Abstract
This study analyzes how human participants combine saccadic and pursuit gaze movements when they track an oscillating target moving along a randomly oriented straight line with the head free to move. We found that to track the moving target appropriately, participants triggered more saccades with increasing target oscillation frequency to compensate for imperfect tracking gains. Our sinusoidal paradigm allowed us to show that saccade amplitude was better correlated with internal estimates of position and velocity error at saccade onset than with those parameters 100 ms before saccade onset as head-restrained studies have shown. An analysis of saccadic onset time revealed that most of the saccades were triggered when the target was accelerating. Finally, we found that most saccades were triggered when small position errors were combined with large velocity errors at saccade onset. This could explain why saccade amplitude was better correlated with velocity error than with position error. Therefore, our results indicate that the triggering mechanism of head-unrestrained catch-up saccades combines position and velocity error at saccade onset to program and correct saccade amplitude rather than using sensory information 100 ms before saccade onset.
Keywords: gaze shifts, head-free, saccade-pursuit interactions
Introduction
To keep an object of interest on the fovea during everyday life activities, humans track moving targets with combined eye-head movements (called gaze movements, gaze = eye-in-space = eye-in-head + head-in-space). Two types of eye movements ensure clear vision while tracking an object: saccades and smooth pursuit. Initially, saccades (e.g., Bahill, Clark, & Stark, 1975; Becker & Jürgens, 1990; Dodge, 1903) and pursuit (e.g., Dodge, 1903; Kettner, Leung, & Peterson, 1996; Leung & Kettner, 1997) have been studied independently in head-restrained conditions. In contrast, the present study investigates how these two types of eye movements interact in a more natural setting, with head movement.
Pursuit gain (ratio between eye velocity and target velocity) is normally smaller than one during head-restrained pursuit (Meyer, Lasker, & Robinson, 1985), thus the eye would increasingly lag behind the target if no correcting movements occurred. To cope with this problem, saccades (called “catch-up saccades”) are triggered by the central nervous system (CNS) to compensate for the increasing position error due to the imperfect pursuit gain (initially pointed out by Dodge, 1903). If the saccadic system only took into account the retinal error (difference between target and eye position) to program the amplitude of catch-up saccades, they would not be accurate because of the target displacement during the saccade. To ensure saccade accuracy, the saccadic system uses a measure of velocity error (or retinal slip, difference between target and eye velocity) to compensate for the displacement of the target (Blohm, Missal, & Lefèvre, 2003; de Brouwer, Missal, Barnes, & Lefèvre, 2002; de Brouwer, Missal, & Lefèvre, 2001; de Brouwer, Yuksel, Blohm, Missal, & Lefèvre, 2002). These studies and others (Barmack, 1970; Blohm, Missal, & Lefèvre, 2005; Carl & Gellman, 1987; Fleuriet & Goffart, 2012; Fleuriet, Hugues, Perrinet, & Goffart, 2011; Morris & Lisberger, 1987; Pola & Wyatt, 1980) have demonstrated that in head-restrained conditions, pursuit and saccades are not working independently but influence each other. The pursuit and the saccadic systems appear to be “different outcomes from a shared cascade of sensory–motor functions” (Krauzlis, 2004); they share information and act in synergy during gaze reorientation (see Orban de Xivry & Lefèvre, 2007 for a review on saccade-pursuit interactions in head-restrained conditions).
All the above studies were conducted in head-restrained conditions. Studies of saccades (e.g., Freedman & Sparks, 1997; Goossens & Van Opstal, 1997; Guitton, Munoz, & Galiana, 1990; Tomlinson & Bahra, 1986) and pursuit (e.g., Collins & Barnes, 1999; Daye, Blohm, & Lefèvre, 2012; Lanman, Bizzi, & Allum, 1978) with the head free to move did not account for saccade-pursuit interactions, except for Daye, Blohm, and Lefèvre (2010) and Herter and Guitton (1998). The present study sought to analyze saccade-pursuit interactions in head-unrestrained conditions, which introduces several challenges. First, because the eyes and head have different dynamics, the command timing to the eye and the neck muscles is crucial to ensure an accurate gaze trajectory. Second, with the head free to move, the CNS must account for the vestibulo-ocular reflex (VOR). To keep a clear vision, any head movement that generates a perturbation of the gaze goal must be negated. However, the VOR can be counterproductive in numerous situations (e.g., when gaze and head are moving in the same direction). Two mechanisms have been proposed to explain the modulation of the VOR. The first assumes that an opposite signal is added to the VOR to cancel the counter-rolling of the eye that would be caused by gaze movements (VOR cancellation, during pursuit, Lanman et al., 1978). The second decreases the gain of the VOR when it would be counterproductive (VOR suppression, during gaze shifts, Cullen, Huterer, Braidwood, & Sylvestre, 2004; Lefèvre, Bottemanne, & Roucoux, 1992). Finally, although gaze and head movements usually have similar trajectories, they can also be controlled independently (Collins & Barnes, 1999). Therefore, the goals of the head and the gaze movements could partly be in conflict, e.g., when head movement is out of phase with the target movement.
This study analyzes catch-up saccades while participants pursued a target oscillating along a straight, randomly oriented line in head-unrestrained conditions. We show that saccade amplitude is explained by a multiple regression with position error and velocity error at saccade onset. This suggests that the programming of saccade amplitude was made through an efference copy coming from an internal model of target position used to control the pursuit movement instead of delayed sensory signals, as previously proposed (Blohm et al., 2003; de Brouwer, Missal et al., 2002; de Brouwer et al., 2001; de Brouwer, Yuksel et al., 2002; Schreiber, Missal, & Lefèvre, 2006).
Methods
Experimental setup
The experimental setup used in this study has been described elsewhere (Daye et al., 2010, 2012). The analyses in this study used the same dataset as in Daye et al. (2010, 2012). Briefly, human participants sat in front of a 1-m distant translucent tangential screen in a completely dark room after giving informed consent. The screen spanned ±40 of the horizontal and vertical visual field. Eight participants (four male, four female, aged 22–32 years) were recorded for the experiment. None of them had any known oculomotor nor visual abnormalities. Three participants (S4, S5, and S7) were completely naive about oculomotor research, and five participants (S1, S2, S3, S6, and S8) were knowledgeable about general oculomotor studies. All procedures were in compliance with the Declaration of Helsinki.
Two laser spots (0.2° diameter, red and green) were back-projected onto the screen. A dedicated real-time computer (PXI-8186 RT, National Instruments, Austin, TX) running LabView (National Instruments, Austin, TX) controlled the position of the targets (1 kHz) via mirror-galvanometers (GSI Lumonics, Billerica, LA). Horizontal and vertical eye movements were recorded at 200 Hz by a Chronos head-mounted video eye-tracker (Chronos Vision GmbH, Berlin, Germany). Two three-dimensional (3-D) optical infrared cameras (Codamotion System, Charnwood Dynamics, Leicestershire, UK) measured (200 Hz) the position of a set of six infrared light-emitting diodes (IREDs) mounted onto the Chronos helmet. The position and the orientation of the head were computed offline from the position of the IREDs.
Calibration
The calibration procedure has been described previously (Daye et al., 2010). Briefly, three calibrations were carried out during a session of recordings: one before, one in the middle, and one at the end of the session. Five series of five fixation targets (2 s per fixation) were successively presented. Subjects were instructed to maintain their head fixed during a series of target presentations. Between the series, they were asked to realign their head to a new position. Gaze orientation was reconstructed from the eye-in-head orientation and the head position and orientation (Ronsse, White, & Lefèvre, 2007).
Paradigm
A recording session was composed of eight blocks of 25 trials. The paradigm used in this study is the same as in Daye et al. (2010, 2012) and was composed of two parts. The first part was the pursuit part. Briefly, after a 500-ms fixation at the center of the screen, the red laser started to move along a randomly oriented straight line with a sinusoidal velocity (frequency: [0.6..1.2]1 Hz; peak to peak displacement amplitude: [40..60]°) for a random duration ([3000..3750] ms). With this range of parameters, 1.8 to 4.5 cycles of motion were presented to the subject. Around the end of the red target motion, a second green target was briefly presented (flash target, duration: 10 ms) at a random position inside a virtual annulus around current pursuit target position (inner radius of 15°; outer radius of 30°). The trial ended with a red fixation at the center of the screen for 500 ms. The second part of the protocol started at the flash presentation or at pursuit target extinction, whichever occurred first and lasted up to trial end. A trial lasted 6 s, and we had a 0.5-s intertrial interval. Thus a block lasted 162 s, and we allowed subjects to pause for ∼30–45 s between two blocks. Therefore a recording session never lasted more than 30 min. Finally, no subject did more than one recording session per day to avoid fatigue.
