Abstract
There are no clinical guidelines addressing the management of depression after traumatic brain injury (TBI). The objectives of this study were to (1) describe depression treatment patterns among Medicare beneficiaries with a diagnosis of depression post-TBI; (2) compare them with depression treatment patterns among beneficiaries with a diagnosis of depression pre-TBI; and (3) quantify the difference in prevalence of use. We conducted a retrospective analysis of Medicare beneficiaries hospitalized with TBI during 2006–2010. We created two cohorts: beneficiaries with a new diagnosis of depression pre-TBI (n=4841) and beneficiaries with a new diagnosis of depression post-TBI (n=4668). We searched for antidepressant medications in Medicare Part D drug event files and created variables indicating antidepressant use in each 30-day period after diagnosis of depression. We used provider specialty and current procedural terminology to identify psychotherapy in any location. We used generalized estimating equations to quantify the effect of TBI on receipt of depression treatment during the year after diagnosis of depression. Average monthly prevalence of antidepressant use was 42% among beneficiaries with a diagnosis of depression pre-TBI and 36% among those with a diagnosis post-TBI (p<0.001). Beneficiaries with a diagnosis of depression post-TBI were less likely to receive antidepressants compared with a depression diagnosis pre-TBI (adjusted odds ratio [OR] 0.87; 95% confidence interval [CI] 0.82, 0.92). There was no difference in receipt of psychotherapy between the two groups (OR 1.08; 95% CI 0.93, 1.26). Depression after TBI is undertreated among older adults. Knowledge about reasons for this disparity and its long-term effects on post-TBI outcomes is limited and should be examined in future work.
Key words: : antidepressants, depression, older adults, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a significant health problem among older adults that results in increased cognitive and functional disability, nursing home placement, healthcare utilization, and mortality.1–8 Depression is common after TBI, with prevalence in older adults ranging from 10–42%.9–14 In addition to causing significant morbidity on its own, depression is also associated with decreased patient adherence to medical regimens, which may further exacerbate neuropsychological impairment and slow the pace of cognitive recovery after TBI.10,15–18
Despite the potential health impact of depression after TBI, there is a paucity of evidence-based studies to guide the treatment of depression post-TBI.19,20 Because of the multifactorial biological and psychosocial contributors to depression post-TBI, standard antidepressant medications may not have the same effectiveness and tolerability in patients with TBI as in patients without neurological insult. As a result, antidepressant medication in this population is considered off-label.18,20 Psychotherapy is effective in the treatment of geriatric depression and may provide benefit following TBI but to date there have been very few investigations focusing on psychotherapy for the treatment of depression post-TBI.20,21
While there is a lack of adequate randomized controlled studies of depression post-TBI, a few small open-label studies conducted in younger patients with TBI suggest that selective serotonin reuptake inhibitors (SSRIs) are both effective and well-tolerated in management of depression, while tricyclic antidepressants (TCAs) are not well-tolerated in this population, with concerns raised both with regard to treatment resistance and seizure rates.20,22–26 There are no such studies among older adults in whom depression develops post-TBI, and the convergence of age-associated characteristics (e.g., reduced cognition, multimorbidity) and the physiological effects of the injured brain may render this population more susceptible to adverse drug events and therefore affect physician choice to prescribe antidepressants as well as the class of antidepressant used.
Documenting current patterns of depression treatment among older adults with TBI will provide valuable information regarding treatment decisions and a basis for comparative effectiveness studies. The objectives of this study were to describe antidepressant and psychotherapy usage patterns in a national sample of Medicare beneficiaries with a new diagnosis of depression post-TBI, compare them to usage patterns among a pre-TBI cohort of Medicare beneficiaries with a new diagnosis of depression, and quantify the difference in prevalence of use. Given the lack of guidance regarding depression treatment post-TBI and the reduced risk of adverse events associated with psychotherapy, we hypothesized that those with a diagnosis of depression post-TBI would be less likely to use antidepressants but more likely to use psychotherapy compared with the pre-TBI cohort.
Methods
Study design
We conducted a retrospective cohort study of Medicare administrative claims data from 2006–2010 to describe depression treatment patterns and quantify differences in the prevalence of depression treatment between Medicare beneficiaries with a new diagnosis of depression after hospital discharge for TBI and a pre-TBI cohort of Medicare beneficiaries with a new diagnosis of depression. The pre-TBI cohort comprised Medicare beneficiaries in whom depression developed during the study period but before hospitalization for TBI. Medicare beneficiaries who experience TBI have a greater burden of comorbid illness compared with the average Medicare beneficiary.27 This unique study design allowed comparison of two cohorts with similar demographic and clinical characteristics.
Data source
Medicare administrative data obtained from the Centers for Medicare & Medicaid Services (CMS) Chronic Condition Data Warehouse (CCW) were the primary sources of data for this study. Medicare is a federal health insurance program in the United States that covers 93% of persons aged 65 and older. Part A covers hospitalizations, skilled nursing facility (SNF) stays under 101 days, home health, and hospice care. Part B covers most outpatient care. Part D covers prescription medications. Part C, also known as Medicare Advantage, allows private insurers to provide Medicare benefits, typically with Parts A, B, and D bundled together. The percentage of Medicare beneficiaries enrolled in a Medicare Advantage plan increased from 16% to 24% during the study period.28
Study sample
We used the Centers for Disease Control and Prevention's (CDC) case definition for TBI, which previously has been reported to have a sensitivity of 89% to detect severe TBI and a positive predictive value of 93% to correctly identify TBI-related hospitalizations.29,30 The dataset comprised all Medicare beneficiaries with a hospital discharge diagnosis of TBI (International Classification of Disease, 9th Revision Clinical Modification [ICD-9CM] codes 800.xx, 801.xx, 803.xx, 804.xx, 850.xx- 854.1x, 950.1-950.3, 959.01) in any position on an inpatient claim between 1/1/2006 and 12/31/2010. This study was approved by the Institutional Review Board of the University of Maryland, Baltimore.
