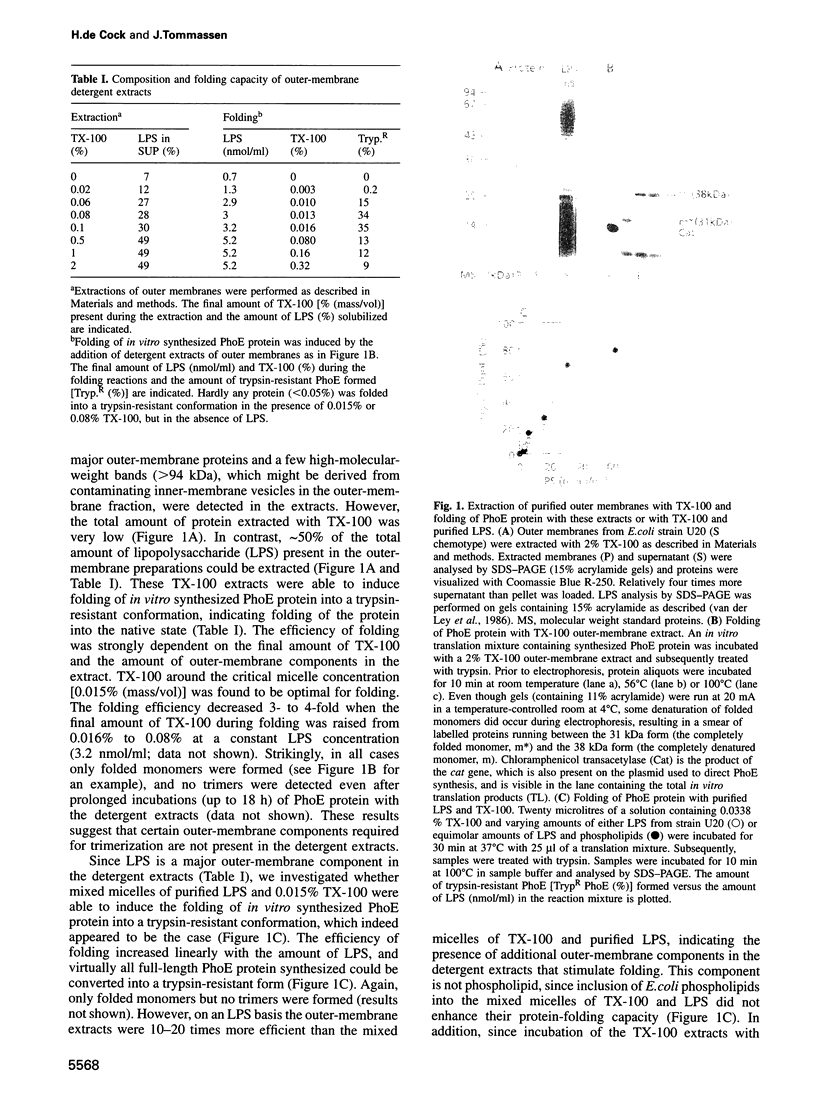
Abstract
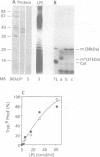
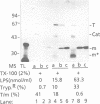
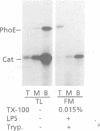
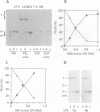
To identify the requirements for the biogenesis of outer-membrane proteins in Gram-negative bacteria, the sorting and assembly of the trimeric, pore-forming protein PhoE was studied in vitro. Purified lipopolysaccharide (LPS) in combination with low amounts of Triton X-100 and divalent cations induced the formation of folded monomers. LPS of deep-rough strains was far less efficient in the formation of folded monomers than wild-type LPS was. These folded monomers could be converted into heat-stable trimers upon addition of outer membranes and higher amounts of Triton X-100. Trimerization could precede the insertion step. These in vitro data suggest that the assembly in vivo proceeds sequentially by (i) formation of a folded monomer by interaction with LPS; (ii) sorting of the folded monomers to assembly sites in the outer membrane; (iii) trimerization; and (iv) insertion.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Spudich E. N., Nikaido H. Protein composition of the outer membrane of Salmonella typhimurium: effect of lipopolysaccharide mutations. J Bacteriol. 1974 Feb;117(2):406–416. doi: 10.1128/jb.117.2.406-416.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bocquet-Pages C., Lazdunski C., Lazdunski A. Lipid-synthesis-dependent biosynthesis (or assembly) of major outer-membrane proteins of Escherichia coli. Eur J Biochem. 1981 Aug;118(1):105–111. doi: 10.1111/j.1432-1033.1981.tb05491.x. [DOI] [PubMed] [Google Scholar]
- Bolla J. M., Lazdunski C., Pagès J. M. The assembly of the major outer membrane protein OmpF of Escherichia coli depends on lipid synthesis. EMBO J. 1988 Nov;7(11):3595–3599. doi: 10.1002/j.1460-2075.1988.tb03237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch D., Leunissen J., Verbakel J., de Jong M., van Erp H., Tommassen J. Periplasmic accumulation of truncated forms of outer-membrane PhoE protein of Escherichia coli K-12. J Mol Biol. 1986 Jun 5;189(3):449–455. doi: 10.1016/0022-2836(86)90316-5. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Cowan S. W., Schirmer T., Rummel G., Steiert M., Ghosh R., Pauptit R. A., Jansonius J. N., Rosenbusch J. P. Crystal structures explain functional properties of two E. coli porins. Nature. 1992 Aug 27;358(6389):727–733. doi: 10.1038/358727a0. [DOI] [PubMed] [Google Scholar]
- De Vrije T., Tommassen J., De Kruijff B. Optimal posttranslational translocation of the precursor of PhoE protein across Escherichia coli membrane vesicles requires both ATP and the protonmotive force. Biochim Biophys Acta. 1987 Jun 12;900(1):63–72. doi: 10.1016/0005-2736(87)90278-1. [DOI] [PubMed] [Google Scholar]