Participants' instructions
Participants were instructed to track the red target with gaze movements during the first part of the protocol. Then they were required to look at the flash as soon as they saw it. The target parameters (frequency and velocity) were such that combined eye-head movements had to be generated to track the target. As soon as they saw the flash, they had to look at its remembered position. Finally, participants had to maintain their gaze at this position until the appearance of the end-of-trial fixation at the center of the screen. Whereas Daye et al. (2012) analyzed the tracking performance of the participants, this study focuses on the catch-up saccades during the pursuit part of the movement before the flash presentation or the offset of the pursuit target, whichever occurred first.
Data analysis
Eye and IREDs positions were stored on a computer hard drive for off-line analysis. All the analysis algorithms were implemented in Matlab (The MathWorks, Natick, MA). Position signals were low-pass filtered (cutoff frequency: 50 Hz) by a zero-phase infinite impulse response (IIR) third-order digital filter. Velocity and acceleration were derived using a central difference algorithm (±10-ms window). Data were rotated with respect to target direction at the onset of the movement of the pursuit target (red laser spot). After this rotation, each target moved horizontally and started its movement to the right. We defined rightward (leftward) movements as being positive (negative) values in our analyses. This normalization induced a new coordinate system; the movement was decomposed into the direction parallel to pursuit (X-axis in text and figures) and the direction perpendicular to pursuit (Y-axis in text and figures) target direction.
Saccades detection
Gaze saccades were detected using a generalized likelihood ratio (GLR) algorithm as in Daye et al. (2010, 2012). Every trial was visually inspected; if a saccade was not detected, the acceleration threshold was decreased from 750°/s2 to 500°/s2. All the trials were aligned with respect to the onset of the pursuit target movement.
Saccade onset phase
Because in our paradigm the pursuit target frequency varied for each trial, it was impossible to use saccade latency with respect to the initiation of pursuit target movement to study the strategies used by the participants to trigger a saccade during a cycle. Thus we expressed saccadic onset time (LON) as a phase in a cycle of target motion (ϕON) using:
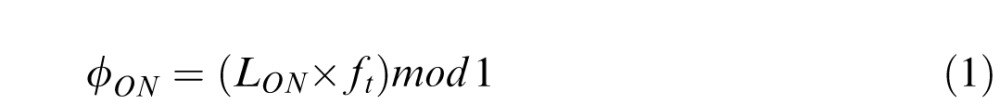 |
ft in Equation 1 represents target oscillation frequency. Following this conversion, each saccade had a phase onset inside a [0..1] range within a cycle.
Sensory signals
We computed for each gaze saccade the position error (PE100) and the retinal slip (RS100) 100 ms before saccade onset as in de Brouwer, Missal et al. (2002). Additionally, due to the predictive nature of our protocol, we computed the acceleration error (AE100) as the difference between the target and gaze accelerations 100 ms before saccade onset.
Prediction variables
We also computed for each saccade the predicted position error (PPEON), the predicted velocity error (PVEON), and the predicted acceleration error (PAEON) at the onset of the gaze shift as the difference between the target position, velocity, and acceleration and gaze position, velocity and acceleration. We qualified these variables as “predicted” in opposition to the “sensory” variables of the previous paragraph because they could not come from a direct reading of a sensory input (because of internal delays). We postulate that they must be generated by an internal model. This point is developed in the Discussion.
Sign correction as a function target velocity
The position error (retinal slip) is usually computed using the difference between target position (velocity, acceleration) and gaze position (velocity, acceleration). Therefore, a positive error corresponds to gaze lagging behind the target, and a negative error corresponds to gaze in advance with respect to the target. Because in our paradigm the target movement was periodic, the interpretation of the sign of position error (retinal slip, acceleration error) is different depending on the direction of the target movement. A positive PE100 could correspond to either a lag (target moving to the right, with target velocity > 0) or an advance of the gaze with respect to the target (target moving to the left, with target velocity < 0). The same kind of reasoning can be applied to interpret retinal slips and acceleration errors. Therefore, the sign of PE100, RS100, AE100, PPEON, PVEON, and PAEON was corrected by the sign of target velocity at the specified time (100 ms before saccade onset or at saccade onset). Following this normalization, a positive (negative) value of PE100 or PPEON always corresponds to gaze behind (ahead of) the target. Similarly, a positive (negative) value of RS100 or PVEON always corresponds to gaze moving slower (faster) than the target while a positive (negative) value of AE100 or PAEON always corresponds to gaze accelerating (decelerating) with respect to the target.
Regressions
When all the variables in a regression are experimental measurements, they all contain a noise resulting from the experimental measure. Regular regressions are used when one of the variables (the independent variable) is assumed not to be polluted by measurement noise. If all the variables are measured, using regular regressions induces a bias in the results. To avoid this, we used orthogonal regressions based on principal component analysis (PCA). Orthogonal regression requires that the variances of the measurement noise in each variable be similar. In practice, we don't have repeated measures of pairs of variables. Therefore, when variables in the regression had the same units, we assumed that the measurement noise was similar. When variables had different units, we used regular regressions.
To ensure that the units are similar when regressing saccade amplitude (°) with either velocity error (°/s) or acceleration error (°/s2), we assumed that the velocity (acceleration) error was integrated to correct saccade amplitude and ensure an accurate saccadic displacement, as shown during head-fixed pursuit (de Brouwer et al., 2001; Schreiber et al., 2006). Therefore, we multiplied the velocity error either by saccade duration SDur for predicted signals or by saccade duration plus 100 ms (SDur + 0.1) for sensory inputs and used those signals to predict saccade amplitude. Similarly, we multiplied acceleration errors by S2Dur/2 for predicted signals or by (SDur + 0.1)2/2 for sensory signals. To test our regression method, we generated a linear two-dimensional (2-D) dataset polluted with Gaussian random noise with different variances on each dimension. Then, we applied the orthogonal regression method and the regular least-square method on the same dataset and compared the results. The orthogonal regression always approximated better the slope of the noise-free dataset than the regular least-square method. For example, when the noise on the regressand was 16 times larger than the noise on the regressor, the slope was underestimated by ∼3% with the total least-square and by ∼7% by the regular least-square. This test was used to validate our method.
When using orthogonal regressions, standard metrics of goodness-of-fit can be misleading because all the variables contain noise. An alternative is to check how well a regression model trained on some of the data predicts the rest of the data. Therefore, the quality of orthogonal regressions was tested using the mean of a 10-fold cross-validation on the sum of the orthogonal squared errors (cvMSE). For each regression, we bootstrapped the computation of cvMSE. Because cvMSE is never negative (and thus is not normal), we present the percentiles 5, 10, and 95 of the generated cvMSE distribution for each orthogonal regression. cvMSE = 0 means that the computed regression is a perfect predictor of the observed behavior. We used two-tailed Wilcoxon rank sum tests to compare regressions. The p value of the rank sum tests is given for each comparison. A smaller cvMSE represents a better predictor of the observed behavior. In contrast, we used vaf (variance-accounted-for: the proportion of regressand variance explained by the regression) to express the quality of regular regressions.
Finally, to give an indication of the effect of the dependent variable on the independent one for first order orthogonal regressions, we computed the correlation between those variables (represented by ρ in first order regressions).
Data set
The same dataset as in Daye et al. (2012) is used for these analyses. We collected 6,533 trials out of which 5,748 were valid trials (88%). Trials were removed from the analyses if a participant did not follow the protocol instructions (10%) or if the IREDs were out of sight for a camera (2%) during the pursuit part of the paradigm. Every trial was visually inspected. Forty-thousand three hundred and seventy-three saccades were detected by the GLR algorithm and analyzed in this article.
