Abstract
To analyze the genetic and biomolecular mechanisms underlying cartilage repair, an optimized mouse model of osteochondral repair is required. Although several models of articular cartilage injury in mice have recently been established, the articular surface in adult C57Bl/6 mice heals poorly. Since C57Bl/6 mice are the most popular strain of genetically manipulated mice, an articular cartilage repair model using C57Bl/6 mice would be helpful for analysis of the mechanisms of cartilage repair. The purpose of this study was to establish a cartilage repair model in C57Bl/6 mice using immature animals. To achieve this goal, full-thickness injuries were generated in 3-week-old (young), 4-week-old (juvenile), and 8-week-old (adult) C57Bl/6 mice. To investigate the reproducibility and consistency of full-thickness injuries, mice were sacrificed immediately after operation, and cartilage thickness at the patellar groove, depth of the cartilage injury, cross-sectional width, and cross-sectional area were compared among the three age groups. The depth of cartilage injury/cartilage thickness ratio (%depth) and the coefficient of variation (CV) for each parameter were also calculated. At 8 weeks postoperatively, articular cartilage repair was assessed using a histological scoring system. With respect to the reproducibility and consistency of full-thickness injuries, cartilage thickness, depth of cartilage injury, and cross-sectional area were significantly larger in young and juvenile mice than in adult mice, whereas cross-sectional width and %depth were almost equal among the three age groups. CVs of %depths were less than 10% in all groups. With respect to articular cartilage repair, young and juvenile mice showed superior results. In conclusion, we established a novel cartilage repair model in C57Bl/6 mice. This model will be valuable in achieving mechanistic insights into the healing process of the joint surface, as it will facilitate the use of genetically modified mice, which are most commonly developed on a C57Bl/6 background.
Introduction
Articular cartilage injuries are common, especially in the working population.1–3 Such injuries may result in osteoarthritis (OA) and cause joint pain and limitations to daily activities, working capabilities, and sports and can thus impact the quality of life of patients.4 Since articular cartilage injuries are known to have a poor capacity for repair,5,6 previous studies of articular cartilage injury have mainly focused on surgical interventions such as osteochondral grafts or cartilage tissue engineering. Recently, molecular cartilage research has reported that molecules promoting the selective differentiation of multipotent mesenchymal stem cells into chondrocytes may stimulate the repair of damaged cartilage.7This report showed the possibility that enhancement of self-regeneration can prevent joint degeneration, and therefore, the repair process of osteochondral tissue is attracting attention. Elucidation of the cartilage repair process is essential to ensure efficient manipulation of the potential for cartilage healing. However, analysis of the repair process has not been described previously.
To better understand the cartilage repair process, an optimized animal model of an osteochondral repair process is needed. In particular, genetically modified animals offer powerful tools to investigate the biological mechanisms of cartilage repair. Although full-thickness cartilage injury models have already been established in dogs, rabbits, and horses,8–10 genetic modification is relatively difficult in such animals. The biological analysis of articular cartilage repair using such animals is thus limited due to the lack of appropriate articular cartilage repair models with genetic modification.11,12
The capacity of articular cartilage repair in mice is known to differ among strains. Although cartilage repair models in mice have been recently established in a limited number of strains,11–13 superior cartilage repair is not obtained in other strains, including C57Bl/6 mice. Since C57Bl/6 mice are the most popular strain as a background for genetic manipulation in mice, an articular cartilage repair model in C57Bl/6 mice would be extremely helpful in the analysis of the mechanisms underlying cartilage repair. Establishment of a cartilage repair model in C57Bl/6 mice is thus required.
Immature individuals or embryos are available for the purpose of analyzing repair processes in tissues with poor healing potential.14–17 To overcome the poor cartilage healing potential of C57Bl/6 mice, younger mice were used to establish an articular cartilage repair model. The purpose of this study was to establish a novel articular cartilage repair model in C57Bl/6 mice that would clarify the biological processes of cartilage repair in genetically manipulated mice in the future.
