Krueger SA, Martin L, et al. Determining if low dose hyper-radiosensitivity (HRS) can be exploited to provide a therapeutic advantage: A cell line study in four glioblastoma multiforme (GBM) cell lines. Int J Radiat Biol. 2013;89:1009–1016. doi: 10.3109/09553002.2013.825061. [DOI] [PubMed] [Google Scholar]
- 65.Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: Final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47:291–298. doi: 10.1016/s0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- 66.Balagamwala EH, Chao ST, Suh JH. Principles of radiobiology of stereotactic radiosurgery and clinical applications in the central nervous system. Technol Cancer Res Treat. 2012;11:3–13. doi: 10.7785/tcrt.2012.500229. [DOI] [PubMed] [Google Scholar]
- 67.Kirkpatrick JP, Meyer JJ, Marks LB. The linear-quadratic model is inappropriate to model high dose per fraction effects in radiosurgery. Semin Radiat Oncol. 2008;18:240–243. doi: 10.1016/j.semradonc.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 68.Kirkpatrick JP, Brenner DJ, Orton CG. Point/Counterpoint. The linear-quadratic model is inappropriate to model high dose per fraction effects in radiosurgery. Med Phys. 2009;36:3381–3384. doi: 10.1118/1.3157095. [DOI] [PubMed] [Google Scholar]
- 69.Souhami L, Seiferheld W, Brachman D, et al. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: Report of Radiation Therapy Oncology Group 93-05 protocol. Int J Radiat Oncol Biol Phys. 2004;60:853–860. doi: 10.1016/j.ijrobp.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 70.Cho KH, Hall WA, Gerbi BJ, et al. Single dose versus fractionated stereotactic radiotherapy for recurrent high-grade gliomas. Int J Radiat Oncol Biol Phys. 1999;45:1133–1141. doi: 10.1016/s0360-3016(99)00336-3. [DOI] [PubMed] [Google Scholar]
- 71.Hall WA, Djalilian HR, Sperduto PW, et al. Stereotactic radiosurgery for recurrent malignant gliomas. J Clin Oncol. 1995;13:1642–1648. doi: 10.1200/JCO.1995.13.7.1642. [DOI] [PubMed] [Google Scholar]
- 72.Kong DS, Lee JI, Park K, et al. Efficacy of stereotactic radiosurgery as a salvage treatment for recurrent malignant gliomas. Cancer. 2008;112:2046–2051. doi: 10.1002/cncr.23402. [DOI] [PubMed] [Google Scholar]
- 73.Shrieve DC, Alexander E, 3rd, Wen PY, et al. Comparison of stereotactic radiosurgery and brachytherapy in the treatment of recurrent glioblastoma multiforme. Neurosurgery. 1995;36:275–282. doi: 10.1227/00006123-199502000-00006. [discussion 282-274] [DOI] [PubMed] [Google Scholar]
- 74.Fogh SE, Andrews DW, Glass J, et al. Hypofractionated stereotactic radiation therapy: An effective therapy for recurrent high-grade gliomas. J Clin Oncol. 2010;28:3048–3053. doi: 10.1200/JCO.2009.25.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moeller BJ, Cao Y, Li CY, et al. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: Role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5:429–441. doi: 10.1016/s1535-6108(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 76.Moeller BJ, Dewhirst MW. Raising the bar: How HIF-1 helps determine tumor radiosensitivity. Cell Cycle. 2004;3:1107–1110. [PubMed] [Google Scholar]
- 77.Moeller BJ, Dreher MR, Rabbani ZN, et al. Pleiotropic effects of HIF-1 blockade on tumor radiosensitivity. Cancer Cell. 2005;8:99–110. doi: 10.1016/j.ccr.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 78.Boothe D, Young R, Yamada Y, et al. Bevacizumab as a treatment for radiation necrosis of brain metastases post stereotactic radiosurgery. Neuro Oncol. 2013;15:1257–1263. doi: 10.1093/neuonc/not085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Levin VA, Bidaut L, Hou P, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79:1487–1495. doi: 10.1016/j.ijrobp.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Torcuator R, Zuniga R, Mohan YS, et al. Initial experience with bevacizumab treatment for biopsy confirmed cerebral radiation necrosis. J Neurooncol. 2009;94:63–68. doi: 10.1007/s11060-009-9801-z. [DOI] [PubMed] [Google Scholar]
- 81.Cabrera AR, Cuneo KC, Desjardins A, et al. Concurrent stereotactic radiosurgery and bevacizumab in recurrent malignant gliomas: A prospective trial. Int J Radiat Oncol Biol Phys. 2013;86:873–879. doi: 10.1016/j.ijrobp.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 82.Cuneo KC, Vredenburgh JJ, Sampson JH, et al. Safety and efficacy of stereotactic radiosurgery and adjuvant bevacizumab in patients with recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2012;82:2018–2024. doi: 10.1016/j.ijrobp.2010.12.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gutin PH, Iwamoto FM, Beal K, et al. Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2009;75:156–163. doi: 10.1016/j.ijrobp.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hundsberger T, Brugge D, Putora PM, et al. Re-irradiation with and without bevacizumab as salvage therapy for recurrent or progressive high-grade gliomas. J Neurooncol. 2013;112:133–139. doi: 10.1007/s11060-013-1044-3. [DOI] [PubMed] [Google Scholar]