Results
Typical trials
Figure 1 shows the behavior of a participant (S2) for a high frequency oscillating target (target frequency = 0.905 Hz) with a 42° amplitude. We only represented the velocity component along the direction parallel to pursuit in Figure 1B because the main displacement occurred along this direction (compare first and second rows of Figure 1A). The gaze movement started with a saccade towards the pursuit target (latency with respect to initiation of target movement = 125 ms). The initial saccade ended on the target but directly after a second saccade was triggered that pushed the gaze away from the target. Then, a third saccade was triggered by the subject to bring the gaze back on the target. Gaze velocity did not match target velocity yet. After the initiation phase (approximately after one cycle of target motion ∼1 s), the movement reached the steady-state regime. Nevertheless the gaze velocity in Figure 1 remained on average smaller than the velocity of the target for the rest of the trial. As a result, several saccades were triggered to compensate for the position error induced by the gaze pursuit gain being smaller than one. Remarkably, saccades were triggered when the target velocity was high. It can also be observed in Figure 1 that the head started its movement more slowly compared to target velocity. After a cycle of target motion (around 1 s), the head movement was in a stationary regime; the amplitude of head velocity was close to the amplitude of target velocity, and the head led the target by 13 ± 6.2 ms (see Daye et al., 2012 for the computation of head phase time).
Figure 1.

Typical high frequency trial. Panel A represents the time course of target (red line), gaze (black line), eye-in-head (green line), and head (gray line) positions. X represents the direction parallel to target motion. Y represents the direction perpendicular to target motion. Panel B represents target, gaze, eye-in-head, and head velocity along the target trajectory (Ẋ) as a function of time. Same color convention as in Panel A. Thick black lines represent gaze saccades. Time origin is the initiation of the target movement.
Proportion of saccadic time
Daye et al. (2012) showed that the pursuit performance decreases with an increase of target oscillation frequency. To compensate for the reduction of the pursuit gain while keeping gaze close to the target, the CNS had to trigger more saccades with high frequency targets than with low frequency targets. To validate this assumption, we computed the proportion of time during which participants executed a saccade and called this parameter “proportion of saccadic time”, Tsacc,prop. Tsacc,prop was defined as the ratio of the sum of the duration of all saccades in a trial to the duration of the corresponding trial. A linear regression between target oscillation frequency and proportion of saccadic time resulted in:
 |
Equation 2 shows that the proportion of saccadic time doubled on the range of observed frequencies (from 0.126 at 0.6 Hz to 0.279 at 1.2 Hz). Slopes of regression (Equation 2) for individual participants varied from 0.048 ± 0.012 to 0.351 ± 0.014. Therefore, Equation 2 shows that with an increase of target frequency, participants increased the proportion of time during which they executed a saccade, validating our assumption.
Catch-up saccades amplitude
In this section, we studied which parameters could be used to predict the amplitude of catch-up gaze saccades. It has been shown that the amplitude of head-restrained catch-up saccades is better explained with both position error and retinal slip 100 ms before saccade than with simple regressions using either of these variables separately (de Brouwer, Missal et al., 2002; de Brouwer et al., 2001; de Brouwer, Yuksel et al., 2002). To test this observation in our protocol, we regressed catch-up gaze saccade amplitude with position error and retinal slip 100 ms before saccade onset. We also computed regressions with the same parameters at saccade onset to test if they generate a better estimation of catch-up saccades amplitude. Those signals could come from the output of an internal predictive model used to correct adequately catch-up saccade amplitude (this will be elaborated on in the Discussion section).
Position error, retinal slip, and acceleration error 100 ms before saccade onset
In this section, we tested if the amplitude of head-unrestrained catch-up saccades can be explained by sensory signals (position error and retinal slip) 100 ms before the onset of the saccade as in head-restrained conditions (de Brouwer, Missal et al., 2002; de Brouwer et al., 2001; de Brouwer, Yuksel et al., 2002).
We computed a regression between saccade amplitude (SA) and the position error 100 ms before saccade onset:
 |
Equation 3 shows that saccade amplitude is sensitive to a change of position error (represented by the slope of the regression). However, the correlation between PE100 and SA is very weak. Thus the computed slope could not be used trustfully to characterize saccade amplitude as a function of PE100.
The CNS must compensate for the target displacement during the saccade execution to ensure an accurate saccadic displacement. If the saccade amplitude is programmed using the sensory information 100 ms before saccade onset, the displacement of the target during the saccade preparation and execution periods should also be taken into account. Therefore, as in de Brouwer, Missal et al. (2002), de Brouwer et al. (2001), and de Brouwer, Yuksel et al. (2002), we postulated that the CNS could integrate the sensory information from the retinal slip to correct the amplitude of the saccade. This integration would start 100 ms before saccade onset up to saccade offset, resulting in a factor (SDur + 0.1) in the following regressions:
 |
 |
When the amplitude of the gaze shift was modeled as a function of the retinal slip 100 ms before saccadic movement onset, regression (Equation 4) shows a significant cvMSE increase compared to (Equation 3; two-tailed Wilcoxon rank sum test, PE100 ≪ ≫ RS100: p < 0.001). Therefore, despite the higher correlation between RS100 and saccade amplitude, the quality of the predictor (measured by cvMSE) is worse between RS100 and saccade amplitude than between PE100 and SA. When both PE100 and RS100 are used to model the amplitude of the gaze shift, regression (Equation 5) shows a significant large increase of the quality of the regression with respect to RS100 but a small decrease with respect to PE100 (two-tailed Wilcoxon rank sum test, PE100 ≪ ≫ Multi100: p = 0.0105, RS100 ≪ ≫ Multi: p < 0.001).
Because the stimulus moved sinusoidally in our experiment, the velocity was not constant during cycles of motion. Therefore, we assumed that AE100 provides information about a change of target velocity that could be used by the CNS to correct saccade amplitude. As for the retinal slip, the integration of the acceleration error would start 100 ms before saccade onset up to saccade offset (resulting in a factor (SDur + 0.1)2/2 in the following regressions). First, we computed a first order regression between AE100 and SA:
 |
Regression (Equation 6) shows that a first order regression using the acceleration error taken 100 ms before saccade onset was bad predictor of saccade amplitude. Nevertheless, the acceleration term could be used to compensate for the velocity error 100 ms before saccade onset, thus building a better predictor of saccade amplitude. To test this hypothesis, a multiple regression including was AE100 computed:
 |
Regression (Equation 7) shows that the addition of the acceleration error term does not affect significantly cvMSE (two-tailed Wilcoxon rank sum test, MultiAE100 ≪ ≫ Multi100: p = 0.528. MultiAE100 ≪ ≫ PE100: p = 0.029). Therefore, this analysis showed that, contrary to our hypothesis, the addition of an acceleration term to the regression did not increase significantly the quality of the prediction for the multiple regression with RS100 and PE100.
Finally, we computed regressions (Equations 3–7) individually for each subject to test if the observed trends were reproduced by each subject. Quality criteria for the quality of single-parameter regressions are shown in Table 1, while quality criteria for multiple regressions are shown in Table 2. We computed the mean value of the mean of each subject's quality criterion as a measurement of the population behavior. Table 1 shows that at the population level, saccade amplitude is better explained by RS100 or AE100 than by PE100 (the population correlation is significantly different than zero only for RS100 and AE100), confirming our observation when all the saccades were pooled together. At the subject level, table 2 shows that the quality of the regression increases between single regressions with PE100 and multiple regressions with PE100 and RS100 for five out of eight subjects. Seven subjects showed a statistically significant increase of the quality of the fit when we compared the single regression with PE100 to the multiple regression with PE100, RS100, and AE100. Only two subjects showed a statistically significant increase of the quality of the fit when we compared both multiple regressions. Finally, we did not see any statistically significant increase of the quality of the fits at the population level. This shows that the sensory signals, measured 100 ms before saccade onset poorly represent saccade amplitude.
Table 1.
Cross-validation on the mean squared error, p(5), p(50), p(95), and correlation (ρ, * = p < 0.05, ** = p < 0.01, *** = p < 0.001) for single parameter regressions between sensory errors and the amplitude of catch-up saccades for each subject, for the mean of all subjects and for all the saccades pooled together.
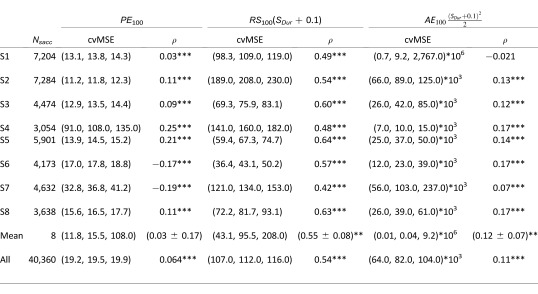
Table 2.
Cross-validation on the mean squared error, p(5), p(50), p(95), for multiple parameters regressions between sensory errors and the amplitude of catch-up saccades for each subject, for the mean of all subjects and for all the saccades pooled together.

