Abstract
Respiratory syncytial virus (RSV) is responsible for majority of infant hospitalizations due to viral infections. Despite its clinical importance, no vaccine against RSV or effective antiviral therapy is available. Several structural classes of small-molecule RSV entry inhibitor have been described and one compound has advanced to clinical testing. Mutations in either one of two resistance hot spots in the F protein mediate unusual pan-resistance to all of these inhibitor classes. Based on the biochemical characterization of resistant viruses and structural insight into the RSV F trimer, we propose a kinetic escape model as the origin of pan-resistance. Since a resistant RSV remained pathogenic in the mouse model, pan-resistance mutations could emerge rapidly in circulating RSV strains. We evaluate clinical implications and discuss consequences for the design of future RSV drug discovery campaigns.
Introduction
Pathogens of the paramyxovirus family such as respiratory syncytial virus (RSV), measles virus, mumps virus, or zoonotic Nipah virus are responsible for major human diseases. RSV is the leading cause of infant hospitalization due to viral infection in the United States, and worldwide RSV disease-related deaths are estimated at 66,000–199,000 among children below 5 years of age (Nair et al., 2010). At the highest risk of developing severe RSV disease are prematurely born infants, children with congenital heart defects or bronchopulmonary dysplasia, the elderly, and the immunosuppressed.
Like most paramyxoviruses, RSV is a highly contagious airborne pathogen that spreads through the respiratory route. While RSV elicits innate and adaptive immune responses, the virus is poorly immunogenic overall and neutralizing antibody titers wane quickly postinfection (Collins and Crowe, 2007), allowing repeated reinfection throughout life (Collins and Crowe, 2007) and challenging the vaccine development. Palivizumab immunoprophylaxis can be administered to high-risk patients, but its high cost burden is prohibitive (Kamal-Bahl et al., 2002; Broor et al., 2007; Weiner et al., 2012; Mahadevia et al., 2012a, 2012b, Hampp et al., 2011). Ribavirin, although approved for RSV treatment, has little value due to efficacy and toxicity issues (Anderson et al., 1990; Hall et al., 1993). Current disease management is therefore largely limited to supportive care, such as oxygen therapy, generating an urgent clinical need for effective therapies (Bjornson and Johnson, 2008; Johnson, 2009).
Developmental Status of Next-Generation Anti-RSV Therapeutics
Of the many experimental RSV inhibitors identified in recent years, two orally available drug candidates have advanced to phase 2 clinical trials. The nucleoside compound ALS-8176 is a competitive inhibitor of the RSV RNA-dependent RNA polymerase (RdRp) complex (Wang et al., 2015), while Gilead's compound GS-5806 functions as an allosteric blocker of the fusion (F) protein, preventing viral entry (Devincenzo et al., 2014; Mackman et al., 2015). Both candidates decreased the viral load and disease symptoms when administered in challenge studies to healthy adult volunteers, although the rate of virus load reduction by ALS-8176 seemed to exceed that of GS-5806 (Devincenzo et al., 2014).
In addition to Gilead's GS-5806, a panel of seven structurally distinct RSV entry inhibitors have been identified and characterized in recent years (Wyde et al., 1998; McKimm-Breschkin, 2000; Razinkov et al., 2001; Andries et al., 2003; Douglas et al., 2003; Wyde et al., 2003; Yu et al., 2003; Cianci et al., 2004; Yan et al., 2014). The RSV cell-entry machinery consists of two envelope protein complexes, a glycoprotein (G) tetramer in addition to the F protein trimer. Remarkably, viral resistance mutations to this diverse panel of compounds mapped in all cases to two distinct microdomains of the F protein, spanning residues 392–401 and 486–489, respectively (Yan et al., 2014). Considering the very diverse chemical and structural nature of the different entry inhibitor classes, this overlapping resistance profile is unexpected.