- 85.Park KJ, Kano H, Iyer A, et al. Salvage gamma knife stereotactic radiosurgery followed by bevacizumab for recurrent glioblastoma multiforme: A case-control study. J Neurooncol. 2012;107:323–333. doi: 10.1007/s11060-011-0744-9. [DOI] [PubMed] [Google Scholar]
- 86.Torcuator RG, Thind R, Patel M, et al. The role of salvage reirradiation for malignant gliomas that progress on bevacizumab. J Neurooncol. 2010;97:401–407. doi: 10.1007/s11060-009-0034-y. [DOI] [PubMed] [Google Scholar]
- 87.Shapiro LQ, Beal K, Goenka A, et al. Patterns of failure after concurrent bevacizumab and hypofractionated stereotactic radiation therapy for recurrent high-grade glioma. Int J Radiat Oncol Biol Phys. 2013;85:636–642. doi: 10.1016/j.ijrobp.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 88.Patel M, Siddiqui F, Jin JY, et al. Salvage reirradiation for recurrent glioblastoma with radiosurgery: Radiographic response and improved survival. J Neurooncol. 2009;92:185–191. doi: 10.1007/s11060-008-9752-9. [DOI] [PubMed] [Google Scholar]
- 89.Niyazi M, Ganswindt U, Schwarz SB, et al. Irradiation and bevacizumab in high-grade glioma retreatment settings. Int J Radiat Oncol Biol Phys. 2012;82:67–76. doi: 10.1016/j.ijrobp.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 90.McKenzie JT, Guarnaschelli JN, Vagal AS, et al. Hypofractionated stereotactic radiotherapy for unifocal and multifocal recurrence of malignant gliomas. J Neurooncol. 2013;113:403–409. doi: 10.1007/s11060-013-1126-2. [DOI] [PubMed] [Google Scholar]
- 91.Hall EJ, Giaccia AJ. Radiobiology for the radiologist. 7th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 92.Combs SE, Thilmann C, Edler L, et al. Efficacy of fractionated stereotactic reirradiation in recurrent gliomas: Long-term results in 172 patients treated in a single institution. J Clin Oncol. 2005;23:8863–8869. doi: 10.1200/JCO.2005.03.4157. [DOI] [PubMed] [Google Scholar]
- 93.Combs SE, Widmer V, Thilmann C, et al. Stereotactic radiosurgery (SRS): Treatment option for recurrent glioblastoma multiforme (GBM) Cancer. 2005;104:2168–2173. doi: 10.1002/cncr.21429. [DOI] [PubMed] [Google Scholar]
- 94.Biswas T, Okunieff P, Schell MC, et al. Stereotactic radiosurgery for glioblastoma: Retrospective analysis. Radiat Oncol. 2009;4:11. doi: 10.1186/1748-717X-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sirin S, Oysul K, Surenkok S, et al. Linear accelerator-based stereotactic radiosurgery in recurrent glioblastoma: A single center experience. Vojnosanit Pregl. 2011;68:961–966. doi: 10.2298/vsp1111961s. [DOI] [PubMed] [Google Scholar]
- 96.Elliott RE, Parker EC, Rush SC, et al. Efficacy of gamma knife radiosurgery for small-volume recurrent malignant gliomas after initial radical resection. World Neurosurg. 2011;76:128–140. doi: 10.1016/j.wneu.2010.12.053. [discussion 161-122] [DOI] [PubMed] [Google Scholar]
- 97.Skeie BS, Enger PO, Brogger J, et al. Gamma knife surgery versus reoperation for recurrent glioblastoma multiforme. World Neurosurg. 2012;78:658–669. doi: 10.1016/j.wneu.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 98.Kim B, Soisson E, Duma C, et al. Treatment of recurrent high grade gliomas with hypofractionated stereotactic image-guided helical tomotherapy. Clin Neurol Neurosurg. 2011;113:509–512. doi: 10.1016/j.clineuro.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 99.Lederman G, Wronski M, Arbit E, et al. Treatment of recurrent glioblastoma multiforme using fractionated stereotactic radiosurgery and concurrent paclitaxel. Am J Clin Oncol. 2000;23:155–159. doi: 10.1097/00000421-200004000-00010. [DOI] [PubMed] [Google Scholar]
- 100.Larson DA, Prados M, Lamborn KR, et al. Phase II study of high central dose Gamma Knife radiosurgery and marimastat in patients with recurrent malignant glioma. Int J Radiat Oncol Biol Phys. 2002;54:1397–1404. doi: 10.1016/s0360-3016(02)03743-4. [DOI] [PubMed] [Google Scholar]
- 101.Minniti G, Scaringi C, De Sanctis V, et al. Hypofractionated stereotactic radiotherapy and continuous low-dose temozolomide in patients with recurrent or progressive malignant gliomas. J Neurooncol. 2013;111:187–194. doi: 10.1007/s11060-012-0999-9. [DOI] [PubMed] [Google Scholar]