Position error, velocity error, and acceleration error at saccade onset
Because of internal delays between visual inputs and the motor command, it has been shown that participants need an internal predictive representation of target movement to ensure accurate tracking in head-restrained conditions (Stark, Vossius, & Young, 1962; Westheimer, 1954). In this section, we wanted to test if the amplitude of catch-up saccades could be better explained using internal predictive signals of position error, velocity error, and acceleration error instead of their corresponding delayed sensory signals. To that goal, we computed simple regressions between SA and PPEON, PVEONSDur, or PAEON(S2Dur/2) as independent variables. We also computed a second-order regression between saccade amplitude and both PPEON and PVEONSDur. Finally, we computed a multiple regression that includes also the acceleration error at saccade onset (PAEON) to account for the sinusoidal target movement. The linear regressions resulted in:
 |
 |
 |
 |
 |
cvMSE of first-order regressions (Equations 8 and 9) clearly show that the variability of the amplitude of a saccade in our paradigm was better explained by predicted velocity error than by predicted position error (two-tailed Wilcoxon rank sum test, PPE ≪ ≫ PVE: p < 0.001). Additionally, two-tailed Wilcoxon rank sum test between cvMSE of simple regression (Equation 12) and multiple regression (Equation 11) confirmed that the addition of retinal slip significantly increased the quality of the fit (two-tailed Wilcoxon rank sum test, PPE ≪ ≫ Multi: p < 0.001). Two-tailed Wilcoxon rank sum test between cvMSE of simple regression (Equation 9) and multiple regression (Equation 11) did not show a statistical difference between the two regressions (two-tailed Wilcoxon rank sum test, PVE ≪ ≫ Multi: p = 0.143). The addition of the acceleration term in regression (Equation 12) generated a significantly better predictor than Equation 9 and Equation 11 (two-tailed Wilcoxon rank sum test, PVE ≪ ≫ MultiAE: p < 0.001, Multi ≪ ≫ MultiAE: p < 0.001).
A two-tailed Wilcoxon rank sum test showed a statistically significant improvement of regression (Equation 12) compared to the corresponding regression (Equation 7) or to the best 100-ms regression (Equation 3; two-tailed Wilcoxon rank sum test, MultiAE ≪ ≫ MultiAE100: p < 0.001. MultiAE ≪ ≫ PE100: p < 0.001). This analysis shows that the amplitude of a saccade is better explained by a regression using position, velocity and acceleration errors at saccade onset than any model with errors 100 ms before saccade onset.
There is still the possibility that the observed behavior was driven by a subset of our subjects. Therefore, as for the previous section, we computed regressions (Equations 8–12) for each subject individually.
Table 3 shows the quality criteria for each subject for all the simple regressions. Contrarily to what we computed with the sensory parameters in the previous section, all the subjects presented a statistically significant correlation between all the individual variables (PPEON, PVEON, and PAEON) and saccade amplitude. This was also the case at the population level, the correlation with PVEONSDur being the highest.
Table 3.
Cross-validation on the mean squared error, p(5), p(50), p(95), and correlation (ρ, * = p < 0.05, ** = p < 0.01, *** = p < 0.001, for single parameter regressions between predicted errors and the amplitude of catch-up saccades for each subject, for the mean of all subjects and for all the saccades pooled together.
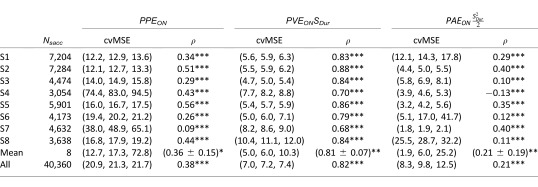
Table 4 shows the quality criterion we used for multiple regressions. All the subjects showed a statistically significant increase of the quality of the multiple regressions using either PPEON and PVEON or PPEON, PVEON, and PAEON; this increase remained statistically significant at the population level. In addition, all the subjects showed a statistically significant increase of the quality of the regressions between the multiple regressions, which was also statistically significant at the population level.
Table 4.
Cross-validation on the mean squared error, p(5), p(50), p(95), for multiple parameters regressions between predicted errors and the amplitude of catch-up saccades for each subject, for the mean of all subjects and for all the saccades pooled together.

Therefore, our analyses point towards the use of predictive signals to correct catch-up saccade amplitude (this will be elaborated on in the Discussion section). Finally, because saccade amplitude was better explained by a model using parameters at saccade onset, we used those parameters for the following analyses.
Time-course of cvMSE
Figure 1 shows that the first saccades after the onset of target movement could be inaccurate. These errors appear to be corrected later during the movement. To test if there was an evolution of the quality of the prediction of saccade amplitude as a function of the latency, we bootstrapped the computation of cvMSE (N = 50) for saccade latency bins of 50 ms. cvMSE was computed as in Equation 12 with PPEON, PVEON, and PAEON as independent variables. Then we computed the mean cvMSE during the first second following the onset of target motion (pursuit initiation) and between 2 and 3 s following the onset of target motion (steady-state pursuit). We observed a significant increase of the quality (decrease of cvMSE) of the model between the two time bins (two-tailed Wilcoxon rank sum test, p < 0.001). Finally, we checked if this difference was observed for different frequency ranges of target motion. To do so, we divided the dataset into target frequency bins of 0.1 Hz. Then we repeated the bootstrap analysis of cvMSE. Finally, we compared the quality of the fit across the different frequency bins. Table 5 shows the results of these tests inside the different frequency bins. There was a significant increase of the fit quality for all comparisons.
Table 5.
Results of Wilcoxon rank sum tests between bootstrapped cvMSE on saccades with LON < 1.0 [s] (init) and bootstrapped cvMSE on saccades with 2.0 ≤ LON ≤ 3.0 [s] (steady-state).

Head-restrained versus head-unrestrained
We tested if the release of the head could explain the increase of fit quality when sampling the parameters at saccade onset instead of the same parameters sampled 100 ms before saccade onset as in de Brouwer, Missal et al. (2002). Because the head is free to move in our experiment, we computed the head contribution to the gaze saccadic displacement (HC) as:
 |
In Equation 13, SA,H corresponds to the head displacement during the gaze saccade and SA corresponds to the amplitude of the gaze saccade. We extracted percentiles 20 (range for the subjects: 0.22–0.55) and 80 (range for the subjects: 0.44–0.89) of each subject's HC distribution. Then, we computed regressions (Equations 7 and 12) separately for small HC (HC smaller than percentile 20) and large HC (larger than percentile 80) for each subject. We compared the quality of the fit for the corresponding conditions (small HC: MultiAE100 ≪ ≫ MultiAE and large HC: MultiAE100 ≪ ≫ MultiAE). The amplitude of the saccades was always better explained by a regression using the parameters sampled at saccade onset (corresponding to regression Equation 12) than by a regression using the parameters sampled at 100 ms before saccade onset (corresponding to regression Equation 7). This analysis demonstrates that the difference between our study and the previous study of de Brouwer, Missal et al. (2002) is not explained by the release of the head since it is valid for the whole range of head contribution.
Distribution of catch-up saccade onset phase
Next, we looked for a pattern in the frequency of catch-up saccades triggered by subjects during head-unrestrained pursuit of an oscillating target. To that goal, we plotted the distribution of the number of saccades as a function of their onset phase (see Equation 1 for the computation of the saccade onset phase) for all the subjects and all the frequencies pooled together in Figure 2A. Figure 2A shows that the distribution of the number of saccades was modulated by the amplitude of target velocity. A larger number of saccades were triggered for high target velocities than for small ones. Additionally, the distribution of phase onset was not symmetric with respect to maximum target velocity (around 0.5, 0, and 1). Figure 2A shows that the number of saccades was largest when the target accelerated (saccade onset phase between 0.35 and 0.5 or between 0.85 and 1) and smallest during the deceleration part of the movement (saccade onset phase between 0.5 and 0.65 or between 0 and 0.15).
Figure 2.
Saccade onset phase. Panel A represents distribution of saccade onset phase for all participants across all frequencies. The thick black line under the distribution corresponds to the absolute value of the target velocity plotted as a function of the position in the sine function (arbitrary amplitude). Dotted lines represent the time of the maximum target velocity. Thin black lines represent the time when the target velocity was equal to zero. Panel B represents distribution of saccade onset phase for target frequency bins of 0.1 Hz. Panel C represents distribution of saccade onset phase for each subject separately.