RSV Envelope Glycoproteins
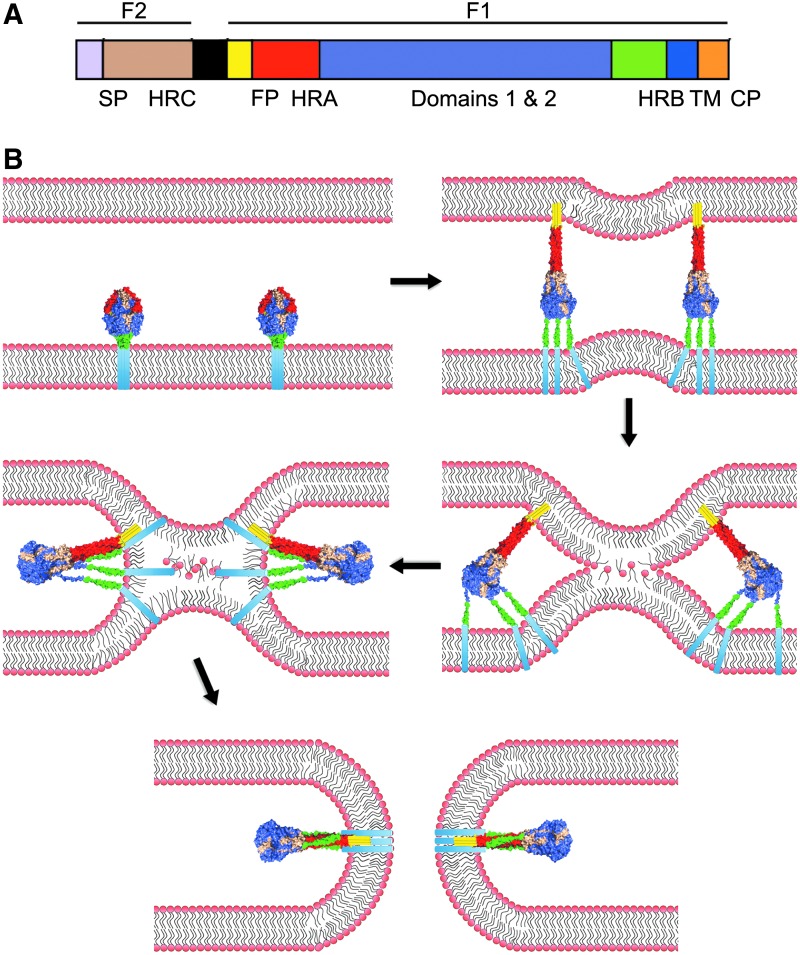
Paramyxovirus F proteins belong to the class I of viral fusion proteins that also includes, among others, HIV Env and Ebola virus GP (Lamb and Parks, 2007). Newly synthesized RSV F trimers initially fold into a metastable prefusion conformation (Fig. 1 shows a schematic of the RSV F organization and refolding process.). Proteolytic maturation by furin-type proteases generates a new amino-terminus of the larger F1 subunit, which also contains the transmembrane domain. This newly liberated N-terminus is located at the beginning of a hydrophobic domain, the fusion peptide, which represents the membrane attack group.
FIG. 1.
The respiratory syncytial virus (RSV) fusion machinery. (A) Schematic of the domain structure of the RSV fusion protein. RSV F is proteolytically cleaved into F1 and F2 subunits. The F1 subunit consists of the fusion peptide (FP; yellow), heptad-repeat A (HR-A; red), domains 1 and 2 (violet), heptad-repeat B (HR-B; green), a transmembrane domain (TM; blue), and the cytoplasmic tail (CP; orange). The F2 subunit consists of heptad-repeat C (HRC; tan). (B) For the viral entry, RSV F undergoes a series of conformational changes that result in the formation of the HR-A central trimeric coil and insertion of the fusion peptide into the target membrane. The HR-B domains then fold into the grooves of the HR-A triple helix, inducing local negative curvature in the membranes that promotes merger of the outer leaflets of the bilayers (hemifusion stage). Assembly of the 6HB leads to opening of a fusion pore. Color-coding of individual F domains as in (A).
