Abstract
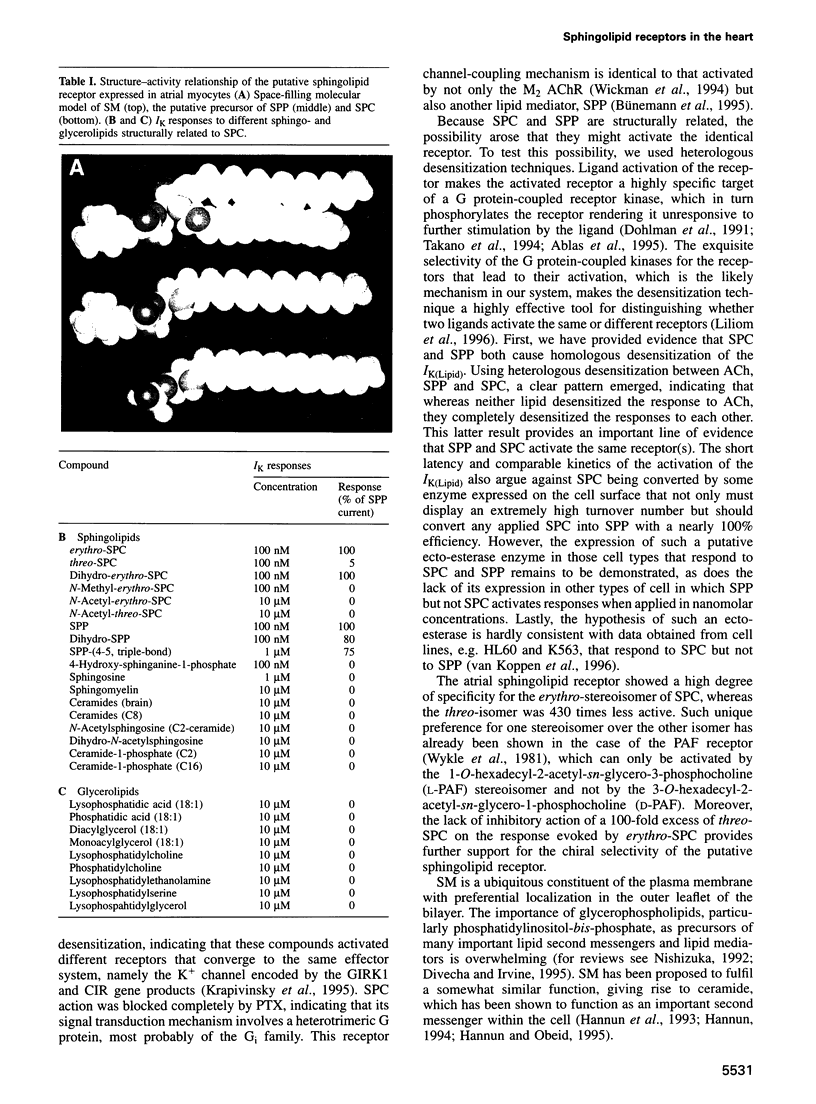
Activation of IK(ACh) is the major effect of the vagal neutrotransmitter acetylcholine in the heart. We report that both lysosphingomyelin (D-erythro-sphingosyl-phosphorylcholine; SPC) and sphingosine 1-phosphate (SPP) activate IK(ACh) in guinea pig atrial myocytes through the same receptor with an EC50 of 1.5 and 1.2 nM, respectively. Pertussis toxin abolished the activation of IK(ACh) by either lipid. The putative receptor showed an exquisite stereoselectivity for the naturally occurring D-erythro-(2S,3R)-SPC stereoisomer, the structure of which was confirmed by mass spectroscopy and NMR. These lipids caused complete homologous and heterologous desensitization with each other but not with ACh, indicating that both act on the same receptor. This receptor displays a distinct structure-activity relationship: it requires an unsubstituted amino group because N-acetyl-SPC, lysophosphatidic acid and lysophosphatidylcholine were inactive. Because SPP and SPC are naturally occurring products of membrane lipid metabolism, it appears that these compounds might be important extracellular mediators acting on a family of bona fide G protein-coupled receptors. Expression of these receptors in the heart raises the possibility that sphingolipids may be a part of the physiological and/or pathophysiological regulation of the heart. Based on their ligand selectivity we propose a classification of the sphingolipid receptors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alblas J., van Etten I., Khanum A., Moolenaar W. H. C-terminal truncation of the neurokinin-2 receptor causes enhanced and sustained agonist-induced signaling. Role of receptor phosphorylation in signal attenuation. J Biol Chem. 1995 Apr 14;270(15):8944–8951. doi: 10.1074/jbc.270.15.8944. [DOI] [PubMed] [Google Scholar]
- Banach K., Bünemann M., Hüser J., Pott L. Serum contains a potent factor that decreases beta-adrenergic receptor-stimulated L-type Ca2+ current in cardiac myocytes. Pflugers Arch. 1993 May;423(3-4):245–250. doi: 10.1007/BF00374402. [DOI] [PubMed] [Google Scholar]