The shape of the distribution in Figure 2A could be present for high target oscillation frequencies but not for small ones. To test this, we plotted in Figure 2B the distribution of the number of saccades as a function of their onset phase for target frequency bins of 0.1 Hz (centered at 0.65, 0.75, 0.85, 0.95, 1.05, and 1.15 Hz). Figure 2B shows that the shape of the distributions of saccade onset phase is similar for the different frequency bins but that the peaks of the distributions are higher for higher target frequencies.
Finally, we tested if the subjects had a similar strategy to trigger their saccades. Figure 2C shows the distribution of saccade onset phase for the different subjects. Figure 2C shows that seven out of eight subjects shared a similar strategy to trigger saccades in our protocol: Most of the saccades were triggered while the target was accelerating. S4 triggered most of the saccades between the apexes of target movement as the others. However, S4's saccade onset phase distribution was symmetrical with respect to target peak velocity, contrarily to all the other subjects.
Catch-up saccade triggering parameters
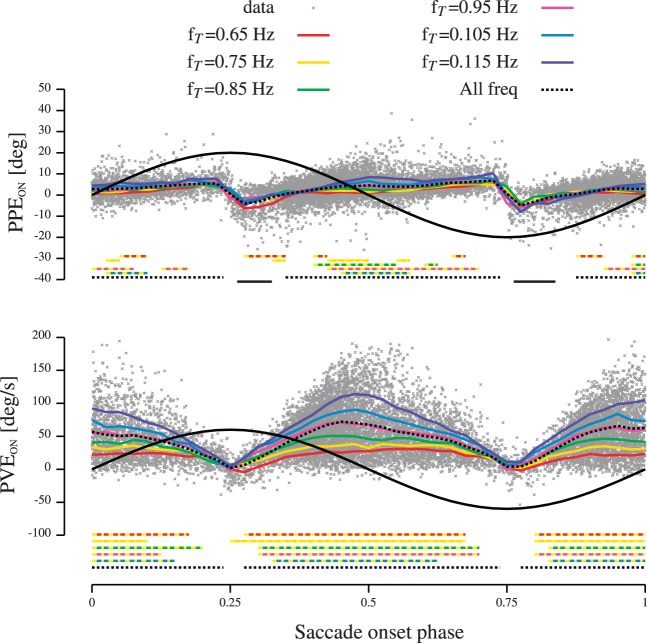
Figure 2 shows that participants triggered saccades at specific moments. In this section, we analyze if this onset phase distribution could be related to the parameters used to explain saccade amplitude. To that goal, we plotted PPEON and PVEON (see Methods) as a function of the saccadic onset phase in Figure 3. Solid black lines in Figure 3 represent a typical frequency-normalized position signal (arbitrary amplitude). Thin dotted lines represent the mean over 0.025 phase onset bins of either PPEON (upper row in Figure 3) or PVEON (lower row in Figure 3) for all target frequencies pooled together. It must be noted that the steps observed in the upper row of Figure 3 appeared because of the sign change of the target velocity that influenced the computation of predicted position error (see Methods). Figure 3 shows that gaze position lagged the target during a large part of the movement (PPEON > 0). However, inside the [0.25..0.325] and [0.75..0.85] ranges, gaze was, on average, in phase or leading the target at saccade onset (PPEON ≤ 0). The lower row in Figure 3 shows that gaze moved on average more slowly than the target during the movement at saccade onset (PVEON > 0). This is supplementary evidence that the head-unrestrained tracking gain is inferior to one, as reported by Daye et al. (2012). To quantify this observation, the horizontal thick black lines under each row represent the time when PPEON or PVEON were significantly higher than zero (dashed black lines, one-sided t test, P < 0.05) or significantly smaller than zero (solid black lines, one-sided t test, p < 0.05). Importantly, the bottom row of Figure 3 shows that PVEON was never statistically smaller than zero (there is no horizontal solid black lines under the bottom row in Figure 3). In contrast, the predicted position error (upper row of Figure 3) shows that there were parts of the cycle for which gaze was on average in advance with respect to the target.
Figure 3.
PPEON and PVEON as a function of saccade onset phase. The upper row represents the corrected predicted position error at saccade onset as a function of the relative saccadic onset time. The lower row represents the corrected predicted velocity error at saccade onset as a function of the relative saccadic onset time. Gray dots represent individual data. Thick black lines represent target position during one cycle (arbitrary amplitude). Thin dotted black lines represent the mean PPEON (upper row) or the mean PVEON (lower row) as a function of the normalized onset time for all the frequencies pooled together. Thin color lines correspond to the mean PPEON (upper row) or the mean PVEON (lower row) as a function of the normalized onset time for different bins of target frequency. Thick horizontal dashed color lines under the graphs correspond to the portion of time during which the two signals represented by the two colors were significantly different. Dashed (solid) horizontal black lines under the graphs correspond to the period during which the average signals were significantly larger (smaller) than 0.
Then, we looked at the sensitivity of PPEON and PVEON to target oscillation frequency. Thin solid color lines in Figure 3 represent the mean over 0.025 phase bins of either PPEON (upper row in Figure 3) or PVEON (lower row in Figure 3) for target oscillation frequency bins of 0.1 Hz (centered at either 0.65, 0.75, 0.85, 0.95, 1.05, or 1.15 Hz). One can clearly see that target oscillation frequency does not modulate PPEON (color lines are superimposed in upper row of Figure 3) but has a profound effect on PVEON (color lines are distinct in lower row of Figure 3). To validate this observation, we computed the time during which PPEON for two successive frequency bins (0.65 Hz with 0.75 Hz, 0.75 Hz with 0.85 Hz, etc.) were statistically different (t test, p < 0.05). The same procedure was applied to compare PVEON for two successive frequency bins. Thick horizontal dashed colored lines under the upper row of Figure 3 represent the times during which PPEON for two successive frequency bins (represented by color components of the dashed lines) were statistically different. Similarly, thick horizontal dashed colored lines under the bottom row of Figure 3 represent the times during which PVEON for two successive frequency bins (represented by color components of the dashed lines) were statistically different. Therefore, these analyses show that the distribution of predicted position error was not modulated by target oscillation frequency (no clear pattern emerges from the frequency comparison) while the predicted velocity error increased with increasing target oscillation frequency.
As a last step, we studied the link between the normalized number of saccades and either PPEON (red markers) in Figure 4E or PVEON (blue markers) in Figure 4D. To represent the relationships between PPEON and PVEON independently of the bins' width, we first normalized the area under the histogram represented in Figure 2 to obtain the distribution represented in Figure 4A. Then we separated the data into two pools: one grouped positive values of PPEON (gaze lagged behind the target) and the corresponding PVEON (filled markers). The second group pooled the negative values of PPEON (gaze led the target) and the corresponding PVEON (open markers). Finally, to build Figure 4D, we plotted the mean PVEON at a relative onset time (e.g., Y1 in Figure 4B) as a function of the normalized number of saccades (e.g., X in Figure 4A) at the same relative onset time. The same procedure was used to build Figure 4E (e.g., using X and Y2).
Figure 4.
Normalized number of saccades as a function of PVEON and PPEON. Panel A represents the averaged normalized distribution of the number of saccades as a function of the normalized onset time (black line) as well as the evolution around the mean of the standard error of the normalized distribution of the number of saccades between subjects (gray lines). Panel B (C) represents PVEON (PPEON) as a function of the normalized onset time. Panel D (E) represents the relationship between the relative number of saccades and PVEON (PPEON). Open (filled) markers correspond to negative (positive) values of PPEON. Solid lines correspond to an exponential fit between PVEON (PPEON) and the normalized number of saccades for positive values of PPEON. Dashed lines correspond to the same fits for the negative values of PPEON.
A symmetric trend of the number of saccades triggered as a function of PPEON for the positive and the negative values of PPEON is shown in Figure 4E. A similar trend of the number of saccades as a function of PVEON is represented in Figure 4D. With an increase of the velocity error, there was an increase of the number of saccades. Surprisingly, the increase of PVEON was accompanied by a decrease of the PPEON. Therefore with a small velocity error, the saccadic system triggered few saccades and tolerated a larger position error. On the other hand, a larger number of saccades were triggered with a small position error and a large velocity error. We fitted an exponential function on PVEON and PPEON for the two pools of data (positive and negative values of PPEON). The four fits resulted in:
 |
 |
 |
 |
Solid lines in Figure 4D and E represent the fits (Equations 14–15) for the positive values of PPEON. Dashed lines represent the fits (Equations 16–17) corresponding to the negative values of PPEON. The fits quantitatively confirm our interpretation above: The number of triggered saccades increased with the retinal slip, even if the position error decreased. This analysis shows that velocity error at saccade onset was the major parameter to trigger saccades in our head-unrestrained paradigm. It also confirms that saccade amplitude is better correlated with velocity error than with position error.