Upon fusion activation, the prefusion F head domain reorganizes into an extended trimeric coil composed of heptad repeat (HR)-A helices, propelling the fusion peptide toward the target membrane. Through folding of shorter HR-B helices, located adjacent to the transmembrane domains, into the grooves of the extended central trimeric coil, a thermodynamically highly stable 6-helix bundle (6HB) forms, through which the F transmembrane domains, fusion peptides, and consequently the viral envelope and target membrane are brought into proximity. While this 6HB “fusion core” must not necessarily be fully closed for opening of a fusion pore (Brindley et al., 2014), the initial engagement of the HR-A helices by the refolding HR-Bs is essential for fusion.
Proposed Mechanism of Activity of Small-Molecule RSV Entry Inhibitors
Biochemical positive-target identification campaigns were launched for three structurally distinct classes of RSV entry inhibitors. Using a tritium-labeled version of the nanomolar blocker VP-14637, specific binding of the compound to the RSV F protein was demonstrated (Douglas et al., 2003). This interaction was markedly reduced at a lower temperature, suggesting that the compound recognizes a refolding intermediate that transiently emerges as F advances toward the postfusion conformation. Resistance mutations supported F targeting by the compound, since escape hot spots mapped to F residues 400 and 488 (Douglas et al., 2005).
Target-site identification was furthermore attempted for BMS-433771 and TMC-353131, two highly potent (EC50 7.9 and 0.13 nM, respectively) RSV entry inhibitors that are structurally distinct from each other and VP-14367 (Cianci et al., 2004; Roymans et al., 2010). Radio-labeled photoactivatable analogs of both compounds cross-linked with RSV F residue Y198, suggesting that this residue is located within or near the physical compound target site (Cianci et al., 2004; Roymans et al., 2010). Residue Y198 lines a hydrophobic pocket that transiently emerges in the extended HR-A central trimeric coil of the prehairpin F structure (Fig. 2). During hairpin formation, cognate residues in HR-B, including the VP-14637 resistance hot spot 488, insert into this pocket for 6HB assembly.
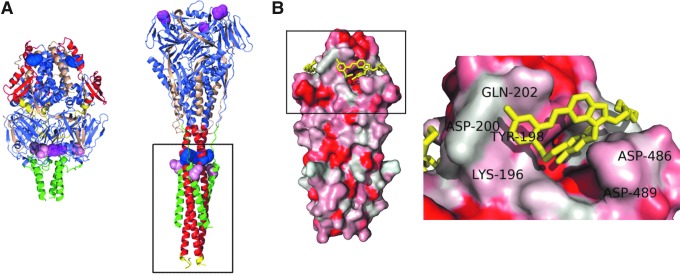
FIG. 2.
Structure of the RSV F protein. (A) Crystal structures of RSV F in the prefusion (PDB ID:4MMQ) and postfusion (PDB ID: 4RRR) conformations. Pan-resistance hot spots D401 (magenta) and D489 (pink) and residue Y198 (blue) identified in covalent inhibitor cross-linking studies are highlighted. F domains are color-coded as in Figure 1A. (B) Cocrystal structures of synthetic RSV F 6HB [boxed out region in (A)] and TMC-353121 (yellow) (PDB ID: 3KPE). The close-up shows TMC-353121 filling the hydrophobic HR-A pocket typically occupied by HR-B residues 486 and 487. 6HB coloring is based on hydrophobicity, red being most hydrophobic. Selected surrounding HR-A and HR-B residues are labeled.
The RSV Entry Inhibition Conundrum
These findings appear to spotlight a straight-forward mechanism of antiviral activity of these entry inhibitor classes (Douglas et al., 2005): the compounds are thought to occupy the hydrophobic pocket that transiently emerges in the central trimeric coil in the prehairpin state (Fig. 1B) of refolding F, preventing subsequent insertion of the cognate HR-B residues and therefore the initiation of 6HB formation. However, four lines of evidence challenge this model.