- Freudl R., Schwarz H., Klose M., Movva N. R., Henning U. The nature of information, required for export and sorting, present within the outer membrane protein OmpA of Escherichia coli K-12. EMBO J. 1985 Dec 16;4(13A):3593–3598. doi: 10.1002/j.1460-2075.1985.tb04122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Klose M., Schwarz H., MacIntyre S., Freudl R., Eschbach M. L., Henning U. Internal deletions in the gene for an Escherichia coli outer membrane protein define an area possibly important for recognition of the outer membrane by this polypeptide. J Biol Chem. 1988 Sep 15;263(26):13291–13296. [PubMed] [Google Scholar]
- Koplow J., Goldfine H. Alterations in the outer membrane of the cell envelope of heptose-deficient mutants of Escherichia coli. J Bacteriol. 1974 Feb;117(2):527–543. doi: 10.1128/jb.117.2.527-543.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Nakayama H., Mitsui T., Nishihara M., Kito M. Relation between growth temperature of E. coli and phase transition temperatures of its cytoplasmic and outer membranes. Biochim Biophys Acta. 1980 Sep 2;601(1):1–10. doi: 10.1016/0005-2736(80)90508-8. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Reid J. Biogenesis of prokaryotic pores. Experientia. 1990 Feb 15;46(2):174–180. [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pages C., Lazdunski C., Lazdunski A. The receptor of bacteriophage lambda: evidence for a biosynthesis dependent on lipid synthesis. Eur J Biochem. 1982 Feb;122(2):381–386. doi: 10.1111/j.1432-1033.1982.tb05892.x. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Control of protein exit from the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:1–23. doi: 10.1146/annurev.cb.05.110189.000245. [DOI] [PubMed] [Google Scholar]
- Ried G., Hindennach I., Henning U. Role of lipopolysaccharide in assembly of Escherichia coli outer membrane proteins OmpA, OmpC, and OmpF. J Bacteriol. 1990 Oct;172(10):6048–6053. doi: 10.1128/jb.172.10.6048-6053.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen K., Nikaido H. In vitro trimerization of OmpF porin secreted by spheroplasts of Escherichia coli. Proc Natl Acad Sci U S A. 1990 Jan;87(2):743–747. doi: 10.1073/pnas.87.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen K., Nikaido H. Lipopolysaccharide structure required for in vitro trimerization of Escherichia coli OmpF porin. J Bacteriol. 1991 Jan;173(2):926–928. doi: 10.1128/jb.173.2.926-928.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen K., Nikaido H. Trimerization of an in vitro synthesized OmpF porin of Escherichia coli outer membrane. J Biol Chem. 1991 Jun 15;266(17):11295–11300. [PubMed] [Google Scholar]