Inclusion criteria were: age ≥65 years at the time of TBI, 6 months of continuous Medicare Parts A and B with no Part C (Medicare Advantage) coverage before hospitalization for TBI, no depression diagnosis recorded in Medicare claims before the start of the study period (1/1/2006), 12 months of continuous Medicare Parts A and B with no Part C coverage before first depression diagnosis, and 6 months continuous Part D coverage with no antidepressant use before first depression diagnosis. The last two criteria were applied to isolate newly diagnosed depression so that we could compare antidepressant usage patterns during the year after first diagnosis of depression. Beneficiaries with a Medicare Advantage plan were excluded because these plans are not required to file claims for medical services.
The CCW contains information on 27 comorbid conditions including depression.31 The CCW contains an annual flag for each condition meeting CCW-defined criteria for that year as well as the date of first diagnosis. Only one date of first diagnosis is noted regardless of how many diagnoses have been made. Depression is defined in the CCW by the presence of any of the following ICD-9 codes: 296.20, 296.21, 296.22, 296.23, 296.24, 296.25, 296.26, 296.30, 296.31, 296.32, 296.33, 296.34, 296.35,296.36, 296.51, 296.52, 296.53, 296.54, 296.55, 296.56, 296.60, 296.61, 296.62, 296.63, 296.64, 296.65, 296.66, 296.89, 298.0, 300.4, 309.1, or 311 in any position on any inpatient, SNF, home health care, hospice, or Carrier (outpatient) claim.
We created two nonoverlapping cohorts of beneficiaries: those in whom depression developed during the study period but before experiencing a TBI and those in whom depression developed post-TBI. We defined incident depression pre-TBI as the date of first diagnosis of depression occurring after the start of the study period (1/1/2006) and before the hospital admission date for TBI. We defined incident depression post-TBI as the date of first diagnosis of depression occurring after the hospital discharge date for TBI but before the end of the study period (12/31/10).
Antidepressant medications
We searched for antidepressant medications in the Medicare Part D prescription drug events file and defined antidepressant therapy as a medication prescription event involving at least one of the medications listed below. We initially created seven categories of antidepressant medications: (1) SSRI (citalopram, escitalopram, escitalopram oxalate, fluoxetine, fluvoxamine, paroxetine, and sertraline); (2) TCAs (amitriptyline, amoxapine, clomipramine, desipramine, doxepin, imipramine, nortriptyline, protriptyline, and trimipramine); (3) serotonin-norepinephrine reuptake inhibitors (desvenlafaxine, duloxetine, and venlafaxine); (4) monoamine oxidase inhibitors (isocarboxazid, phenelzine, and tranylcypromine); (5) phenylpiperazine antidepressants (nefazodone and trazodone); (6) tetracyclic antidepressants (maprotiline and mirtazapine), and (7) buproprion.
SSRIs accounted for more than 75% of the antidepressants in our sample; therefore, we created an “other antidepressant” (OAD) category composed of all antidepressants except SSRIs. We also created an “any” antidepressant variable. We divided beneficiary follow-up time into 30-day periods starting the date of depression diagnosis, and recorded filled prescriptions and proportion of days covered (number of daily doses in the prescription/number of days in the period) for all antidepressants in each 30-day period with Part D coverage. Because some prescriptions are written for 90 rather than 30 days, it is possible for a beneficiary to have no prescription fills during a period but still have a proportion of days covered by that prescription greater than zero.
Antidepressant use during each 30-day period post-depression diagnosis was defined as either a filled prescription for an antidepressant or a proportion of days covered by an antidepressant greater than 0. Missing antidepressant information could result from a lapse in Medicare Part D coverage or SNF or hospital stays. We did not require Medicare Part D coverage in each period, but rather report prevalence of antidepressant use as a percentage of the number of depression cases in each 30-day period without missing data.
We were interested in comparing antidepressant management of depression between a cohort of beneficiaries with a diagnosis of depression post-TBI and a pre-TBI incident depression cohort with similar demographic and clinical characteristics. Beneficiaries with a diagnosis of depression during the study period but before the TBI hospitalization formed an excellent comparison group. To maintain independence of the cohorts, beneficiaries with a diagnosis of depression pre-TBI were no longer followed once they were hospitalized for TBI.
Depression diagnoses occurred at different times during the study period. To accurately report antidepressant use among incident depression patients, we created a person-month file with an indicator variable for incident depression pre- or post-TBI and an indicator for diagnosis of depression in each 30-day period (1 month).
Nonpharmacological management of depression
We searched Medicare Parts A & B claims for events associated with non-pharmacologic management of depression and created two variables representing treatment: hospitalization and psychotherapy. We identified hospitalizations with a primary diagnosis of depression using ICD-9 codes 296.2x, 296.3x, or 311.xx. We identified admission to psychiatric hospitals using provider identification codes and admission to a psychiatric unit using revenue center codes (0114, 0124, 0134, 0144, 0154, 0204). For psychiatric hospital and psychiatric unit stays, we further required a primary or a secondary diagnosis of depression. We combined the three types of hospitalizations (inpatient admission with depression diagnosis, psychiatric hospital admission, and admission to the psychiatric unit) into a variable indicating any psychiatric hospitalization.