First, the crosslinking data suggest that at least two compound classes directly dock into the hydrophobic pocket and thus physically interact with HR-A, but curiously none of the identified escape mutations are localized in the pocket or HR-A at large. Rather, a broad cross-resistance against all of the entry inhibitors mapped to residues 486–489 in HR-B. This pattern could be due to nonsaturated resistance site mapping or general interference of HR-A mutations with class I fusion protein functionality. However, at least five RSV adaptation campaigns were carried out by different laboratories with different compounds in past years, creating confidence that the available data, in aggregate, represent a comprehensive profile of molecular escape pathways. Furthermore, the peptidic HIV entry inhibitor enfuvirtide, which targets the central trimeric core in refolding HIV gp41, analogous to the model for RSV entry inhibitors, induces primary resistance mutations in the core itself (Mink et al., 2005). This precedence argues against a hard mechanistic block preventing class I fusion protein core changes.
Second, the picomolar RSV blocker TMC-353121 bound to synthetic HR-A-derived peptides only in the presence of HR-B peptides, and in vitro data revealed that the compound promoted, rather than disrupted, 6HB formation (Roymans et al., 2010). To explain this counterintuitive finding, the study authors hypothesized that compound-stabilized 6HBs may be nonproductive, since synthetic peptides showed a small disruption in the 6HB zipper near the HR-B N-terminus. However, it is unclear whether this disturbance is equally present when the 6HB is in its native F protein environment and the question remains why the compound is unable to occupy the HR-A pocket in the absence of the HR-B domain.
Third, a viral escape from all seven well-characterized RSV entry inhibitors reported in the literature can also be achieved through mutations in a second F microdomain-spanning residues 392–401 (summarized in Yan et al., 2014). In postfusion F, this second cross-resistance hot spot maps to the membrane distal-most end of the protein, approximately 100 Å from the F 486–489 microdomain and the 6HB fusion core (Fig. 2). There is no structural explanation for resistance mutations at positions 392–401 if the compounds dock into the HR-A pocket, and previous cross-linking studies did not develop a comprehensive hypothesis that considers all known resistance hot spots.
Finally, if the resistance mediated by mutations in the 486–489 microdomain indicates compound docking into the HR-A pocket, at least seven structurally distinct and mostly extremely potent compounds would be able to form high-affinity interactions with this domain. While a common target for this diverse panel of compounds cannot be ruled out categorically, such a universal drug-binding site would be unusual. Well-behaved inhibitors typically engage their target through a limited set of high-affinity interactions [typically three to four contact points (Arkin et al., 2014)], making it challenging to reconcile how compounds with different three-dimensional geometries and distinct functionalities could all be accommodated by the same microdomain.
Kinetic Versus Thermodynamic Resistance
We identified and characterized another small-molecule RSV entry inhibitor class that is sensitive to resistance mutations in the 392–401 and 486–489 microdomains. Substitutions in either resistance hot spots were associated with an F hyperfusion activity (Yan et al., 2014). This raises the question whether resistance due to substitutions in residues 486–489 is indeed due to a thermodynamic effect—mutations in the physical binding site eliminate high-affinity interactions—or is rather the result of faster F refolding kinetics. In the kinetic resistance model, accelerated F refolding is expected to narrow the window of opportunity for compound interference with transiently emerging target sites, resembling the escape mechanism proposed for second-generation peptidic inhibitors of HIV env-mediated membrane fusion (Reeves et al., 2002; Hermann et al., 2009; De Feo et al., 2014; Su et al., 2015).
When we located the two resistance hot spots in the recently solved crystal structure of prefusion RSV F (McLellan et al., 2013), we found that residues in either domain were located in immediate proximity to each other at the interface between the top of the prefusion F stalk and the base of the head domain (Fig. 2). This interface is stabilized by a network of noncovalent interactions in other paramyxovirus F proteins such as measles virus and parainfluenzavirus 5 F (Doyle et al., 2006; Yin et al., 2006; Bose et al., 2013). Resembling these related F proteins, RSV F resistance mutations induced hyperfusogenicity after transient F expression and when incorporated into recombinant virions and biochemical assays demonstrated a higher propensity for F refolding into the postfusion conformation (Yan et al., 2014).