Materials and Methods
Experimental animals
All procedures were approved by the Institutional Animal Care and Use Committee of our institution. C57Bl/6 mice were purchased from Japan SLC (Shizuoka, Japan). Mice were used after 7-day acclimatization following transportation.18 All purchased mice recovered normal behavior within 24 h after transportation. Mice were housed in a temperature- and humidity-controlled environment under 12-h light/12-h dark conditions and fed a standard rodent diet in accordance with our institutional guidelines for the care and use of laboratory animals.
Operative procedure for full-thickness injuries in mice
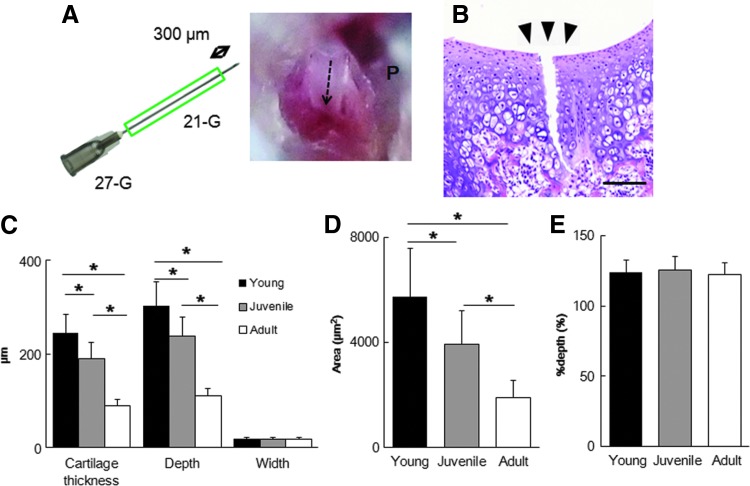
Full-thickness injuries were generated in 3-week-old C57Bl/6 (young) mice, 4-week-old C57Bl/6 (juvenile) mice, and 8-week-old C57Bl/6 (adult) mice. These articular cartilage injuries were made in one knee, with a sham operation performed in the other knee. A modified version of a previously reported full-thickness injury model in mice was applied in this study.11 The two ends of 21G needles were cut as an outer cylinder adjusted to be approximately 300 μm shorter than a 27G needle, and this device was used to create the injury (Fig. 1A). We measured cartilage thicknesses in young mice before starting our study, showing that thickness was less than 300 μm. We therefore chose a fixed depth of 300 μm for this study. Under general anesthesia, the hind limbs were disinfected. A medial parapatellar skin incision slightly less than 1 cm in length was then made using a microsurgical scalpel. The joint capsule was opened, and the patella was dislocated laterally to expose the articular surface of the trochlear groove. With the femoral epicondyles gently fixed by surgical tweezers, a longitudinal full-thickness injury was made in the patellar groove using the ends of the needles of the device described above (Fig. 1B). These microsurgical procedures were performed under a surgical microscope (SZX16; Olympus, Tokyo, Japan). Penetration to the subchondral bone was confirmed by bleeding from the cartilage injury site. After irrigation with normal saline to remove the debris, the knee dislocation was reduced. The joint capsule and skin were sutured in separate layers. Postoperatively, the mice were warmed until they recovered from anesthesia.
FIG. 1.
(A) Schematic illustration of a full-thickness cartilage injury. The patellar groove (P) is scratched using a 27G needle covered with a 21G needle as a sheath. The dashed arrow shows the track of the full-thickness cartilage injury. (B) Representative histology of the injured patellar groove in young mice. Black triangles show the position of the osteocartilage injury. Scale bar, 100 μm. (C–E) Measurements of osteocartilage injury in young, juvenile, and adult mice immediately postoperation (n=10). Cartilage thickness, depth of cartilage injury, cross-sectional width (C), cross-sectional area of the cartilage injury site (D), and %depth (E). *p<0.0001. All values are expressed as mean±SD.