We used provider specialty (26,27,62,68,86) and current procedural terminology (CPT) codes (90804–90829, 90845,90847, 90849, 90853, 90857, 90865, 90870, G0409, G0410, G0411) to identify mental health services/psychotherapy in any location (inpatient, outpatient, SNF, hospice, or home health). We merged all treatment events into our person-month file. Finally, we created an indicator variable for “any” treatment of depression, which included antidepressants, psychotherapy, or psychiatric hospitalization.
Covariates
Demographic characteristics were obtained from the CCW enrollment file. Baseline comorbidities at TBI hospitalization were determined using CMS's CCW flagged comorbid conditions.31 These 27 chronic conditions are identified based on the presence of ICD-9 codes on inpatient, skilled nursing, home health, or outpatient claims using algorithms defined by CMS. If the date of first diagnosis of a particular chronic condition was before the date of depression diagnosis, the patient was considered to have that chronic condition at baseline. TBI hospitalization-associated variables included length of hospital stay and discharge to a SNF.
Data analysis
Follow-up time post-depression diagnosis was divided into 30-day periods as previously explained. Beneficiaries were censored at the date of first TBI (for those diagnosed with depression pre-TBI), death, or the end of the study period.
We examined the frequency of baseline and TBI hospitalization-associated characteristics in the total sample and by incident depression status. For each category of antidepressants, we determined the prevalence of antidepressant medication use in each 30-day period over the year after first depression diagnosis among beneficiaries with a diagnosis of depression before and after TBI and report the average monthly prevalence of use. We also examined prevalence of use among individual antidepressant classes. We compared the prevalence of antidepressant use between beneficiaries with a diagnosis of depression pre- and post-TBI and generated p values for comparisons using chi-square tests.
Similarly, we determined the average monthly prevalence of psychotherapy and any treatment for depression in each 30-day period over the year after first depression diagnosis among beneficiaries with a diagnosis of depression before and after TBI and report the prevalence of treatment. We compared the prevalence of depression treatment between beneficiaries with a diagnosis of depression pre- and post-TBI using chi-square tests.
To quantify the effect of TBI on receipt of antidepressant treatment over the year after diagnosis of depression, we used generalized estimating equations with a logistic model to accommodate the correlation between repeated measurements. We used bivariate analysis to identify potential confounders for inclusion in our models. Covariates associated with both the dependent (receipt of treatment for depression) and independent (timing of depression diagnosis) variables at the p<0.001 level were considered for inclusion.
We ran separate models for any antidepressant, SSRIs, and OADs. We tested interactions of time since depression diagnosis, age, race, and sex with the independent variable. Similarly, we constructed regression models for psychotherapy and “any” treatment of depression. We conducted sensitivity analyses to examine the robustness of our results to deviations from our assumptions. These analyses included limiting to beneficiaries with continuous Part D coverage during follow-up post-depression diagnosis, excluding beneficiaries with any antidepressant use 12 months before diagnosis of depression, and limiting follow-up time post-depression diagnosis to 6 months.
Because of large sample size, statistical significance was defined a priori as p<0.001. All analysis was performed with SAS version 9.2 (SAS Institute, Cary, NC).
Results
Between 2006 and 2010, 115,334 Medicare beneficiaries with at least 6 months of continuous Medicare Parts A and B and no Part C (Medicare Advantage) coverage were hospitalized for TBI and 105,432 (91%) survived to hospital discharge. We excluded 33,003 (31%) who had a diagnosis of depression before the start of the study period (1/1/2006), 4729 (4%) without 12 months of Medicare Parts A and B with no Part C before diagnosis of depression, and 11,972 (11%) who either did not have 6 continuous months of Medicare Part D coverage or used antidepressants during the 6 months before diagnosis of depression.
Our final study sample contained 55,728 beneficiaries. Average age was 80.9 (standard deviation [SD] 8.2) years (Table 1). Beneficiaries were predominantly female (58%) and white (85%). The most common comorbidities were ischemic heart disease (65%) and heart failure (45%). Beneficiaries who were excluded from the study were slightly older compared with those who were included (81.2 [SD 7.6] years vs. 80.9 [8.2] years, p<0.001). They were more likely to be female (75% vs. 58%, p<0.001) and had a greater burden of comorbid illness (e.g., Alzheimer disease and related dementias 53% vs. 27%, p<0.001; heart failure 60% vs. 45%, p<0.001).
Table 1.
Characteristics of Medicare Beneficiaries 2006–2010 Hospitalized with Traumatic Brain Injury by Incident Depression Status (n=55,728)
| Characteristic | New depression pre-TBI n=4841 | New depression post-TBI n=4668 | No depression n=46,219 |
|---|---|---|---|
| Mean age1 (years) (standard deviation) | 80.7 (8.0) | 81.7 (7.9) | 81.0 (8.3) |
| Sex, n (%) | |||
| Female | 3170 (65) | 2882 (62) | 26,460 (57) |
| Male | 1671 (35) | 1786 (38) | 19,759 (43) |
| Race, n (%) | |||
| White | 4211 (87) | 4011 (86) | 39,079 (85) |
| Black | 266 (5) | 305 (6) | 3208 (7) |
| Other | 364 (8) | 352 (8) | 3932 (9) |
| History of comorbid disease, n (%) | |||
| Alzheimer and related dementias | 2440 (50) | 1177 (25) | 11,759 (25) |
| Heart failure | 2566 (53) | 1999 (43) | 20,562 (44) |
| Chronic obstructive pulmonary disease | 1984 (41) | 1541 (33) | 14,480 (31) |
| Diabetes | 2117 (44) | 1793 (38) | 17,942 (39) |
| Atrial fibrillation | 1458 (30) | 1201 (26) | 12,489 (27) |
| Chronic kidney disease | 1663 (34) | 1143 (24) | 11,961 (26) |
| Ischemic heart disease | 3511 (73) | 3082 (66) | 29,370 (64) |
| Stroke/transient ischemic attack | 1767 (37) | 1273 (28) | 12,233 (26) |
| Discharge to Skilled Nursing Facility, n (%) | 2205 (46) | 1872 (40) | 15,053 (33) |
| Length of TBI hospital stay, n (%) | |||
| 0–2 days | 1212 (25) | 1063 (23) | 12,380 (27) |
| 3–5 days | 1905 (39) | 1732 (37) | 16,242 (35) |
| 6–8 days | 710 (15) | 777 (17) | 6844 (15) |
| 9 or more days | 1014 (21) | 1096 (24) | 10,753 (23) |
| Mean months follow-up post-depression diagnosis, (standard deviation) | 10.3 (8.6) | 16.4 (12.4) | n/a |
TBI, traumatic brain injury.
