Abstract
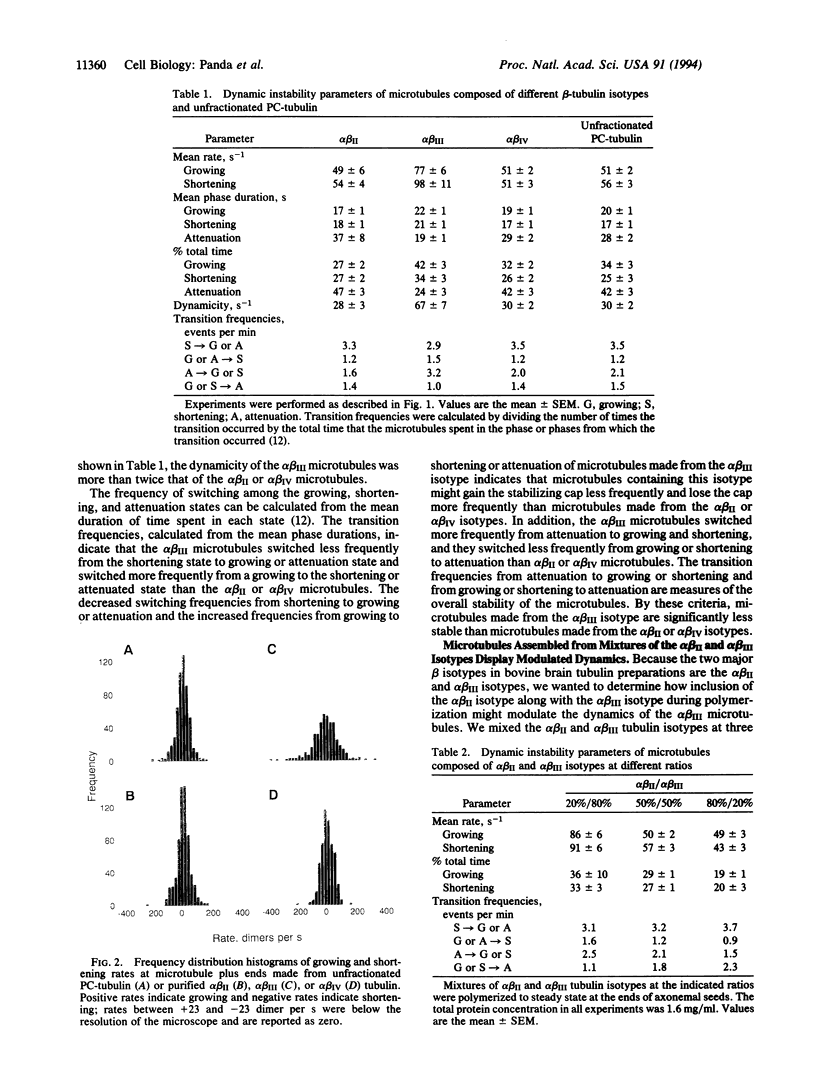
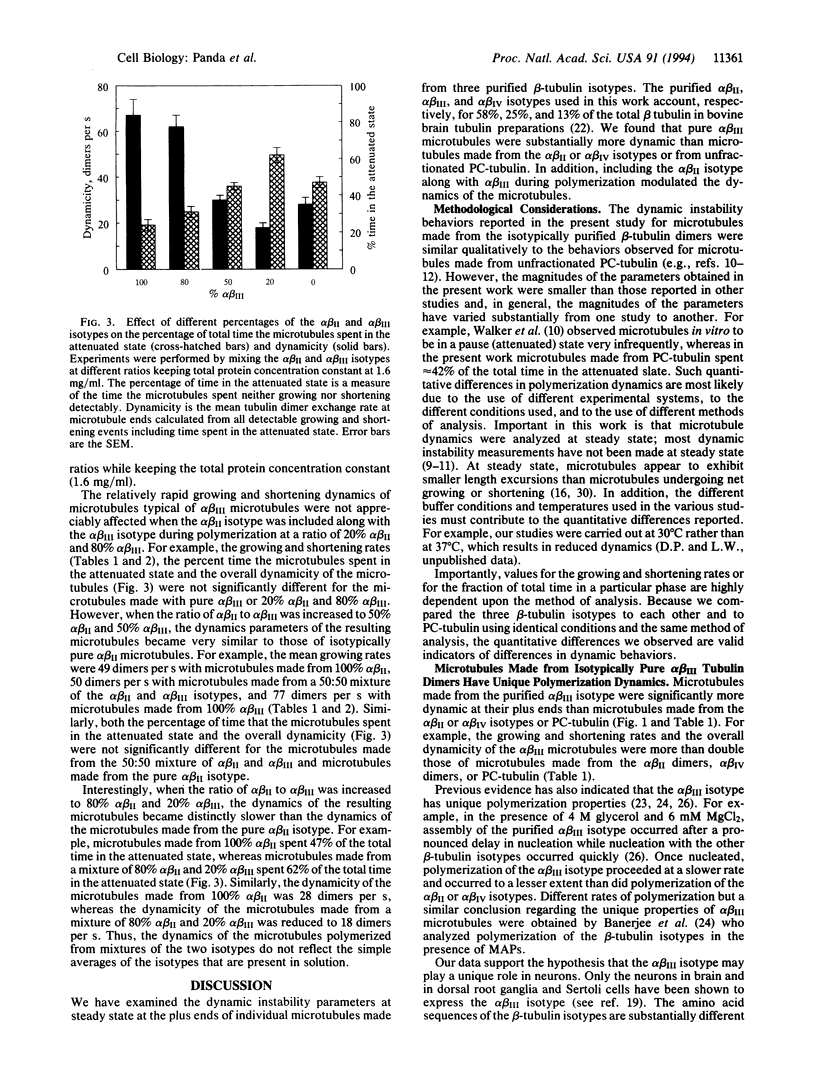
The growing and shortening dynamics of individual bovine brain microtubules at their plus ends at steady state in vitro, assembled from isotypically pure alpha beta II, alpha beta III, or alpha beta IV tubulin dimers, were determined by differential interference contrast video microscopy. Microtubules assembled from the purified alpha beta III isotype were considerably more dynamic than microtubules made from the alpha beta II or alpha beta IV isotypes or from unfractionated phosphocellulose-purified tubulin. Furthermore, increasing the proportion of the alpha beta II isotype in a mixture of the alpha beta II and alpha beta III isotypes suppressed microtubule dynamics, demonstrating that microtubule dynamics can be influenced by the tubulin isotype composition. The data support the hypothesis that cells might determine the dynamic properties and functions of its microtubules in part by altering the relative amounts of the different tubulin isotypes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. E., Hunt D. F., Lee M. K., Shabanowitz J., Michel H., Berlin S. C., MacDonald T. L., Sundberg R. J., Rebhun L. I., Frankfurter A. Characterization of posttranslational modifications in neuron-specific class III beta-tubulin by mass spectrometry. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4685–4689. doi: 10.1073/pnas.88.11.4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A., Luduena R. F. Distinct colchicine binding kinetics of bovine brain tubulin lacking the type III isotype of beta-tubulin. J Biol Chem. 1991 Jan 25;266(3):1689–1691. [PubMed] [Google Scholar]
- Banerjee A., Luduena R. F. Kinetics of colchicine binding to purified beta-tubulin isotypes from bovine brain. J Biol Chem. 1992 Jul 5;267(19):13335–13339. [PubMed] [Google Scholar]
- Banerjee A., Roach M. C., Trcka P., Luduena R. F. Preparation of a monoclonal antibody specific for the class IV isotype of beta-tubulin. Purification and assembly of alpha beta II, alpha beta III, and alpha beta IV tubulin dimers from bovine brain. J Biol Chem. 1992 Mar 15;267(8):5625–5630. [PubMed] [Google Scholar]
- Banerjee A., Roach M. C., Trcka P., Ludueña R. F. Increased microtubule assembly in bovine brain tubulin lacking the type III isotype of beta-tubulin. J Biol Chem. 1990 Jan 25;265(3):1794–1799. [PubMed] [Google Scholar]
- Belmont L. D., Hyman A. A., Sawin K. E., Mitchison T. J. Real-time visualization of cell cycle-dependent changes in microtubule dynamics in cytoplasmic extracts. Cell. 1990 Aug 10;62(3):579–589. doi: 10.1016/0092-8674(90)90022-7. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cross D., Dominguez J., Maccioni R. B., Avila J. MAP-1 and MAP-2 binding sites at the C-terminus of beta-tubulin. Studies with synthetic tubulin peptides. Biochemistry. 1991 Apr 30;30(17):4362–4366. doi: 10.1021/bi00231a036. [DOI] [PubMed] [Google Scholar]