Tissue processing and histology
At each time point, the knee joints were disarticulated and fixed in 10% formalin (Wako, Tokyo, Japan) and then decalcified with ethylenediaminetetraacetic acid. When the sections were embedded in paraffin, the femoral axis was carefully adjusted to be upright against the surface embedding, and serial sections were then created at 5-μm intervals. Histological sections were created as previously reported.11 Briefly, axial sections were made at three points per knee joint (A–C). The first section (A) was 100 mm proximal to the intercondylar notch, the second (B) another 100 mm proximal to Section A, and the third (C) was 100 mm proximal to Section B. During the tissue-processing procedure, true vertical was carefully confirmed against the joint surface to ensure that growth plates in four points per section were kept as landmarks. Sections at each of the points A–C were used for histomorphometry and scoring. In the same manner, the other sections within the (A–C) interval were used for immunochemical analysis. All slides were analyzed using an Olympus DP72 camera and DP2-BSW software (Olympus).
Histomorphometry and scoring for articular cartilage repair
Each section (A–C) was stained using hematoxylin and eosin (HE) and Safranin-O staining. To assess the reproducibility and consistency of the full-thickness injury, cartilage thickness of the patellar groove, the depth of cartilage injury, the cross-sectional width of cartilage injury, and the cross-sectional area of the cartilage injury site on the day of operation were measured using ImageJ software (National Institutes of Health, Bethesda, MD). The depth of the cartilage injury/cartilage thickness ratio (%depth) and the coefficient of variation (CV) for each parameter were also calculated. The histological score for joint surface repair in each section was evaluated as previously reported (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/tec).11,12,19 Measuring and scoring were performed independently by two blinded observers. All data are presented as mean±standard deviation (SD).
Immunohistochemistry
Sections of the mouse knee were deparaffinized, and endogenous peroxidase activity was quenched. After treatment with an N-Histofine MOUSESTAIN KIT (Nichirei, Tokyo, Japan), type II collagen was identified using rabbit polyclonal antibodies against rat type II collagen (Daiichi Fine Chemical, Toyama, Japan).
Statistical analyses
Mean values immediately postoperation and mean histological scores were statistically compared among the three generations by analysis of variance (ANOVA). If ANOVA indicated significance, subsequent multiple comparisons were performed using Tukey's honestly significant difference (Tukey's HSD) post hoc testing to evaluate differences between pairs of groups. Significance was accepted for values of p<0.05. All statistical analyses were performed using JMP Pro version 10.0 statistical software (SAS Institute, Cary, NC).
Results
Reproducibility and consistency of full-thickness injuries
To investigate the reproducibility and consistency of our experimental full-thickness injuries, mice in each age group were sacrificed immediately after operation (males, n=5; females, n=5), and cartilage thickness of the patellar groove, depth of the cartilage injury, cross-sectional width of the cartilage injury, and cross-sectional area of the cartilage injury site were compared among the three age groups. Cartilage thickness, depth of the cartilage injury, and cross-sectional area were significantly larger in young and juvenile mice than in adult mice, whereas cross-sectional width and %depth were almost equal among the three age groups (Table 1 and Fig. 1C–E). All injuries at each section reached the subchondral bone. Although the CVs of cartilage thickness, depth of cartilage injury, and cross-sectional width were over 10%, CVs of %depth were all less than 10%.
Table 1.
Histological Evaluations
| Cartilage thickness (μm) | Depth (μm) | Width (μm) | Area (μm2) | %Depth (%) | |
|---|---|---|---|---|---|
| Young | 244.47±39.95 | 302.40±50.82 | 18.96±3.15 | 5726.66±1842.03 | 123.86±8.83 |
| CV=16.34% | CV=16.80% | CV=16.59% | CV=32.17% | CV=7.13% | |
| Juvenile | 190.62±34.86 | 238.49±40.46 | 18.52±3.50 | 3936.24±1247.41 | 125.68±9.44 |
| CV=18.29% | CV=16.97% | CV=18.93% | CV=31.69% | CV=7.51% | |
| Adult | 90.17±13.30 | 109.88±16.20 | 18.04±3.67 | 1896.91±658.36 | 122.12±8.59 |
| CV=14.75% | CV=14.74% | CV=20.36% | CV=34.71% | CV=7.04% |
All values are expressed as mean±SD.