Age at depression diagnosis or age at TBI for those in whom depression did not develop.
During the study period, 4841/55,728 (8.7%) beneficiaries had a diagnosis of depression pre-TBI and 4668/50,887 (9.2%) previously free of depression received a new diagnosis of depression post-TBI (two separate cohorts). Beneficiaries with a diagnosis of depression post-TBI were older (81.7 [SD 7.9] years vs. 80.7 [8.0] years, Student t test p<0.001) and more likely to be male (38% vs. 35%, chi-square p<0.001) compared with a diagnosis of depression pre-TBI (Table 1). Beneficiaries with a diagnosis of depression post-TBI were less likely to have Alzheimer disease and related dementias (25% vs. 50%, p<0.001), heart failure (43% vs. 53%, p<0.001), and chronic obstructive pulmonary disease (33% vs. 41%, p<0.001) compared with those with a diagnosis of depression pre-TBI. Beneficiaries with a diagnosis of depression post-TBI had longer mean follow-up time than those with a diagnosis pre-TBI (16.4 [SD 12.4] months vs. 10.3 [SD 8.6] months, p<0.001).
During the year after diagnosis of depression, average monthly prevalence of antidepressant use was 42% among those with a diagnosis pre-TBI and 36% among those with a diagnosis post-TBI (p<0.001) (Table 2). SSRIs accounted for 79% of all monthly antidepressant use pre-TBI and 75% post-TBI (p<0.001). Use of OADs was similar pre- and post-TBI (9% pre-TBI vs. 9% post-TBI, p=0.985). Overall, 65% of beneficiaries with a diagnosis of depression pre-TBI and 47% of beneficiaries with a diagnosis of depression post-TBI (56% across the two groups) filled an antidepressant prescription at least once.
Table 2.
Average Monthly Prevalence of Treatment for Depression Over 12 Months after Diagnosis of Depression by Pre- or Post-Traumatic Brain Injury Status, n=76,877 Person Months of Observation
| Depression pre-TBI n=34,328 PM | Depression post-TBI n=42,549 PM | p value1 | |
|---|---|---|---|
| All antidepressants,2 n (%) | 13,562 (42) | 13,578 (36) | <0.001 |
| SSRI,2 n (%) | 10,654 (33) | 10,156 (27) | <0.001 |
| Any antidepressant except SSRI,2 n (%) | 2908 (9) | 3422 (9) | 0.985 |
| Any psychotherapy,3 n (%) | 1859 (5) | 2555 (6) | <0.001 |
| Any treatment for depression,2 n (%) | 14,861 (45) | 15,239 (39) | <0.001 |
TBI, traumatic brain injury; PM, person months, SSRI, selective serotonin reuptake inhibitors.
Chi-square test; 2percentages of those without missing data; 3beneficiaries could receive psychotherapy in addition to/instead of antidepressant treatment.
The most commonly used antidepressants based on monthly prevalence of ≥1% by timing of depression diagnosis were citalopram, escitalopram, and sertraline, all SSRIs (Fig. 1). Mirtazapine, a tetracyclic antidepressant, was the fourth most commonly used antidepressant. Antidepressant use was significantly lower among beneficiaries with a diagnosis of depression post-TBI for all antidepressants (p<0.001 for all) except citalopram, a SSRI, and trazadone, a phenylpiperazine antidepressant. Prevalence of trazadone use was slightly higher post-TBI than pre-TBI (2.2% vs. 1.9%), but this was not statistically significant (p=0.035). Prevalence of mirtazapine use was higher post-TBI than pre-TBI (6.1% vs. 3.9%, p<0.001).
FIG. 1.
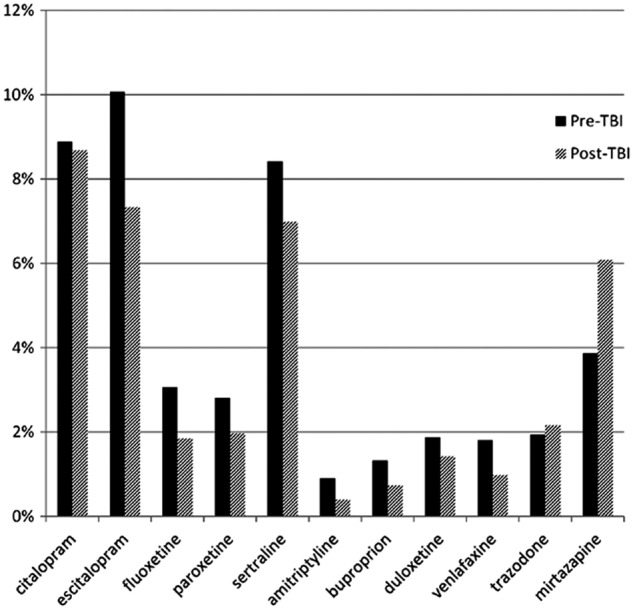
Average monthly prevalence of the most commonly used antidepressants over twelve months following diagnosis of depression by pre- or post-TBI status, n=9, 509. (p<0.001 for all except citalopram [p=0.35] and trazodone [p=0.035].)