Head contribution and saccades accuracy
We computed HC for SA ≥ 5°. The 5, 50, and 95 percentiles of the resulting HC distribution are respectively equal to 0.142, 0.465, and 0.873. This shows that the head contributed on average for about 50% of the gaze saccadic displacement in our experiment.
Then, we tested if the head contribution to the saccadic displacement had an effect on the accuracy of the catch-up saccades. To do so, we computed a linear regression between the corrected position error at the end of the saccade (PPEOFF) and HC. The regression resulted in:
 |
PPEOFF was computed similarly to PPEON using the sign of ṪOFF to correct for the target velocity at the offset of the saccade.
Regression (Equation 18) shows that the contribution of the head to the gaze displacement had no significant effect on the accuracy of the saccade pointing towards a gaze saccade control scheme.
Daye et al. (2012) showed that the gain of head movement during head-unrestrained tracking is slightly bigger for horizontal movements than for vertical movements (see equation 6 in Daye et al., 2012). Thus, horizontal head movements could influence differently the accuracy of catch-up saccades than vertical head movements. To test this assumption, we isolated trials with target orientations comprised between +5° and −5° around the horizontal (1,890 saccades were selected) and vertical (1,995 saccades were selected) axes. Then we computed a similar linear regression to Equation 18 on each population:
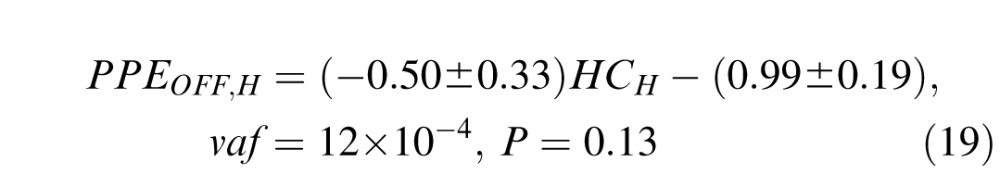 |
 |
As for Equation 18, the low vaf of fits (Equations 19–20) shows that catch-up saccade accuracy was insensitive to the head contribution, even when the gain of head movement was the largest.
Discussion
In this study, we demonstrate that participants used more saccades to compensate for the decrease of tracking performances with an increase of target oscillation frequency. We show that saccade amplitude was better explained by a multiple regression using the position error, velocity error, and acceleration error at saccade onset as the independent variables than with first-order regressions with the position error. Then, we show that the number of saccades was modulated by the velocity of the target: More saccades were triggered when target velocity was large and when the target was accelerating. Our analyses indicate that the majority of the saccades were triggered with a large predicted velocity error and a small predicted position error. Finally, we show that the head contribution to the gaze saccade did not modulate the accuracy of saccades.
Saccade amplitude
Because most of the saccades were triggered with a large velocity error and a small position error, our observation that saccade amplitude was better correlated with PVEON than PPEON is not surprising. In head-restrained conditions, de Brouwer, Missal et al. (2002) showed that catch-up saccade amplitude was better described by a regression on PE100 and RS100 than by a single regression on either PE100 or RS100. Becker and Jürgens (1979) showed that the last time a change of target position can influence saccade amplitude was around 100 ms before saccade onset. Therefore, de Brouwer, Missal et al. (2002), de Brouwer et al. (2001), and de Brouwer, Yuksel et al. (2002) proposed that PE100 and RS100 correspond to sensory inputs delayed by 100 ms. A decade before Becker and Jürgens (1979), Barmack (1970) showed that a step of target velocity occurring 50 ms before saccade onset still modulates the amplitude of catch-up saccades. All those results pointed towards an influence of the retinal slip when programming gaze saccade amplitude in head-restrained conditions but with different latencies between a stimulus change and a modification of the behavior.
As for de Brouwer, Missal et al. (2002), our analyses show that saccade amplitude was better represented by a multiple regression using the position error and the velocity error as independent parameters. However, there are several differences between this study and de Brouwer, Missal et al. (2002). First, our analyses show that saccade amplitude was better explained with the position error and the velocity error at the onset of the gaze saccade than with PE and RS 100 ms before saccade onset (corresponding to sensory information, as in de Brouwer, Missal et al., 2002). We think that this difference arose because our stimulus had an acceleration component that was not present in the previous head-restrained studies with step-ramp stimuli (de Brouwer, Missal et al., 2002; de Brouwer et al., 2001; de Brouwer, Yuksel et al., 2002). Figure 2 shows that most of the saccades were triggered when the stimulus was accelerating. Because of the large target oscillation frequency, several saccades were triggered close to the target position extrema. At those phases in the cycle, the position error and the retinal slip 100 ms before the onset of the movement can have a sign opposite to the amplitude of the saccade (the closer saccade onset is to the position extrema, the more likely the sign will be reversed). In those conditions, the saccadic system would need to know that the sensory information is not correctly aligned with the desired displacement to program accurately the corresponding saccade. Therefore, because of the periodic nature of the task, the addition of the acceleration error 100 ms before saccade onset increases the quality of the regressions. This was predictable because the acceleration term can be used to help correct the sensory information by informing the saccadic system of the nature of the velocity signal (accelerating versus decelerating). However, even if cvMSE decreases when the acceleration term is included, the analyses show that saccade amplitude was always better explained with the parameters at saccade onset than with the same parameter sampled 100 ms before saccade onset. We also tested if the difference between head-restrained and head-unrestrained observations could be explained by the release of the head. All our subjects showed a statistically significant increase of the fit quality independently of the head contribution during saccades when sampling parameters at 0 ms instead of 100 ms prior to saccade onset. This result demonstrates that the differences between our experiment and de Brouwer, Missal et al. (2002), de Brouwer et al. (2001), and de Brouwer, Yuksel et al. (2002) could not be explained by the release of the head. Finally, we show a strong significant decrease of cvMSE as a function of the latency for all target oscillation frequency bins.
Comparing pursuit movements between sinusoidal and unpredictable target motion, Westheimer (1954) concluded that “the pursuit reaction time becomes eliminated” once “the subject becomes familiar with the target motion,” thus “he is able to anticipate the latter [target motion].” Eight years later, Stark et al. (1962) demonstrated that the CNS uses an internal model of the target movement (to anticipate target displacement) to control pursuit movements and overcome visual delays in head-restrained condition. This means that the CNS has access to an estimate of target movement to control the eye movement. In addition to visual delays, the CNS must also account for the large inertia of the head during head-unrestrained pursuit. Thus, we think that head-unrestrained pursuit must rely strongly on internal signals. This is in agreement with the study of Ackerley and Barnes (2011) in which the authors showed that the head movement is more robust to an extinction of the target than gaze.
Recently, it has been demonstrated that gaze and head movements during head-restrained (Barnes & Collins, 2008) and head-unrestrained (Ackerley & Barnes, 2011) pursuit are driven by an internal model of target velocity. With their step-ramp paradigm, the authors showed that an initial presentation of target movement during 100–150 ms was necessary to ensure a proper estimate of a constant velocity target motion. Finally, Bennett, Orban de Xivry, Barnes, and Lefèvre (2007) showed that target acceleration influences subjects smooth pursuit for target presentation longer than ∼500 ms. Therefore, we think that a similar mechanism was used during our experiment with the difference that our target trajectory is nonlinear and thus building the internal model of target velocity takes longer in our experiment compared to Ackerley and Barnes (2011). Nevertheless, the experiment of Ackerley and Barnes (2011) confirmed that an internal model of target velocity is built during head-unrestrained tracking.
Several authors have shown the involvement of the cerebellum during pursuit (see Krauzlis, 2004 for a review of the neural pathways involved during pursuit). Ron and Robinson (1973) reported pursuit eye movements when electrically stimulating lobulus simplex, crus I and crus II of cerebellar cortex. Later, Krauzlis (2000) recorded the activity of the ventral paraflocculus and proposed that it reflects the dynamic compensation of the internal delays by the cerebellum. Ron and Robinson (1973) showed that stimulations of lobes V, VI, and VII of the vermis, as well as lobulus simplex and crus I and II, produce saccadic eye movements, suggesting an implication of these structures in saccadic eye movements. Later, Ritchie (1976) lesioned vermal and paravermal areas of the cerebellar cortex in monkeys and reported saccade dysmetria, demonstrating the involvement of these cerebellar areas during saccades.