CV, coefficient of variation.
Outcome of articular cartilage repairs
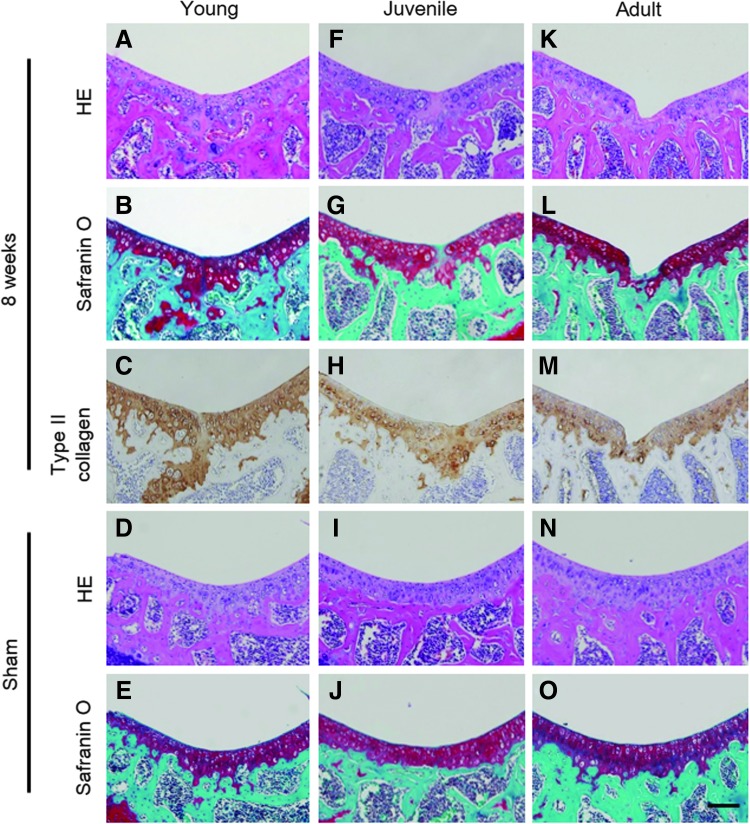
We evaluated the outcome of articular cartilage repair at 2 and 8 weeks postoperatively. Although the defects had already filled with repair tissues in all age groups by 2 weeks postoperatively, repair tissue in young mice differed qualitatively from that in juvenile and adult mice. The defect in young mice was filled with hyaline cartilage-like tissues, whereas that in juvenile and young mice was filled with fibrous tissue (Fig. 2). Young and juvenile mice showed superior articular cartilage repair at 8 weeks postoperatively, whereas adult mice responded inadequately (Fig. 3A–C, F–H, K–M). Immunohistochemistry of young and juvenile mice for type II collagen showed superior staining, whereas that of adult mice showed poor staining at the injury site (Fig. 3C, H, M). Although the thickness of articular cartilage decreased with advancing age in the sham knee, no significant difference between the operated and age-matched sham knees was seen (Fig. 3D, E, I, J, N, O).
FIG. 2.
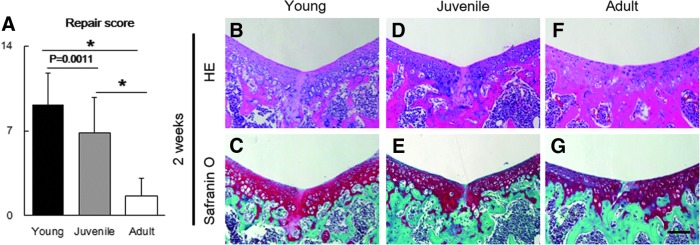
(A) Articular cartilage repair scores at 2 weeks postoperation (n=10). (B–G) Representative histology of full cartilage repair at 2 weeks postoperation. Specimens from young mice (B, C), juvenile mice (D, E), and adult mice (F, G) are stained with hematoxylin and eosin and Safranin-O stains. Scale bar, 100 μm. *p<0.0001.