Receipt of psychotherapy (with or without antidepressant use) was slightly higher among those with a diagnosis of depression post-TBI (5.4% pre-TBI vs. 6.0% post-TBI, p<0.001) (Table 2). Psychiatric hospitalizations were rare events and were combined into “any” treatment for depression. Receipt of any treatment for depression (i.e., antidepressants, psychiatric hospitalization, or psychotherapy) was higher among beneficiaries with a diagnosis of depression pre-TBI than in beneficiaries with a diagnosis of depression post-TBI (45% vs. 39%, p<0.001). Over the year after diagnosis of depression, 71% of beneficiaries with a diagnosis of depression pre-TBI and 56% of beneficiaries with a diagnosis of depression post-TBI (64% across the two groups) received any treatment for depression at least once.
Our adjusted logistic regression model for antidepressants included an indicator variable for post-TBI depression diagnosis, age, sex, race, Alzheimer disease and related dementias, congestive heart failure, chronic kidney disease, chronic obstructive pulmonary disease, ischemic heart disease, stroke/transient ischemic attack, period post-depression diagnosis, total follow-up time post-depression diagnosis, psychiatric hospitalization, and psychotherapy.
Beneficiaries with a diagnosis of depression post-TBI were less likely to receive antidepressants compared with those with a diagnosis of depression pre-TBI (odds ratio [OR] 0.87, 95% confidence interval [CI] 0.82, 0.92) (Table 3). The same was observed for SSRI use (OR 0.81; 95% CI 0.76, 0.87), but not when OAD use was modeled as the dependent variable (OR 1.09; 95% CI 0.95, 1.24). Time, sex, and race had statistically significant interactions with the independent variable. Stratification of the regression models by these variables, however, resulted in statistically significant effect estimates that were all in the same direction and did not differ greatly by strata (data available on request).
Table 3.
Adjusted1 Odds Ratios and 95% Confidence Intervals of Receiving Treatment for Depression among Medicare Beneficiaries with Traumatic Brain Injury with a Diagnosis of Depression Pre- or Post-Traumatic Brain Injury, n=9509
| All antidepressants | SSRI | OAD2 | Psychotherapy3 | Any treatment for depression4 | |
|---|---|---|---|---|---|
| Pre-TBI | Reference | Reference | Reference | Reference | Reference |
| Post-TBI | 0.87 (0.82, 0.92) | 0.81 (0.76, 0.87) | 1.09 (0.95, 1.24) | 1.08 (0.93, 1.26) | 0.89 (0.85, 0.94) |
SSRI, selective serotonin reuptake inhibitors; OAD, other antidepressant; TBI, traumatic brain injury.
Adjusted for age, sex, race, time, Alzheimer disease and related dementias, congestive heart failure, chronic kidney disease, chronic obstructive pulmonary disease, ischemic heart disease, stroke/transient ischemic attack, neurologic disease, liver disease, total follow-up time post-depression diagnosis, hospitalizations for depression, psychotherapy.
Other antidepressants: includes all antidepressants except SSRIs.
Adjusted for age, sex, race, time, antidepressant use.
Adjusted for age, sex, race, time, Alzheimer disease and related dementias, congestive heart failure, chronic kidney disease, chronic obstructive pulmonary disease, ischemic heart disease, neurologic disease, liver disease, stroke/transient ischemic attack, total follow-up time post-depression diagnosis.
Therefore, for simplicity, our results are not stratified and can be interpreted as the average effect of depression diagnosis timing on receipt of antidepressants across strata. Sensitivity analyses confirmed the robustness of our results. Limiting to smaller time intervals post-depression diagnosis did not significantly change our effect estimates.
Our adjusted logistic regression model for psychotherapy included age, sex, race, period post-depression diagnosis, antidepressant use, and total follow-up time post-depression diagnosis. Beneficiaries with a diagnosis of depression post-TBI were more likely to receive psychotherapy compared with those with a diagnosis of depression pre-TBI; however, this association did not reach statistical significance (OR 1.08; 95% CI 0.93, 1.26).
Our adjusted logistic regression model for any treatment for depression (antidepressants, psychotherapy, or psychiatric hospitalizations) included age, sex, race, Alzheimer disease and related dementias, congestive heart failure, chronic kidney disease, chronic obstructive pulmonary disease, ischemic heart disease, stroke/transient ischemic attack, neurologic disease, liver disease, period post-depression diagnosis, and total follow-up time post-depression diagnosis. Beneficiaries with a diagnosis of depression post-TBI were less likely to receive any treatment for depression compared with those with a diagnosis of depression pre-TBI (OR 0.89; 95% CI 0.85, 0.94).
Discussion
Antidepressant therapy is effective for depression in the majority of older adults.32,33 Despite this, depression remains undertreated, with a range of 11%-57% of depressed older adults receiving any treatment.34–38 In our study, 56% of those with a diagnosis of depression received treatment with antidepressants at least once during the year after diagnosis of depression. Previous studies reporting lower treatment rates focused on current rather than any use over a fixed period, which may explain our higher estimate.34,36 Nonetheless, average monthly prevalence of antidepressant use (36–42%) was consistent with previous reports.34–38 In our study, beneficiaries with a diagnosis of depression post-TBI were significantly less likely to receive antidepressant treatment compared with those with a diagnosis of depression pre-TBI. This difference was not offset by a concomitant increase in receipt of psychotherapy.