We propose that the velocity information used to compensate for the displacement of the target during catch-up saccades both in head-restrained and head-unrestrained conditions corresponds to the signal used to control pursuit. Because Ron and Robinson (1973) reported that stimulations of lobulus simplex and crus I and II of the cerebellar cortex produces saccades and smooth pursuit, those sites could be candidates for neural areas in which both velocity and position signals are mixed to correct catch-up saccade amplitude. However, it is clear that further studies should be conducted to define clearly which neural areas are needed to control catch-up saccades amplitude. The same signal could be used for the head-restrained one-dimensional step-ramp paradigm (de Brouwer, Missal et al., 2002; de Brouwer et al., 2001; de Brouwer, Yuksel et al., 2002), orthogonal position and velocity steps paradigm (Fleuriet & Goffart, 2012; Fleuriet et al., 2011), head-unrestrained step-ramp paradigm (Ackerley & Barnes, 2011), and our sinusoidal paradigm. We think the original head-fixed protocol (de Brouwer, Missal et al., 2002; de Brouwer et al., 2001; de Brouwer, Yuksel et al., 2002) could not have isolated the difference between delayed sensory inputs and an internal velocity signal used to control pursuit movements because the target had no velocity change during the pursuit of the ramps. However, when the target jumped the acceleration was infinite. Therefore we think that the better approximation of saccade amplitude with position and velocity errors 100 ms before saccade onset in the original studies (de Brouwer, Missal et al., 2002; de Brouwer et al., 2001; de Brouwer, Yuksel et al., 2002) reflected the time needed by the CNS to update the internal model of the target movement. This delay is consistent (albeit shorter) with the observation of Barnes and Collins (2008) in which the authors showed that a target presentation of ∼150 ms is needed to build an internal representation of target movement in a step-ramp paradigm. The shorter delay (100 ms instead of 150 ms) observed in de Brouwer et al. (2001) could be explained because pursuit was already active in their protocol when the target jumped, while Ackerley and Barnes (2011) and Barnes and Collins (2008) looked at the initiation phase of pursuit. Finally, our results are also in agreement with Fleuriet and Goffart (2012) and Fleuriet et al. (2011) who proposed that the CNS continuously evaluates the position of the target to program catch-up saccade amplitude. Therefore, it is likely that the same mechanism, based on an internal representation of target velocity, is used to correct catch-up saccade amplitude both in head-restrained and head-unrestrained conditions. This internal representation of target trajectory could rely on an internal model based on a Kalman filter as recently proposed for modeling predictive smooth pursuit eye movements (Orban de Xivry, Coppe, Blohm, and Lefèvre, 2013).
Saccade triggering strategy
To quantify what triggered catch-up saccades in our protocol we plotted the evolution of the predicted position error and the predicted velocity error at saccade onset. We show that the amplitude of the position error at saccade onset was not influenced by the frequency of the oscillating target. On the contrary, the predicted velocity error at saccade onset was clearly modulated by the frequency of the pursuit target. This modulation is supplementary evidence for the saturation effect shown by the pursuit system for high target velocities (Meyer et al., 1985).
In the last part of this analysis, we show that to ensure that the mean position error remained low while tracking the target, the CNS had to trigger a saccade sooner than if the velocity error was small. These analyses show that the CNS, as in head-restrained conditions (de Brouwer, Yuksel et al., 2002), triggered head-unrestrained saccades based on both velocity error and position error.
de Brouwer, Yuksel et al. (2002) proposed the eye-crossing time (Txe = −PE/RS) as the parameter used to predict when to trigger a new saccade. As for the other analyses in de Brouwer, Missal et al. (2002), de Brouwer et al. (2001), and de Brouwer, Yuksel et al. (2002), Txe is based on delayed sensory signals evaluated from step-ramp target motion. The calculation of Txe corresponds to a linear prediction of when the eye would intersect target motion. If Txe is too long (or too short), a saccade is triggered. Our new analyses demonstrate that saccades are programmed using an internal signal representing target motion. Thus we would need the state of the internal model of target motion to compute the predictive trigger signal. Unfortunately, we do not have access to this signal. Therefore, we cannot conclude on the validity of Txe for periodic target motion with our experiment. However, data fits (Equations 13–16) show that there was a clear relationship between position and velocity errors to decide when a saccade will be triggered.
Saccade accuracy
With the head free to move, the CNS faces more challenges to control accurately a gaze saccade. One of them is the integration of the sensory information from both the eye and the head to program adequately the amplitude of catch-up saccades. To test the efficiency of this multisensory integration, we analyzed the sensitivity of saccade accuracy to the contribution of the head to the gaze saccadic displacement. If either the eye or the head were not correctly integrated, one can assume that the head contribution would modify the accuracy of the saccade. Regression (Equation 18) clearly shows that the contribution of the head did not have an influence on the accuracy of head-unrestrained gaze saccades in our paradigm. The independence of the saccadic accuracy to the head displacement during gaze shifts could also be explained by a fully functional VOR. It has been shown that the VOR is active but canceled by the pursuit signal during head-unrestrained pursuit (Lanman, Bizzi, & Allum, 1978) and that the VOR is suppressed during large gaze saccades (Cullen et al., 2004; Lefèvre et al., 1992). However, no study has been conducted to analyze the VOR gain during head-unrestrained catch-up saccades.
Conclusion
This paper analyzes catch-up saccades during the pursuit of a periodic target with the head free to move. During head-restrained studies, it has been assumed that the CNS uses sensory signals 100 ms before saccade onset to correct catch-up saccade amplitude. However, our analyses show that saccade amplitude in our periodic paradigm is better correlated with position, velocity, and acceleration errors at saccade onset than with those signals 100 ms before the onset. This is not consistent with the sole use of sensory information because of internal delays. Therefore, we suggest that catch-up saccades (both in head-restrained and head-unrestrained conditions) use an internal representation of target motion instead of sensory inputs 100 ms before saccade onset to account for target displacement during saccades when target trajectory is predictable.
Acknowledgments
Dr. Daye was supported by the Intramural Research Program of the National Eye Institute. Dr. Blohm has been supported by the National Science and Engineering Reserach Council (Canada), the Ontario Research Fund (Canada), the Canadian Foundation for Innovation (Canada), and the Botterell Foundation (Queens University, Kingston, ON, Canada). De. Lefevre has been supported by Fonds National de la Recherche Scientifique, Action de Recherche Concertée (Belgium). This paper presents research results of the Belgian Network Dynamical Systems, Control and Optimization, funded by the Interuniversity Attraction Poles Programmes, initiated by the Belgian State, Science Policy Office.
Commercial relationships: none.
Corresponding author: Pierre M. Daye.
Email: pierre.daye@nih.gov.
Address: NIH, NEI Laboratory of Sensorimotor Research, Bethesda, MD.
Footnotes
Parameters were randomized independently using uniform distributions between the specified boundaries.
Contributor Information
Pierre M. Daye, Email: pierre.daye@gmail.com.
Gunnar Blohm, Email: gunnar.blohm@queensu.ca.
Phillippe Lefèvre, Email: philippe.lefevre@uclouvain.be.