FIG. 3.
(A–I) Representative histology from injured (A–C, F–H, K–M) and sham-operated (D, E, I, J, N, O) joints at 8 weeks postoperation. Specimens from young mice (A–E), juvenile mice (F–J), and adult mice (K–O) are stained with hematoxylin and eosin, Safranin-O, and type 2 collagen stains. Scale bar, 100 μm.
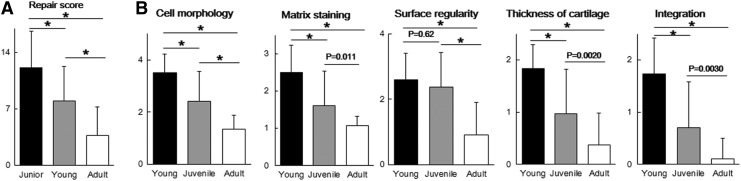
Scores for the articular joint surface were significantly higher in young and juvenile mice than in adult mice at 8 weeks postoperatively (mean±SD; 12.17±4.51 in young mice versus 3.77±3.50 in adult mice [p<0.0001] and 8.03±4.30 in juvenile mice versus 3.77±3.50 in adult mice [p<0.0001]) (males, n=5; females, n=5) (Fig. 4A). CVs for the joint surface score were 10.69% in young mice, 14.3% in juvenile mice, and 7.86% in adult mice. When individual parameters of the histological score were examined, young and juvenile mice clearly showed better cell morphology scores than adult mice. Young mice showed hyaline cartilage repair (3.50±0.73 on cell morphology), juvenile mice showed fibrocartilage repair (2.40±1.16 on cell morphology), and adult mice showed noncartilage repair (1.33±0.55 on cell morphology) (Fig. 4B).
FIG. 4.
(A) Articular cartilage repair scores at 8 weeks postoperation (n=10). (B) Individual parameters for histological score at 8 weeks postoperation. All values are expressed as mean±SD. *p<0.0001.
Discussion
Reproducibility of the surgical procedure is crucial for establishing an animal model.20–22 To assess the reproducibility and consistency of our full-thickness injury model, the sections were investigated on the day of the operation. Although the CVs of cartilage thickness, depth of cartilage injury, and cross-sectional width were more than 10%, CVs of %depth were all less than 10%. Cartilage thickness differs among individuals, and the section angle does not always represent the true vertical compared to the joint surface. Cartilage depth and depth of cartilage injury were thus affected by these factors. To eliminate these biases, %depth was calculated to normalize the depth of the cartilage injury to cartilage thickness. We consider %depth as a standardized value and, therefore, the most important for assessing reproducibility and consistency in this model.
The repair tissue in young mice differed from that of juvenile and adult mice, suggesting that the difference in cartilage repair among age groups started from immediately after injury creation and resulted in the different outcomes of articular cartilage repair among age groups.
The reproducibility of cartilage repair is also important for establishing an animal model. To assess the reproducibility and consistency of the degree of cartilage repair, sections were investigated at 8 weeks postoperatively. In the current model, CVs for the articular repair score were less than 15%. Although no previous reports have shown exact CVs of repair outcomes, estimates based on the available data were at least over 20%.11,12 We thus considered the 14.34% in juvenile mice comparable to the CVs of repair outcomes in previous reports. Based on the reproducibility of the surgical procedure and the consistency of cartilage repair, we believe that the current model is useful for analyzing the biological processes in articular cartilage repair.
Handling immature mice is technically difficult because of their small bodies and fragile cartilage. To overcome these problems, we choose thinner 27G needles in place of the 26G needles selected by Eltawil et al.11 We combined these thinner needles with 21G needles, because 27G needles are easily flexible. This was done to provide an outer stiffener and to maintain the reproducibility of the injury as a stopper or a guide for the cartilage injury. These original devices could be applied to both immature and adult mice. Our method allows easy generation of cartilage injury with high reproducibility without expensive devices or special techniques.