Current evidence suggests that SSRIs are safe and effective in the management of depression after TBI in younger adults, and our results indicate that they are the most commonly used antidepressants among Medicare beneficiaries after TBI.24–26 Still, concerns about increased stroke risk (37–55% potential increase) may discourage physicians from prescribing SSRIs to older adults with TBI, especially because TBI is an independent risk factor for stroke.39–43 Other factors that may influence treatment decisions among older adults after TBI include post-TBI cognitive impairment, polypharmacy, and altered drug metabolism.
Nonetheless, beneficiaries with a diagnosis of depression before TBI have a significant burden of comorbidity. In fact, they have a higher burden than those with a diagnosis of depression post-TBI. Therefore, concerns about comorbidity and polypharmacy would be present pre-TBI, yet these persons still received more antidepressants. Research on safety and effectiveness of antidepressants in older adults after TBI is needed to help guide treatment decisions.
We are reliant on claims data to identify depression, and no indicator of severity is available. Previous studies have used psychiatric hospitalizations, receipt of psychotherapy, and even receipt of antidepressants as proxy measures of depression severity.44–46 Therefore, one interpretation of these data is that beneficiaries with a diagnosis of depression pre-TBI had more severe depression as evidenced by greater antidepressant use. Depression post-TBI, however, is often comorbid with other psychiatric disorders, suggesting potentially increased severity.47–50 It is more likely that the observed disparities in management of depression after TBI are because of a lack of recognition of depression among patients and providers, uncertainty about its duration, and absence of clinical guidelines concerning treatment of depression post-TBI.
Results from this study should be interpreted in light of certain limitations. First, depression is underdiagnosed and underrecorded in billing records, which could have resulted in an underestimate of incident depression and the prevalence of antidepressant treatment. This would likely not differ pre- or post-TBI, however; therefore, the absolute difference in receipt of antidepressant treatment should not be affected. Second, TBI severity was not measured in this study. TBI severity could impact incident depression and the decision to treat with antidepressants, but our analysis was not designed to examine that question. Future investigations should evaluate the impact of TBI severity on treatment decisions after TBI among older adults.
Finally, polypharmacy and potential cognitive impairment among this population could influence the decision to treat patients with depression after TBI. Nonetheless, beneficiaries with a diagnosis of depression before TBI had a higher burden of comorbidity than those with a diagnosis of depression post-TBI, yet these persons still received more antidepressants. Consequences of depression treatment decisions should be examined in subsequent studies.
This is the first study to characterize antidepressant use and quantify the disparity in use between older adults with a diagnosis of depression after TBI and a similar pre-TBI cohort of older adults. It provides important information regarding antidepressant class and prevalence of use that can be used in future comparative effectiveness studies to inform treatment guidelines for post-TBI depression in older adults. Strengths of our study include a unique dataset composed of Medicare beneficiaries hospitalized with TBI. These data contain follow-up information on beneficiaries before the TBI event, permitting comparison of a non-TBI population (the pre-TBI period) and a TBI population (the post-TBI period) with well-matched clinical and demographic characteristics.
This national study of Medicare beneficiaries suggests that among older adults, depression after TBI is undertreated. Knowledge about reasons for this disparity and its long-term effects on post-TBI outcomes is limited and should be examined in future work. Until then, efforts to address the undertreatment of depression after TBI should focus on educating providers and highlighting existing studies supporting safety and effectiveness of antidepressant treatment after TBI.
Acknowledgment
This work was supported by National Institutes of Health grant R21 AG042768-01 (Zuckerman, PI). Dr. Albrecht and Mr. Khokhar are supported by National Institutes of Health grant T32AG000262-14 (Magaziner, PI).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Faul M., Xu L., Wald M.M., and Coronado V. Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths, 2002–2006. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. Atlanta, Ga: Available at: http://www.cdc.gov/traumaticbraininjury/pdf/blue_book.pdf Accessed December/15/13 [Google Scholar]
- 2.Livingston D.H., Lavery R.F., Mosenthal A.C., Knudson M.M., Lee S., Morabito D., Manley G.T., Nathens A., Jurkovich G., Hoyt D.B., and Coimbra R. (2005). Recovery at one year following isolated traumatic brain injury: a Western Trauma Association prospective multicenter trial. J. Trauma 59, 1298–1304 [DOI] [PubMed] [Google Scholar]