- Banach K., Hüser J., Lipp P., Wellner M. C., Pott L. Activation of muscarinic K+ current in guinea-pig atrial myocytes by a serum factor. J Physiol. 1993 Feb;461:263–281. doi: 10.1113/jphysiol.1993.sp019513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bünemann M., Brandts B., zu Heringdorf D. M., van Koppen C. J., Jakobs K. H., Pott L. Activation of muscarinic K+ current in guinea-pig atrial myocytes by sphingosine-1-phosphate. J Physiol. 1995 Dec 15;489(Pt 3):701–707. doi: 10.1113/jphysiol.1995.sp021084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D. E. Direct G protein activation of ion channels? Annu Rev Neurosci. 1994;17:441–464. doi: 10.1146/annurev.ne.17.030194.002301. [DOI] [PubMed] [Google Scholar]
- Cohen R., Barenholz Y., Gatt S., Dagan A. Preparation and characterization of well defined D-erythro sphingomyelins. Chem Phys Lipids. 1984 Oct;35(4):371–384. doi: 10.1016/0009-3084(84)90079-3. [DOI] [PubMed] [Google Scholar]
- Desai N. N., Carlson R. O., Mattie M. E., Olivera A., Buckley N. E., Seki T., Brooker G., Spiegel S. Signaling pathways for sphingosylphosphorylcholine-mediated mitogenesis in Swiss 3T3 fibroblasts. J Cell Biol. 1993 Jun;121(6):1385–1395. doi: 10.1083/jcb.121.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai N. N., Spiegel S. Sphingosylphosphorylcholine is a remarkably potent mitogen for a variety of cell lines. Biochem Biophys Res Commun. 1991 Nov 27;181(1):361–366. doi: 10.1016/s0006-291x(05)81427-5. [DOI] [PubMed] [Google Scholar]
- Divecha N., Irvine R. F. Phospholipid signaling. Cell. 1995 Jan 27;80(2):269–278. doi: 10.1016/0092-8674(95)90409-3. [DOI] [PubMed] [Google Scholar]
- Dobrowsky R. T., Jenkins G. M., Hannun Y. A. Neurotrophins induce sphingomyelin hydrolysis. Modulation by co-expression of p75NTR with Trk receptors. J Biol Chem. 1995 Sep 22;270(38):22135–22142. doi: 10.1074/jbc.270.38.22135. [DOI] [PubMed] [Google Scholar]
- Dobrowsky R. T., Werner M. H., Castellino A. M., Chao M. V., Hannun Y. A. Activation of the sphingomyelin cycle through the low-affinity neurotrophin receptor. Science. 1994 Sep 9;265(5178):1596–1599. doi: 10.1126/science.8079174. [DOI] [PubMed] [Google Scholar]
- Dohlman H. G., Thorner J., Caron M. G., Lefkowitz R. J. Model systems for the study of seven-transmembrane-segment receptors. Annu Rev Biochem. 1991;60:653–688. doi: 10.1146/annurev.bi.60.070191.003253. [DOI] [PubMed] [Google Scholar]
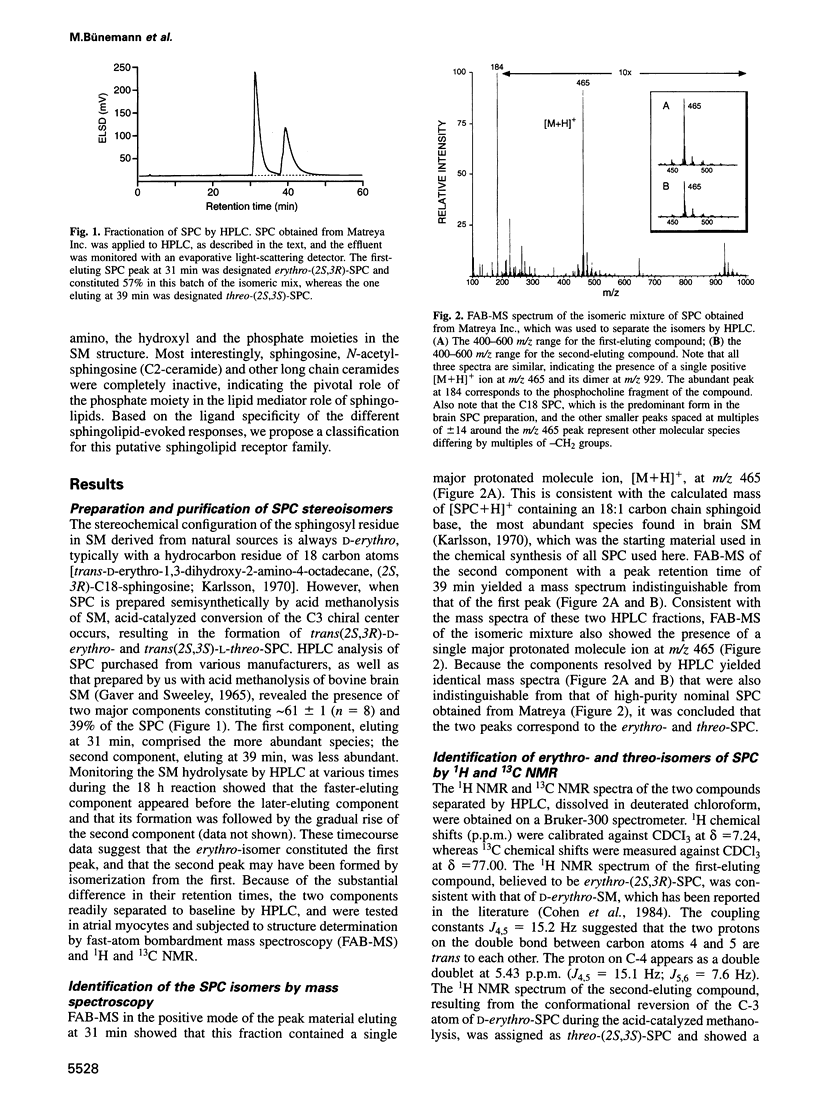