These findings are consistent with the kinetic resistance model, which explains several of the open questions associated with RSV F entry inhibitors such as the unusual pan-resistance effect and the sensitivity of all inhibitors tested to mutations in the HR-B domain and in the microdomain spanning residues 392–401. In this view, pan-resistance is a consequence of the destabilizing effect of the mutations on prefusion F and not an altered affinity of HR-B residues to the central HR-A trimeric coil. Consequently, the proximity of F residues 489 and 189 in the fully assembled final 6HB structure may be, although tantalizing, a coincidence rather than an indicator of the compound target site. Although the mystery remains that resistance mutations have never emerged in linear proximity to residue 198, we consider physical docking of BMS-433771 and TMC-353131 into the HR-A pocket most likely, based on the TMC-353131 cross-linking to RSV F HR domain-derived synthetic peptides (Roymans et al., 2010).
Target site identification becomes less clear for the structurally unrelated RSV entry inhibitors that are also sensitive to mutations in microdomains 392–401 and 486–489. We propose that the resistance resulting from substitutions in these microdomains alone provides little evidence for physical compound docking into the HR-A hydrophobic pocket. Rather, these compounds may dock into unrelated F target sites, since the kinetic-driven pan-resistance mechanism affords a viral escape from all entry inhibitors that act through stabilizing an F prefusion conformation or refolding intermediate.
Clinical Implications and Consequences for RSV Drug Screening Campaigns
While one may anticipate that reduced conformational stability of the prefusion F trimer results in viral attenuation, RSV recombinants harboring a resistance mutation at position 401 were as pathogenic as the wild-type virus in a mouse model of RSV infection (Yan et al., 2014). Should resistant RSV strains remain virulent also in the human host, pan-resistance may quickly emerge in circulating RSV strains, eliminate the clinical impact of entry inhibitors such as GS-5806, and may exclude combination therapy attempts employing different entry inhibitors. Combination with polymerase inhibitors may be more fruitful, but only until preexisting pan-resistance is widespread in circulating strains.
The correlation between RSV F protein fusogenicity and viral pathogenesis is poorly understood, but RSV recombinants have revealed that an increased F fusion activity coincides with higher viral loads early after infection and severe lung histopathology in the mouse model of RSV infection (Hotard et al., 2015). Should this observation extend to the human host, a clinical implementation of RSV entry inhibitors could trigger the emergence of resistant viruses that cause enhanced diseases. In addition, palivizumab immunoprophylaxis is based on the neutralization of fusion activity through the antibody binding to the F protein. The consequences of pan-resistance mutations for palivizumab inhibition are untested thus far, but the problem of enhanced disease would be aggravated should pan-resistance compromise palivizumab efficacy.
The surprising number of structurally distinct RSV entry inhibitor classes reported in the literature suggests that open anti-RSV drug discovery campaigns are biased for the identification of F blockers. To avoid the pan-resistance trap in future campaigns, anti-RSV drug screens should be proactively designed to eliminate hits early that are sensitive to this escape mechanism. As a minimum, counterscreens against RSV strains carrying the signature escape mutations should be integrated into the hit-validation process. Better, RSV reporter strains should be used for primary screens that harbor these resistance mutations, thus preventing the hit discovery pipeline from being clogged with undesirable entry of inhibitor candidates. This approach should shift the hit pool toward viral polymerase inhibitors or blockers of particle assembly and release. Alternatively, this strategy may identify novel RSV entry inhibitor candidates that are insensitive to F pan-resistance. These compounds would have high developmental potential and be mechanistically intriguing, but have remained elusive thus far in our recent screening campaigns with pan-resistant RSV reporter strains. While impossible to tell without further screening data, it may be mechanistically very challenging for a small-molecule inhibitor to overcome the RSV F pan-resistance mechanism.
Acknowledgments
We thank A. L. Hammond for critical reading of the article. Research of the authors is supported, in part, by Public Health Service Grants AI071002, AI083402, and HD079327 from the NIH/NIAID to R.K.P.
Disclosure Statement
No competing financial interests exist.