- Falconer M. M., Echeverri C. J., Brown D. L. Differential sorting of beta tubulin isotypes into colchicine-stable microtubules during neuronal and muscle differentiation of embryonal carcinoma cells. Cell Motil Cytoskeleton. 1992;21(4):313–325. doi: 10.1002/cm.970210407. [DOI] [PubMed] [Google Scholar]
- Farrell K. W., Jordan M. A., Miller H. P., Wilson L. Phase dynamics at microtubule ends: the coexistence of microtubule length changes and treadmilling. J Cell Biol. 1987 Apr;104(4):1035–1046. doi: 10.1083/jcb.104.4.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gildersleeve R. F., Cross A. R., Cullen K. E., Fagen A. P., Williams R. C., Jr Microtubules grow and shorten at intrinsically variable rates. J Biol Chem. 1992 Apr 25;267(12):7995–8006. [PubMed] [Google Scholar]
- Hotani H., Horio T. Dynamics of microtubules visualized by darkfield microscopy: treadmilling and dynamic instability. Cell Motil Cytoskeleton. 1988;10(1-2):229–236. doi: 10.1002/cm.970100127. [DOI] [PubMed] [Google Scholar]
- Hoyle H. D., Raff E. C. Two Drosophila beta tubulin isoforms are not functionally equivalent. J Cell Biol. 1990 Sep;111(3):1009–1026. doi: 10.1083/jcb.111.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hush J. M., Wadsworth P., Callaham D. A., Hepler P. K. Quantification of microtubule dynamics in living plant cells using fluorescence redistribution after photobleaching. J Cell Sci. 1994 Apr;107(Pt 4):775–784. doi: 10.1242/jcs.107.4.775. [DOI] [PubMed] [Google Scholar]
- Jordan M. A., Thrower D., Wilson L. Effects of vinblastine, podophyllotoxin and nocodazole on mitotic spindles. Implications for the role of microtubule dynamics in mitosis. J Cell Sci. 1992 Jul;102(Pt 3):401–416. doi: 10.1242/jcs.102.3.401. [DOI] [PubMed] [Google Scholar]
- Littauer U. Z., Giveon D., Thierauf M., Ginzburg I., Ponstingl H. Common and distinct tubulin binding sites for microtubule-associated proteins. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7162–7166. doi: 10.1073/pnas.83.19.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q., Luduena R. F. In vitro analysis of microtubule assembly of isotypically pure tubulin dimers. Intrinsic differences in the assembly properties of alpha beta II, alpha beta III, and alpha beta IV tubulin dimers in the absence of microtubule-associated proteins. J Biol Chem. 1994 Jan 21;269(3):2041–2047. [PubMed] [Google Scholar]
- Ludueña R. F. Are tubulin isotypes functionally significant. Mol Biol Cell. 1993 May;4(5):445–457. doi: 10.1091/mbc.4.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzati M., Clavilier L., Pere-Aubert G., Slonimski P. P. Contrôle biochimique de la recombinaison chez Saccharomyces cerevisiae. I. Inhibition par la L-histidine de la recombinaison intragenique mitotique au locus ad 3. Mol Gen Genet. 1971;111(2):120–137. doi: 10.1007/BF00267787. [DOI] [PubMed] [Google Scholar]