- GAVER R. C., SWEELEY C. C. METHODS FOR METHANOLYSIS OF SPHINGOLIPIDS AND DIRECT DETERMINATION OF LONG-CHAIN BASES BY GAS CHROMATOGRAPHY. J Am Oil Chem Soc. 1965 Apr;42:294–298. doi: 10.1007/BF02540132. [DOI] [PubMed] [Google Scholar]
- Goodemote K. A., Mattie M. E., Berger A., Spiegel S. Involvement of a pertussis toxin-sensitive G protein in the mitogenic signaling pathways of sphingosine 1-phosphate. J Biol Chem. 1995 Apr 28;270(17):10272–10277. doi: 10.1074/jbc.270.17.10272. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hannun Y. A., Bell R. M. Lysosphingolipids inhibit protein kinase C: implications for the sphingolipidoses. Science. 1987 Feb 6;235(4789):670–674. doi: 10.1126/science.3101176. [DOI] [PubMed] [Google Scholar]
- Hannun Y. A., Obeid L. M. Ceramide: an intracellular signal for apoptosis. Trends Biochem Sci. 1995 Feb;20(2):73–77. doi: 10.1016/s0968-0004(00)88961-6. [DOI] [PubMed] [Google Scholar]
- Hannun Y. A., Obeid L. M., Wolff R. A. The novel second messenger ceramide: identification, mechanism of action, and cellular activity. Adv Lipid Res. 1993;25:43–64. [PubMed] [Google Scholar]
- Hannun Y. A. The sphingomyelin cycle and the second messenger function of ceramide. J Biol Chem. 1994 Feb 4;269(5):3125–3128. [PubMed] [Google Scholar]
- Jalink K., Hengeveld T., Mulder S., Postma F. R., Simon M. F., Chap H., van der Marel G. A., van Boom J. H., van Blitterswijk W. J., Moolenaar W. H. Lysophosphatidic acid-induced Ca2+ mobilization in human A431 cells: structure-activity analysis. Biochem J. 1995 Apr 15;307(Pt 2):609–616. doi: 10.1042/bj3070609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson K. A. On the chemistry and occurrence of sphingolipid long-chain bases. Chem Phys Lipids. 1970 Oct;5(1):6–43. doi: 10.1016/0009-3084(70)90008-3. [DOI] [PubMed] [Google Scholar]
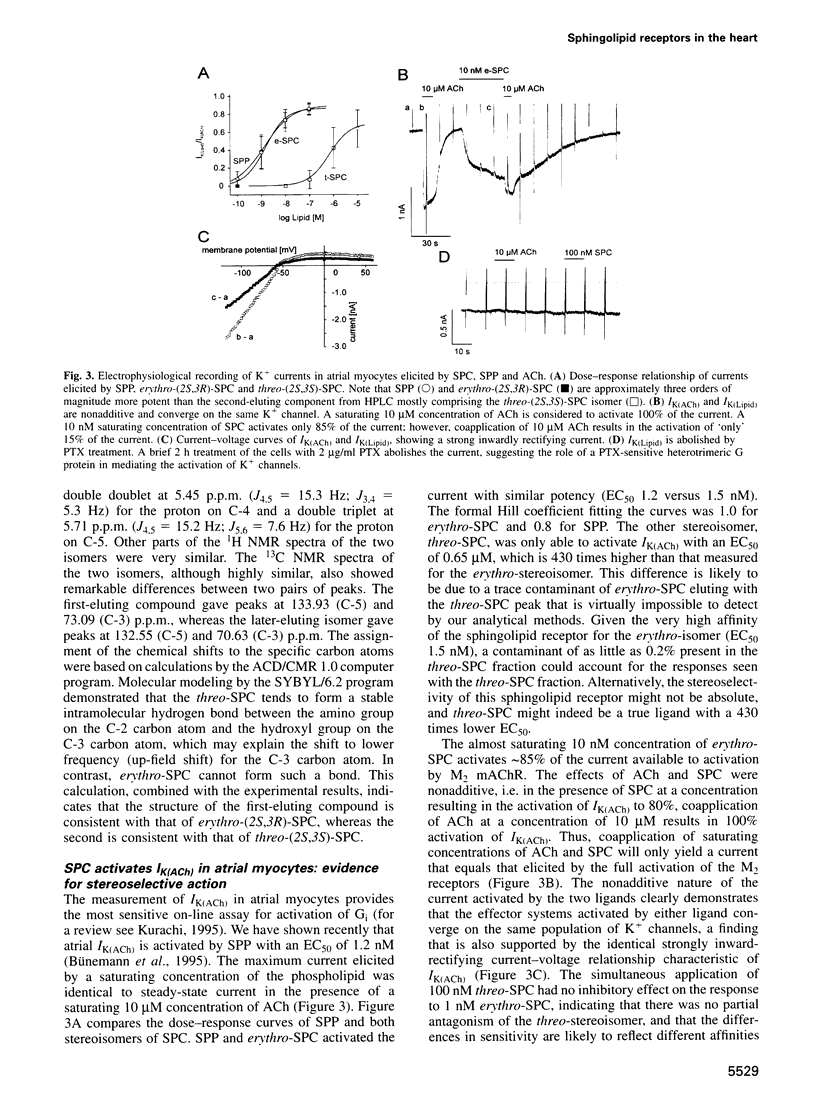
- Krapivinsky G., Gordon E. A., Wickman K., Velimirović B., Krapivinsky L., Clapham D. E. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K(+)-channel proteins. Nature. 1995 Mar 9;374(6518):135–141. doi: 10.1038/374135a0. [DOI] [PubMed] [Google Scholar]