- 3.Susman M., DiRusso S.M., Sullivan T., Risucci D., Nealon P., Cuff S., Haider A., and Benzil D. (2002). Traumatic brain injury in the elderly: increased mortality and worse functional outcome at discharge despite lower injury severity. J. Trauma 53, 219–224 [DOI] [PubMed] [Google Scholar]
- 4.Utomo W.K., Gabbe B.J., Simpson P.M., and Cameron P.A. (2009). Predictors of in-hospital mortality and 6-month functional outcomes in older adults after moderate to severe traumatic brain injury. Injury 40, 973–977 [DOI] [PubMed] [Google Scholar]
- 5.Selassie A.W., Zaloshnja E., Langlois J.A., Miller T., Jones P., and Steiner C. (2008). Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J. Head Trauma Rehabil. 23, 123–131 [DOI] [PubMed] [Google Scholar]
- 6.Gubler K.D., Davis R., Koepsell T., Soderberg R., Maier R.V., and Rivara F.P. (1997). Long-term survival of elderly trauma patients. Arch. Surg. 132, 1010–1014 [DOI] [PubMed] [Google Scholar]
- 7.Thompson H.J., Weir S., Rivara F.P., Wang J., Sullivan S.D., Salkever D., and MacKenzie E.J. (2012). Utilization and costs of health care after geriatric traumatic brain injury. J. Neurotrauma 29, 1864–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mosenthal A.C., Lavery R.F., Addis M., Kaul S., Ross S., Marburger R., Deitch E.A, and Livingston D.H. (2002). Isolated traumatic brain injury: age is an independent predictor of mortality and early outcome. J. Trauma 52, 907–911 [DOI] [PubMed] [Google Scholar]
- 9.Menzel J.C. (2008). Depression in the elderly after traumatic brain injury: a systematic review. Brain Inj. 22, 375–380 [DOI] [PubMed] [Google Scholar]
- 10.Rosenthal M., Christensen B.K., and Ross T.P. (1998). Depression following traumatic brain injury. Arch. Phys. Med. Rehabil. 79, 90–103 [DOI] [PubMed] [Google Scholar]
- 11.Rapoport M.J., Kiss A., and Feinstein A. (2006). The impact of depression on outcome following mild-to-moderate traumatic brain injury in older adults. J. Affect. Disord. 92, 273–276 [DOI] [PubMed] [Google Scholar]
- 12.Dikman S.S., Bombardier C.H., Machamer J.E., Fann J.R., and Temkin N.R. (2004). Natural history of depression in traumatic brain injury. Arch. Phys. Med. Rehabil. 85, 1457–1464 [DOI] [PubMed] [Google Scholar]
- 13.Jorge R.E., Robinson R.G., Starkstein S.E., and Arndt S.V. (1994). Influence of major depression on 1-year outcome in patients with traumatic brain injury. J. Neurosurg 81, 726–733 [DOI] [PubMed] [Google Scholar]
- 14.Fedoroff J.P., Starkstein S.E., Forrester A.W., Geisler F.H., Jorge R.E., Arndt S.V., and Robinson R.G. (1992) Depression in patients with acute traumatic brain injury. Am. J. Psychiatry 149, 918–923 [DOI] [PubMed] [Google Scholar]
- 15.DiMatteo M.R. Lepper H.S., and Croghan T.W. (2000). Depression is a risk factor for noncompliance with medical treatment: Meta-analysis of the effects of anxiety and depression on patient adherence. Arch. Intern. Med. 160, 2101–2107 [DOI] [PubMed] [Google Scholar]
- 16.Grenard J.L., Munjas B.A., Adams J.L., Suttorp M., Maglione M., McGlyn E.A., and Gellad W.F. (2011). Depression and medication adherence in the treatment of chronic diseases in the United States: a meta-analysis. J. Gen. Intern. Med. 26, 1175–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osterberg L, Blaschke T. (2005) Adherence to medication. N. Engl. J. Med. 353, 487–497 [DOI] [PubMed] [Google Scholar]
- 18.Rapoport M.J. (2012). Depression following traumatic brain injury: epidemiology, risk factors and management. CNS Drugs 26, 111–121 [DOI] [PubMed] [Google Scholar]
- 19.Warden D.L., Gordon B., McAllister T.W., Silver J.M., Barth J.T., Bruns J., Drake A., Gentry T., Jagoda A., Katz D.I., Kraus J., Labbate L.A., Ryan L.M., Sparling M.B., Walters B., Whyte J., Zapata A., and Zitnay G. (2006). Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J. Neurotrauma 23, 1468–501 [DOI] [PubMed] [Google Scholar]
- 20.Fann J.R., Hart T., and Schomer K.G. (2009). Treatment for depression after traumatic brain injury: a systematic review. J. Neurotrauma 26, 2383–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollon S.D., Jarrett R.B., Nierenberg A.A., Thase M.E., Trivedi M., and Rush A.J. (2005). Psychotherapy and medication in the treatment of adult and geriatric depression: which monotherapy or combined treatment? J. Clin. Psychiatry 66, 455–468 [DOI] [PubMed] [Google Scholar]
- 22.Fann J.R., Uomoto J.M., and Katon W.J. (2000). Sertraline in the treatment of major depression following mild traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 12, 226–232 [DOI] [PubMed] [Google Scholar]
- 23.Turner-Stokes L., Hassan N., Pierce K., and Clegg F. (2002). Managing depression in brain injury rehabilitation: the use of an integrated care pathway and preliminary report of response to sertraline. Clin. Rehabil, 16, 261–268 [DOI] [PubMed] [Google Scholar]
- 24.Perino C., Rago R., Cicolini A., Torta R., and Monaco F. (2001). Mood and behavioural disorders following traumatic brain injury: clinical evaluation and pharmacological management. Brain Inj. 15, 139–148 [DOI] [PubMed] [Google Scholar]
- 25.Dinan T.G., and Mobayed M. (1992). Treatment resistance of depression after head injury: A preliminary study of amitriptyline response. Acta Psychiatr. Scand. 85, 292–294 [DOI] [PubMed] [Google Scholar]
- 26.Wroblewski B.A., Joseph A.B., and Cornblatt R.R. (1996). Antidepressant pharmacotherapy and the treatment of depression in patients with severe traumatic brain injury: a controlled, perspective study. J. Clin. Psychiatry 57, 582–587 [DOI] [PubMed] [Google Scholar]