- Struyvé M., Moons M., Tommassen J. Carboxy-terminal phenylalanine is essential for the correct assembly of a bacterial outer membrane protein. J Mol Biol. 1991 Mar 5;218(1):141–148. doi: 10.1016/0022-2836(91)90880-f. [DOI] [PubMed] [Google Scholar]
- Thom J. R., Randall L. L. Role of the leader peptide of maltose-binding protein in two steps of the export process. J Bacteriol. 1988 Dec;170(12):5654–5661. doi: 10.1128/jb.170.12.5654-5661.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommassen J., Lugtenberg B. Localization of phoE, the structural gene for outer membrane protein e in Escherichia coli K-12. J Bacteriol. 1981 Jul;147(1):118–123. doi: 10.1128/jb.147.1.118-123.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gelder P., De Cock H., Tommassen J. Detergent-induced folding of the outer-membrane protein PhoE, a pore protein induced by phosphate limitation. Eur J Biochem. 1994 Dec 15;226(3):783–787. doi: 10.1111/j.1432-1033.1994.00783.x. [DOI] [PubMed] [Google Scholar]
- Wickner W., Driessen A. J., Hartl F. U. The enzymology of protein translocation across the Escherichia coli plasma membrane. Annu Rev Biochem. 1991;60:101–124. doi: 10.1146/annurev.bi.60.070191.000533. [DOI] [PubMed] [Google Scholar]
- de Cock H., Hekstra D., Tommassen J. In vitro trimerization of outer membrane protein PhoE. Biochimie. 1990 Feb-Mar;72(2-3):177–182. doi: 10.1016/0300-9084(90)90143-5. [DOI] [PubMed] [Google Scholar]
- de Cock H., Hendriks R., de Vrije T., Tommassen J. Assembly of an in vitro synthesized Escherichia coli outer membrane porin into its stable trimeric configuration. J Biol Chem. 1990 Mar 15;265(8):4646–4651. [PubMed] [Google Scholar]
- de Cock H., Meeldijk J., Overduin P., Verkleij A., Tommassen J. Membrane biogenesis in Escherichia coli: effects of a secA mutation. Biochim Biophys Acta. 1989 Nov 3;985(3):313–319. doi: 10.1016/0005-2736(89)90418-5. [DOI] [PubMed] [Google Scholar]
- de Cock H., van Blokland S., Tommassen J. In vitro insertion and assembly of outer membrane protein PhoE of Escherichia coli K-12 into the outer membrane. Role of Triton X-100. J Biol Chem. 1996 May 31;271(22):12885–12890. doi: 10.1074/jbc.271.22.12885. [DOI] [PubMed] [Google Scholar]
- van Alphen L., Verkleij A., Leunissen-Bijvelt J., Lugtenberg B. Architecture of the outer membrane of Escherichia coli. III. Protein-lipopolysaccharide complexes in intramembraneous particles. J Bacteriol. 1978 Jun;134(3):1089–1098. doi: 10.1128/jb.134.3.1089-1098.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ley P., de Graaff P., Tommassen J. Shielding of Escherichia coli outer membrane proteins as receptors for bacteriophages and colicins by O-antigenic chains of lipopolysaccharide. J Bacteriol. 1986 Oct;168(1):449–451. doi: 10.1128/jb.168.1.449-451.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]