References
- Anderson L.J., Parker R.A., and Strikas R.L. (1990). Association between respiratory syncytial virus outbreaks and lower respiratory tract deaths of infants and young children. J Infect Dis 161, 640–646 [DOI] [PubMed] [Google Scholar]
- Andries K., Moeremans M., Gevers T., Willebrords R., Sommen C., Lacrampe J., et al. (2003). Substituted benzimidazoles with nanomolar activity against respiratory syncytial virus. Antiviral Res 60, 209–219 [DOI] [PubMed] [Google Scholar]
- Arkin M.R., Tang Y., and Wells J.A. (2014). Small-molecule inhibitors of protein-protein interactions: progressing toward the reality. Chem Biol 21, 1102–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornson C.L., and Johnson D.W. (2008). Croup. Lancet 371, 329–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose S., Heath C.M., Shah P.A., Alayyoubi M., Jardetzky T.S., and Lamb R.A. (2013). Mutations in the parainfluenza virus 5 fusion protein reveal domains important for fusion triggering and metastability. J Virol 87, 13520–13531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindley M.A., Plattet P., and Plemper R.K. (2014). Efficient replication of a paramyxovirus independent of full zippering of the fusion protein six-helix bundle domain. Proc Natl Acad Sci U S A 111, E3795–E3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broor S., Parveen S., Bharaj P., Prasad V.S., Srinivasulu K.N., Sumanth K.M., et al. (2007). A prospective three-year cohort study of the epidemiology and virology of acute respiratory infections of children in rural India. PLoS One 2, e491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianci C., Yu K.L., Combrink K., Sin N., Pearce B., Wang A., et al. (2004). Orally active fusion inhibitor of respiratory syncytial virus. Antimicrob Agents Chemother 48, 413–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P.L., and Crowe J.E., Jr. (2007). Respiratory syncytial virus and metapneumoviruses. In Fields Virology. Knipe D.M., and Howley P.M., eds. (Lippincott, Williams, & Wilkins, Philadelphia: ), pp. 1601–1645 [Google Scholar]
- De Feo C.J., Wang W., Hsieh M.L., Zhuang M., Vassell R., and Weiss C.D. (2014). Resistance to N-peptide fusion inhibitors correlates with thermodynamic stability of the gp41 six-helix bundle but not HIV entry kinetics. Retrovirology 11, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devincenzo J., Fathi H., Mcclure M., Westland C., Harrison L., Symons J., et al. (2014). Treatment with Oral ALS-008176, a Nucleoside Analog, Rapidly Reduces RSV Viral Load and Clinical Disease Severity in a Healthy Volunteer Challenge Study. Open Forum Infect Dis 1, S66–S69 [Google Scholar]
- Douglas J.L., Panis M.L., Ho E., Lin K.Y., Krawczyk S.H., Grant D.M., et al. (2003). Inhibition of respiratory syncytial virus fusion by the small molecule VP-14637 via specific interactions with F protein. J Virol 77, 5054–5064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas J.L., Panis M.L., Ho E., Lin K.Y., Krawczyk S.H., Grant D.M., et al. (2005). Small molecules VP-14637 and JNJ-2408068 inhibit respiratory syncytial virus fusion by similar mechanisms. Antimicrob Agents Chemother 49, 2460–2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle J., Prussia A., White L.K., Sun A., Liotta D.C., Snyder J.P., et al. (2006). Two domains that control prefusion stability and transport competence of the measles virus fusion protein. J Virol 80, 1524–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C.B., Granoff D.M., Gromisch D.S., Halsey N.A., Kohl S., Marcuse E.K., et al. (1993). Use of Ribavirin in the Treatment of Respiratory Syncytial Virus-Infection. Pediatrics 92, 501–504 [PubMed] [Google Scholar]