- 27.Centers for Medicare & Medicaid Services. (2011). Chronic Conditions among Medicare Beneficiaries, Chart Book: Baltimore, MD [Google Scholar]
- 28.The Henry J. Kaiser Family Foundation. Fact Sheet: Medicare Advantage. May 2014. Available at: http://kff.org/medicare/fact-sheet/medicare-advantage-fact-sheet Accessed October/8/14
- 29.Thurman DJ. (1995). Guidelines for surveillance of central nervous system injury. National Center for Injury Prevention and Control (U.S.), U.S. Department of Health and Human Services, Public Health Service: Atlanta, Ga [Google Scholar]
- 30.Marr A, Coronado V. (2004). Central nervous system injury surveillance data submission standards—2002. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control (U.S.), U.S. Department of Health and Human Services, Public Health Service: Atlanta, Ga [Google Scholar]
- 31.Centers for Medicare and Medicaid Services Chronic Condition Data Warehouse. Available at: http://www.ccwdata.org/web/guest/condition-categories Accessed March/18/14
- 32.Nelson JC, Delucchi K, Schneider LS. (2008). Efficacy of second generation antidepressants in late-life depression: a meta-analysis of the evidence. Am. J. Geriatr. Psychiatry 16, 558–567 [DOI] [PubMed] [Google Scholar]
- 33.Tedeschini E., Levkovitz Y., Iovieno N., Ameral V.E., Nelson J.C., and Papakostas G.I. (2011). Efficacy of antidepressants for late-life depression: a meta-analysis and meta-regression of placebo-controlled randomized trials. J. Clin. Pyschiatry 72, 1660–1668 [DOI] [PubMed] [Google Scholar]
- 34.Wilson KC, Copeland JR, Taylor S, Donoghue J, McCracken CF. (1999) Natural history of pharmacotherapy of older depressed community residents: The MRC-ALPHA Study. Br. J. Psychiatry 175, 439–443 [DOI] [PubMed] [Google Scholar]
- 35.Sanglier T., Saragoussi D., Milea D., Auray J.P., Valuck R.J., and Tournier M. (2011). Comparing antidepressant treatment patterns in older and younger adults: a claims database analysis. J. Am. Geriatr. Soc. 59, 1197–1205 [DOI] [PubMed] [Google Scholar]
- 36.Steffens D.C., Skoog I., Norton M.C., Hart A.D., Tschanz J.T., Plassman B.L., Wyse B.W., Welsh-Bohmer K.A., and Breitner J.C. (2000). Prevalence of depression and its treatment in an elderly population. Arch. Gen. Psychiatry 57, 601–607 [DOI] [PubMed] [Google Scholar]
- 37.Unützer J. (2007). Clinical practice. Late-life depression. N. Engl. J. Med. 357, 2269–2276 [DOI] [PubMed] [Google Scholar]
- 38.Barry L.C., Abou J.J., Simen A.A., and Gill T.M. (2012). Under-treatment of depression in older persons. J. Affect. Disord. 136, 789–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Albrecht J.S., Liu X., Smith G.S., Baumgarten M., Rattinger G.B., Gambert S.R., Langenberg P., and Zuckerman I.H. (2015). Stroke incidence following traumatic brain injury in older adults. J. Head Trauma Rehabil. Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y.H., Kang J.H., and Lin H.C. (2011). Patients with traumatic brain injury population-based study suggests increased risk of stroke. Stroke 42, 2733–2739 [DOI] [PubMed] [Google Scholar]
- 41.Trifiro G., Dieleman J., Sen E.F., Gambassi G., and Sturkenboomi MC. (2010). Risk of ischemic stroke associated with antidepressant drug use in elderly persons. J. Clin. Psychopharmacol. 30, 252–258 [DOI] [PubMed] [Google Scholar]
- 42.Wu C.S., Wang S.C., Cheng Y.C., and Gau S.S. (2011). Association of cerebrovascular events with antidepressant use: a case-crossover study. Am. J. Psychiatry 168, 511–521 [DOI] [PubMed] [Google Scholar]
- 43.Coupland C, Dhiman P, Morriss R, Arthur A, Barton G, and Hippisley-Cox J. (2011). Antidepressant use and risk of adverse outcomes in older people: population based cohort study. BMJ 343, d4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qian J., Simoni-Wastila L., Langenberg P., Rattinger G.B., Zuckerman H., Lehmann S., and Terrin M. (2013). Effects of depression diagnosis and antidepressant treatment on mortality in Medicare beneficiaries with chronic obstructive pulmonary disease. J. Am. Geriatr. Soc. 61, 754–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Milea D., Guelfucci F., Bent-Ennakhil N., Toumi M., and Auray J.P. (2010). Antidepressant monotherapy: a claims database analysis of treatment changes and treatment duration. Clin. Ther. 32, 2057–2072 [DOI] [PubMed] [Google Scholar]
- 46.Valuck R.J., Orton H.D., and Libby A.M. (2009). Antidepressant discontinuation and risk of suicide attempt: a retrospective, nested case-control study. J. Clin. Psychiatry 70, 1069–1077 [DOI] [PubMed] [Google Scholar]
- 47.Bombardier C.H., Fann J.R., Temkin N.R., Esselman P.C., Barber J., and Dikmen S.S. (2010). Rates of major depressive disorder and clinical outcomes following traumatic brain injury. JAMA 303, 1938–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bryant R.A., O'Donnell M.L., Creamer M., McFarlane A.C., Clark C.R., and Silove D. (2010). The psychiatric sequelae of traumatic injury. Am. J. Psychiatry 167, 312–320 [DOI] [PubMed] [Google Scholar]
- 49.Vaishnavi S., Rao V., and Fann J.R. (2009). Neuropsychiatric problems after traumatic brain injury: unraveling the silent epidemic. Psychosomatics 50, 198–205 [DOI] [PubMed] [Google Scholar]
- 50.Whenlan-Goodinson R., Ponsford J., Johnston L., and Grant F. (2009). Psychiatric disorders following traumatic brain injury: their nature and frequency. J. Head Trauma Rehabil. 24, 324–332 [DOI] [PubMed] [Google Scholar]