The healing potential of mice is known to differ among strains.23,24 In particular, C57Bl/6 mice show a poor capacity for cartilage repair.11,12 Although adult C57Bl/6 mice showed poor cartilage repair, as in previous reports, the articular cartilage of young and juvenile mice showed superior cartilage repair compared with that of adult mice. The present results show that the articular cartilage of young and juvenile C57Bl/6 mice healed equivalently in comparison with that of previously established strains such as MRL/MpJ mice and DBA/1 mice. Since the knee joint in young mice was smaller than that in juvenile and adult mice, the defect size was relatively larger in young mice that in juvenile and adult mice. Regarding the relative defect size among age groups, the ability to achieve cartilage repair in young mice is much superior to that in juvenile and adult mice.
MRL/MpJ mice have been reported to have an impaired inflammatory response.25 This strain shows super-healing characteristics, not only of articular cartilage but also ear punch wounds and neonatal digit amputation.26 Eltawil et al. reported that DBA/1 mice also showed high potential for cartilage repair.11 DBA/1 mice have high sensitivity in inflammatory arthritis models such as type II collagen-induced arthritis.27 The characteristics of the two strains indicate that inflammatory mechanisms play an important role in articular cartilage repair. Further studies will resolve the differences in the articular healing potential among mouse strains.
Several limitations to this study must be considered when interpreting the present results. First, the current repair model is only applicable to immature mice, whereas patients with articular cartilage injuries in common clinical situations are usually adults. Although the mechanisms of articular cartilage repair may differ between younger and adult individuals, clarification of the healing mechanisms underlying articular cartilage in younger individuals is expected to provide important insights that will facilitate the manipulation of cartilage healing in adult humans. Second, this model includes a microsurgical procedure and requires training to obtain sufficient reproducibility. However, the accessibility of this model is acceptable, because the operation techniques are very simple and familiar to orthopedic researchers and our original devices were created from commercially available supplies. Third, we should take the processes of articular cartilage maturation and degeneration into consideration. Particularly in young individuals, cartilage maturation affects cartilage thickness. We created full-thickness injuries in all age groups, meaning that all samples had the same proportion (100%) of injury at the initial time point. We therefore consider the effects of cartilage maturation as minimal. Regarding adult mice, the effect of progressive degeneration should be considered in our results. However, distinguishing degradation from unrepaired tissue was extremely difficult in this study.
In the current model, young mice showed hyaline cartilage repair, while juvenile mice showed fibrocartilage repair. We are of the opinion that young mice offer an appropriate model for investigating the mechanisms of cartilage repair and juvenile mice are appropriate for phenotypic change. Studies using the current model for young mice and juvenile mice are now underway.
In summary, we have established a model of articular cartilage repair in C57Bl/6 mice. This model replicates articular cartilage repair and should prove useful in analyzing the biological mechanisms underlying osteochondral repair.
Supplementary Material
Disclosure Statement
No competing financial interests exist.