References
- Ackerley R., Barnes G. R. (2011). Extraction of visual motion information for the control of eye and head movement during head-free pursuit. Experimental Brain Research, 210 (3), 569–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahill A. T., Clark M. R., Stark L. (1975). The main sequence, a tool for studying human eye movements. Mathematical Biosciences, 24 (3–4), 191–204. [Google Scholar]
- Barmack N. H. (1970). Modification of eye movements by instantaneous changes in the velocity of visual targets. Vision Research, 10 (12), 1431–1441. [DOI] [PubMed] [Google Scholar]
- Barnes G. R., Collins C. J. S. (2008). The influence of briefly presented randomized target motion on the extraretinal component of ocular pursuit. Journal of Neurophysiology, 99 (2), 831–842. [DOI] [PubMed] [Google Scholar]
- Becker W., Jürgens R. (1979). An analysis of the saccadic system by means of double step stimuli. Vision Research, 19 (9), 967–983. [DOI] [PubMed] [Google Scholar]
- Becker W., Jürgens R. (1990). Human oblique saccades: Quantitative analysis of the relation between horizontal and vertical components. Vision Research, 30 (6), 893–920. [DOI] [PubMed] [Google Scholar]
- Bennett S. J., Orban de Xivry J. J., Barnes G. R., Lefèvre P. (2007). Target acceleration can be extracted and represented within the predictive drive to ocular pursuit. Journal of Neurophysiology, 98 (3), 1405–1414. [DOI] [PubMed] [Google Scholar]
- Blohm G., Missal M., Lefèvre P. (2003). Interaction between smooth anticipation and saccades during ocular orientation in darkness. Journal of Neurophysiology, 89 (3), 1423. [DOI] [PubMed] [Google Scholar]
- Blohm G., Missal M., Lefèvre P. (2005). Direct evidence for a position input to the smooth pursuit system. Journal of Neurophysiology, 94 (1), 712–721. [DOI] [PubMed] [Google Scholar]
- Carl J. R., Gellman R. S. (1987). Human smooth pursuit: Stimulus-dependent responses. Journal of Neurophysiology, 57 (5), 1446–1463. [DOI] [PubMed] [Google Scholar]
- Collins C. J. S., Barnes G. R. (1999). Independent control of head and gaze movements during head-free pursuit in humans. Journal of Physiology, 515 (1), 299–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen K. E., Huterer M., Braidwood D. A., Sylvestre P. A. (2004). Time course of vestibuloocular reflex suppression during gaze shifts. Journal of Neurophysiology, 92 (6), 3408–3422. [DOI] [PubMed] [Google Scholar]
- Daye P. M., Blohm G., Lefèvre P. (2010). Saccadic compensation for smooth eye and head movements during head-unrestrained two-dimensional tracking. Journal of Neurophysiology, 103 (1), 543–556. [DOI] [PubMed] [Google Scholar]
- Daye P. M., Blohm G., Lefèvre P. (2012). Target motion direction influence on tracking performance and head tracking strategies in head-unrestrained conditions. Journal of Vision, 12 (1): 12 1–12, http://www.journalofvision.org/content/12/1/23, doi:10.1167/12.1.23. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- de Brouwer S., Missal M., Barnes G. R., Lefèvre P. (2002). Quantitative analysis of catch-up saccades during sustained pursuit. Journal of Neurophysiology, 87 (4), 1772–1780. [DOI] [PubMed] [Google Scholar]
- de Brouwer S., Missal M., Lefèvre P. (2001). Role of retinal slip in the prediction of target motion during smooth and saccadic pursuit. Journal of Neurophysiology, 86 (2), 550–558. [DOI] [PubMed] [Google Scholar]
- de Brouwer S., Yuksel D., Blohm G., Missal M., Lefèvre P. (2002). What triggers catch-up saccades during visual tracking? Journal of Neurophysiology, 87 (3), 1646–1650. [DOI] [PubMed] [Google Scholar]
- Dodge R. (1903). Five types of eye movement in the horizontal meridian plane of the field of regard. American Journal of Physiology, 8 (4), 307–329. [Google Scholar]
- Fleuriet J., Goffart L. (2012). Saccadic interception of a moving visual target after a spatiotemporal perturbation. Journal of Neuroscience, 32 (2), 452–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleuriet J., Hugues S., Perrinet L., Goffart L. (2011). Saccadic foveation of a moving visual target in the rhesus monkey. Journal of Neurophysiology, 105 (2), 883–895. [DOI] [PubMed] [Google Scholar]
- Freedman E. G., Sparks D. L. (1997). Eye-head coordination during head-unrestrained gaze shifts in rhesus monkeys. Journal of Neurophysiology, 77 (5), 2328–2348. [DOI] [PubMed] [Google Scholar]
- Goossens H. H. L. M., Van Opstal A. J. (1997). Human eye-head coordination in two dimensions under different sensorimotor conditions. Experimental Brain Research, 114 (3), 542–560. [DOI] [PubMed] [Google Scholar]
- Guitton D., Munoz D. P., Galiana H. L. (1990). Gaze control in the cat: Studies and modeling of the coupling between orienting eye and head movements in different behavioral tasks. Journal of Neurophysiology, 64 (2), 509–531. [DOI] [PubMed] [Google Scholar]
- Herter T. M., Guitton D. (1998). Human head-free gaze saccades to targets flashed before gaze-pursuit are spatially accurate. Journal of Neurophysiology, 80 (5), 2785–2789. [DOI] [PubMed] [Google Scholar]
- Kettner R. E., Leung H. C., Peterson B. W. (1996). Predictive smooth pursuit of complex two-dimensional trajectories in monkey: Component interactions. Experimental Brain Research, 108 (2), 221–235. [DOI] [PubMed] [Google Scholar]
- Krauzlis R. J. (2000). Population coding of movement dynamics by cerebellar Purkinje cells. Neuroreport, 11 (5), 1045–1050. [DOI] [PubMed] [Google Scholar]
- Krauzlis R. J. (2004). Recasting the smooth pursuit eye movement system. Journal of Neurophysiology, 91 (2), 591–603. [DOI] [PubMed] [Google Scholar]
- Lanman J., Bizzi E., Allum J. (1978). The coordination of eye and head movement during smooth pursuit. Brain Research, 153 (1), 39–53. [DOI] [PubMed] [Google Scholar]
- Lefèvre P., Bottemanne I., Roucoux A. (1992). Experimental study and modeling of vestibulo-ocular reflex modulation during large shifts of gaze in humans. Experimental Brain Research, 91 (3), 496–508. [DOI] [PubMed] [Google Scholar]
- Leung H. C., Kettner R. E. (1997). Predictive smooth pursuit of complex two-dimensional trajectories demonstrated by perturbation responses in monkeys. Vision Research, 37 (10), 1347–1354. [DOI] [PubMed] [Google Scholar]
- Meyer C. H., Lasker A. G., Robinson D. A. (1985). The upper limit of human smooth pursuit velocity. Vision Research, 25 (4), 561–563. [DOI] [PubMed] [Google Scholar]
- Morris E. J., Lisberger S. G. (1987). Different responses to small visual errors during initiation and maintenance of smooth-pursuit eye movements in monkeys. Journal of Neurophysiology, 58 (6), 1351–1369. [DOI] [PubMed] [Google Scholar]
- Orban de Xivry J. J., Coppe S., Blohm G., Lefèvre P. (2013). Kalman filtering naturally accounts for visually guided and predictive smooth pursuit dynamics. Journal of Neuroscience, 33 (44), 17301–17313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban de Xivry J. J., Lefèvre P. (2007). Saccades and pursuit: Two outcomes of a single sensorimotor process. Journal of Physiology, 584 (1), 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pola J., Wyatt H. J. (1980). Target position and velocity: The stimuli for smooth pursuit eye movements. Vision Research, 20 (6), 523–534. [DOI] [PubMed] [Google Scholar]
- Ritchie L. (1976). Effects of cerebellar lesions on saccadic eye movements. Journal of Neurophysiology, 39 (6), 1246–1256. [DOI] [PubMed] [Google Scholar]
- Ron S., Robinson D. A. (1973). Eye movements evoked by cerebellar stimulation in the alert monkey. Journal of Neurophysiology, 36 (6), 1004–1022. [DOI] [PubMed] [Google Scholar]
- Ronsse R., White O., Lefèvre P. (2007). Computation of gaze orientation under unrestrained head movements. Journal of Neuroscience Methods, 159 (1), 158–169. [DOI] [PubMed] [Google Scholar]
- Schreiber C., Missal M., Lefèvre P. (2006). Asynchrony between position and motion signals in the saccadic system. Journal of Neurophysiology, 95 (2), 960–969. [DOI] [PubMed] [Google Scholar]
- Stark L., Vossius G., Young L. R. (1962). Predictive control of eye tracking movements. IRE Transactions on Human Factors in Electronics, 3 (2), 52–57. [Google Scholar]
- Tomlinson R. D., Bahra P. S. (1986). Combined eye-head gaze shifts in the primate. I. Metrics. Journal of Neurophysiology, 56 (6), 1542–1557. [DOI] [PubMed] [Google Scholar]
- Westheimer G. (1954). Eye movement responses to a horizontally moving visual stimulus. Archives of Ophthalmology, 52 (6), 932–941. [DOI] [PubMed] [Google Scholar]