- Liliom K., Murakami-Murofushi K., Kobayashi S., Murofushi H., Tigyi G. Xenopus oocytes express multiple receptors for LPA-like lipid mediators. Am J Physiol. 1996 Mar;270(3 Pt 1):C772–C777. doi: 10.1152/ajpcell.1996.270.3.C772. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992 Oct 23;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Obeid L. M., Hannun Y. A. Ceramide: a stress signal and mediator of growth suppression and apoptosis. J Cell Biochem. 1995 Jun;58(2):191–198. doi: 10.1002/jcb.240580208. [DOI] [PubMed] [Google Scholar]
- Okajima F., Kondo Y. Pertussis toxin inhibits phospholipase C activation and Ca2+ mobilization by sphingosylphosphorylcholine and galactosylsphingosine in HL60 leukemia cells. Implications of GTP-binding protein-coupled receptors for lysosphingolipids. J Biol Chem. 1995 Nov 3;270(44):26332–26340. doi: 10.1074/jbc.270.44.26332. [DOI] [PubMed] [Google Scholar]
- Olivera A., Zhang H., Carlson R. O., Mattie M. E., Schmidt R. R., Spiegel S. Stereospecificity of sphingosine-induced intracellular calcium mobilization and cellular proliferation. J Biol Chem. 1994 Jul 8;269(27):17924–17930. [PubMed] [Google Scholar]
- Postma F. R., Jalink K., Hengeveld T., Moolenaar W. H. Sphingosine-1-phosphate rapidly induces Rho-dependent neurite retraction: action through a specific cell surface receptor. EMBO J. 1996 May 15;15(10):2388–2392. [PMC free article] [PubMed] [Google Scholar]
- Pushkareva MYu, Hannun Y. A. Modulation of cytosolic protein phosphorylation by sphingosylphosphorylcholine. Biochim Biophys Acta. 1994 Mar 10;1221(1):54–60. doi: 10.1016/0167-4889(94)90215-1. [DOI] [PubMed] [Google Scholar]
- Pushkareva M., Obeid L. M., Hannun Y. A. Ceramide: an endogenous regulator of apoptosis and growth suppression. Immunol Today. 1995 Jun;16(6):294–297. doi: 10.1016/0167-5699(95)80184-7. [DOI] [PubMed] [Google Scholar]
- Seufferlein T., Rozengurt E. Sphingosylphosphorylcholine activation of mitogen-activated protein kinase in Swiss 3T3 cells requires protein kinase C and a pertussis toxin-sensitive G protein. J Biol Chem. 1995 Oct 13;270(41):24334–24342. doi: 10.1074/jbc.270.41.24334. [DOI] [PubMed] [Google Scholar]
- Seufferlein T., Rozengurt E. Sphingosylphosphorylcholine rapidly induces tyrosine phosphorylation of p125FAK and paxillin, rearrangement of the actin cytoskeleton and focal contact assembly. Requirement of p21rho in the signaling pathway. J Biol Chem. 1995 Oct 13;270(41):24343–24351. doi: 10.1074/jbc.270.41.24343. [DOI] [PubMed] [Google Scholar]