References
- 1.Hjelle K., Solheim E., Strand T., Muri R., and Brittberg M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy 18, 730, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Widuchowski W., Widuchowski J., and Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee 14, 177, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Aroen A., Loken S., Heir S., Alvik E., Ekeland A., Granlund O.G., and Engebretsen L. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med 32, 211, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Heir S., Nerhus T.K., Rotterud J.H., Loken S., Ekeland A., Engebretsen L., and Aroen A. Focal cartilage defects in the knee impair quality of life as much as severe osteoarthritis: a comparison of knee injury and osteoarthritis outcome score in 4 patient categories scheduled for knee surgery. Am J Sports Med 38, 231, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Hunziker E.B. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage 10, 432, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Hunter W. On the structure and diseases of articulating cartilage. Philos Trans R Soc Lond 42b, 514, 1743 [Google Scholar]
- 7.Johnson K., Zhu S., Tremblay M.S., Payette J.N., Wang J., Bouchez L.C., Meeusen S., Althage A., Cho C.Y., Wu X., and Schultz P.G. A stem cell-based approach to cartilage repair. Science 336, 717, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Shapiro F., Koide S., and Glimcher M.J. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am 75, 532, 1993 [DOI] [PubMed] [Google Scholar]
- 9.Convery F.R., Akeson W.H., and Keown G.H. The repair of large osteochondral defects. An experimental study in horses. Clin Orthop Relat Res 82, 253, 1972 [PubMed] [Google Scholar]
- 10.Breinan H.A., Hsu H.P., and Spector M. Chondral defects in animal models: effects of selected repair procedures in canines. Clin Orthop Relat Res 391 Suppl, S219, 2001 [PubMed] [Google Scholar]
- 11.Eltawil N.M., De Bari C., Achan P., Pitzalis C., and Dell'accio F. A novel in vivo murine model of cartilage regeneration. Age and strain-dependent outcome after joint surface injury. Osteoarthritis Cartilage 17, 695, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitzgerald J., Rich C., Burkhardt D., Allen J., Herzka A.S., and Little C.B. Evidence for articular cartilage regeneration in MRL/MpJ mice. Osteoarthritis Cartilage 16, 1319, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Rai M.F., Hashimoto S., Johnson E.E., Janiszak K.L., Fitzgerald J., Heber-Katz E., Cheverud J.M., and Sandell L.J. Heritability of articular cartilage regeneration and its association with ear wound healing in mice. Arthritis Rheum 64, 2300, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei X., Gao J., and Messner K. Maturation-dependent repair of untreated osteochondral defects in the rabbit knee joint. J Biomed Mater Res 34, 63, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Wei X., and Messner K. Maturation-dependent durability of spontaneous cartilage repair in rabbit knee joint. J Biomed Mater Res 46, 539, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Chaudhury S. Mesenchymal stem cell applications to tendon healing. Muscles Ligaments Tendons J 2, 222, 2012 [PMC free article] [PubMed] [Google Scholar]
- 17.Kuo C.K., Petersen B.C., and Tuan R.S. Spatiotemporal protein distribution of TGF-betas, their receptors, and extracellular matrix molecules during embryonic tendon development. Dev Dyn 237, 1477, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obernier J.A., and Baldwin R.L. Establishing an appropriate period of acclimatization following transportation of laboratory animals. ILAR J 47, 364, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Wakitani S., Goto T., Pineda S.J., Young R.G., Mansour J.M., Caplan A.I., and Goldberg V.M. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am 76, 579, 1994 [DOI] [PubMed] [Google Scholar]
- 20.Buckwalter J.A. Articular cartilage injuries. Clin Orthop Relat Res 402, 21, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Ding C., Cicuttini F., Scott F., Cooley H., Boon C., and Jones G. Natural history of knee cartilage defects and factors affecting change. Arch Intern Med 166, 651, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Wang Y., Ding C., Wluka A.E., Davis S., Ebeling P.R., Jones G., and Cicuttini F.M. Factors affecting progression of knee cartilage defects in normal subjects over 2 years. Rheumatology (Oxford) 45, 79, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Rajnoch C., Ferguson S., Metcalfe A.D., Herrick S.E., Willis H.S., and Ferguson M.W. Regeneration of the ear after wounding in different mouse strains is dependent on the severity of wound trauma. Dev Dyn 226, 388, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Zhou F., He X., Iwakura Y., Horai R., and Stuart J.M. Arthritis in mice that are deficient in interleukin-1 receptor antagonist is dependent on genetic background. Arthritis Rheum 52, 3731, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Kench J.A., Russell D.M., Fadok V.A., Young S.K., Worthen G.S., Jones-Carson J., Henson J.E., Henson P.M., and Nemazee D. Aberrant wound healing and TGF-beta production in the autoimmune-prone MRL/+mouse. Clin Immunol 92, 300, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Heydemann A. The super super-healing MRL mouse strain. Front Biol (Beijing) 7, 522, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wooley P.H., Luthra H.S., Stuart J.M., and David C.S. Type II collagen-induced arthritis in mice. I. Major histocompatibility complex (I region) linkage and antibody correlates. J Exp Med 154, 688, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.