- Margolis R. L., Wilson L. Microtubule treadmills--possible molecular machinery. Nature. 1981 Oct 29;293(5835):705–711. doi: 10.1038/293705a0. [DOI] [PubMed] [Google Scholar]
- Margolis R. L., Wilson L. Opposite end assembly and disassembly of microtubules at steady state in vitro. Cell. 1978 Jan;13(1):1–8. doi: 10.1016/0092-8674(78)90132-0. [DOI] [PubMed] [Google Scholar]
- Matthews K. A., Rees D., Kaufman T. C. A functionally specialized alpha-tubulin is required for oocyte meiosis and cleavage mitoses in Drosophila. Development. 1993 Mar;117(3):977–991. doi: 10.1242/dev.117.3.977. [DOI] [PubMed] [Google Scholar]
- Mitchison T., Kirschner M. Microtubule assembly nucleated by isolated centrosomes. Nature. 1984 Nov 15;312(5991):232–237. doi: 10.1038/312232a0. [DOI] [PubMed] [Google Scholar]
- Saxton W. M., Stemple D. L., Leslie R. J., Salmon E. D., Zavortink M., McIntosh J. R. Tubulin dynamics in cultured mammalian cells. J Cell Biol. 1984 Dec;99(6):2175–2186. doi: 10.1083/jcb.99.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano L., de la Torre J., Maccioni R. B., Avila J. Involvement of the carboxyl-terminal domain of tubulin in the regulation of its assembly. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5989–5993. doi: 10.1073/pnas.81.19.5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelden E., Wadsworth P. Observation and quantification of individual microtubule behavior in vivo: microtubule dynamics are cell-type specific. J Cell Biol. 1993 Feb;120(4):935–945. doi: 10.1083/jcb.120.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan K. F., Cleveland D. W. Identification of conserved isotype-defining variable region sequences for four vertebrate beta tubulin polypeptide classes. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4327–4331. doi: 10.1073/pnas.83.12.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toso R. J., Jordan M. A., Farrell K. W., Matsumoto B., Wilson L. Kinetic stabilization of microtubule dynamic instability in vitro by vinblastine. Biochemistry. 1993 Feb 9;32(5):1285–1293. doi: 10.1021/bi00056a013. [DOI] [PubMed] [Google Scholar]
- Villasante A., Wang D., Dobner P., Dolph P., Lewis S. A., Cowan N. J. Six mouse alpha-tubulin mRNAs encode five distinct isotypes: testis-specific expression of two sister genes. Mol Cell Biol. 1986 Jul;6(7):2409–2419. doi: 10.1128/mcb.6.7.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. A., O'Brien E. T., Pryer N. K., Soboeiro M. F., Voter W. A., Erickson H. P., Salmon E. D. Dynamic instability of individual microtubules analyzed by video light microscopy: rate constants and transition frequencies. J Cell Biol. 1988 Oct;107(4):1437–1448. doi: 10.1083/jcb.107.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]