- Spiegel S., Milstien S. Sphingolipid metabolites: members of a new class of lipid second messengers. J Membr Biol. 1995 Aug;146(3):225–237. doi: 10.1007/BF00233943. [DOI] [PubMed] [Google Scholar]
- Takano T., Honda Z., Sakanaka C., Izumi T., Kameyama K., Haga K., Haga T., Kurokawa K., Shimizu T. Role of cytoplasmic tail phosphorylation sites of platelet-activating factor receptor in agonist-induced desensitization. J Biol Chem. 1994 Sep 2;269(35):22453–22458. [PubMed] [Google Scholar]
- Wickman K. D., Iñiguez-Lluhl J. A., Davenport P. A., Taussig R., Krapivinsky G. B., Linder M. E., Gilman A. G., Clapham D. E. Recombinant G-protein beta gamma-subunits activate the muscarinic-gated atrial potassium channel. Nature. 1994 Mar 17;368(6468):255–257. doi: 10.1038/368255a0. [DOI] [PubMed] [Google Scholar]
- Wu J., Spiegel S., Sturgill T. W. Sphingosine 1-phosphate rapidly activates the mitogen-activated protein kinase pathway by a G protein-dependent mechanism. J Biol Chem. 1995 May 12;270(19):11484–11488. doi: 10.1074/jbc.270.19.11484. [DOI] [PubMed] [Google Scholar]
- Wykle R. L., Miller C. H., Lewis J. C., Schmitt J. D., Smith J. A., Surles J. R., Piantadosi C., O'Flaherty J. T. Stereospecific activity of 1-O-alkyl-2-O-acetyl-sn-glycero-3-phosphocholine and comparison of analogs in the degranulation of platelets and neutrophils. Biochem Biophys Res Commun. 1981 Jun;100(4):1651–1658. doi: 10.1016/0006-291x(81)90708-7. [DOI] [PubMed] [Google Scholar]
- Yang Z., Costanzo M., Golde D. W., Kolesnick R. N. Tumor necrosis factor activation of the sphingomyelin pathway signals nuclear factor kappa B translocation in intact HL-60 cells. J Biol Chem. 1993 Sep 25;268(27):20520–20523. [PubMed] [Google Scholar]
- Yatomi Y., Ruan F., Hakomori S., Igarashi Y. Sphingosine-1-phosphate: a platelet-activating sphingolipid released from agonist-stimulated human platelets. Blood. 1995 Jul 1;86(1):193–202. [PubMed] [Google Scholar]
- van Koppen C., Meyer zu Heringdorf M., Laser K. T., Zhang C., Jakobs K. H., Bünemann M., Pott L. Activation of a high affinity Gi protein-coupled plasma membrane receptor by sphingosine-1-phosphate. J Biol Chem. 1996 Jan 26;271(4):2082–2087. doi: 10.1074/jbc.271.4.2082. [DOI] [PubMed] [Google Scholar]