- Hampp C., Kauf T.L., Saidi A.S., and Winterstein A.G. (2011). Cost-effectiveness of respiratory syncytial virus prophylaxis in various indications. Arch Pediatr Adolesc Med 165, 498–505 [DOI] [PubMed] [Google Scholar]
- Hermann F.G., Egerer L., Brauer F., Gerum C., Schwalbe H., Dietrich U., et al. (2009). Mutations in gp120 contribute to the resistance of human immunodeficiency virus type 1 to membrane-anchored C-peptide maC46. J Virol 83, 4844–4853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotard A.L., Lee S., Currier M.G., Crowe J.E., Jr., Sakamoto K., Newcomb D.C., et al. (2015). Identification of residues in the human respiratory syncytial virus fusion protein that modulate fusion activity and pathogenesis. J Virol 89, 512–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D.W. (2009). Croup. BMJ Clin Evid 3, 321–361 [PMC free article] [PubMed] [Google Scholar]
- Kamal-Bahl S., Doshi J., Campbell J. (2002). Economic analyses of respiratory syncytial virus immunoprophylaxis in high-risk infants: a systematic review. Arch Pediatr Adolesc Med 156, 1034–1041 [DOI] [PubMed] [Google Scholar]
- Lamb R.A., and Parks G.D. (2007). Paramyxoviridae: the viruses and their replication. In Fields Virology. Knipe D.M. and Howley P.M., eds. (Wolters Kluwer/Lippincott Williams & Wilkins, Philadelphia: ), pp. 1449–1496 [Google Scholar]
- Mackman R.L., Sangi M., Sperandio D., Parrish J.P., Eisenberg E., Perron M., et al. (2015). Discovery of an Oral Respiratory Syncytial Virus (RSV) Fusion Inhibitor (GS-5806) and Clinical Proof of Concept in a Human RSV Challenge Study. J Med Chem 58, 1630–1643 [DOI] [PubMed] [Google Scholar]
- Mahadevia P.J., Makari D., and Masaquel A. (2012a). Methodological concerns regarding cost-effectiveness analysis of palivizumab in Florida Medicaid. Arch Pediatr Adolesc Med 166, 968–969; author reply 9–70 [DOI] [PubMed] [Google Scholar]
- Mahadevia P.J., Masaquel A.S., Polak M.J., and Weiner L.B. (2012b). Cost utility of palivizumab prophylaxis among pre-term infants in the United States: a national policy perspective. J Med Econ 15, 987–996 [DOI] [PubMed] [Google Scholar]
- McKimm-Breschkin J. (2000). VP-14637 ViroPharma. Curr Opin Invest Drugs 1, 425–427 [PubMed] [Google Scholar]
- McLellan J.S., Chen M., Leung S., Graepel K.W., Du X., Yang Y., et al. (2013). Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science 340, 1113–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink M., Mosier S.M., Janumpalli S., Davison D., Jin L., Melby T., et al. (2005). Impact of human immunodeficiency virus type 1 gp41 amino acid substitutions selected during enfuvirtide treatment on gp41 binding and antiviral potency of enfuvirtide in vitro. J Virol 79, 12447–12454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair H., Nokes D.J., Gessner B.D., Dherani M., Madhi S.A., Singleton R.J., et al. (2010). Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375, 1545–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razinkov V., Gazumyan A., Nikitenko A., Ellestad G., and Krishnamurthy G. (2001). RFI-641 inhibits entry of respiratory syncytial virus via interactions with fusion protein. Chem Biol 8, 645–659 [DOI] [PubMed] [Google Scholar]
- Reeves J.D., Gallo S.A., Ahmad N., Miamidian J.L., Harvey P.E., Sharron M., et al. (2002). Sensitivity of HIV-1 to entry inhibitors correlates with envelope/coreceptor affinity, receptor density, and fusion kinetics. Proc Natl Acad Sci U S A 99, 16249–16254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roymans D., De Bondt H.L., Arnoult E., Geluykens P., Gevers T., Van Ginderen M., et al. (2010). Binding of a potent small-molecule inhibitor of six-helix bundle formation requires interactions with both heptad-repeats of the RSV fusion protein. Proc Natl Acad Sci U S A 107, 308–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y., Chong H., Qiu Z., Xiong S., and He Y. (2015). Mechanism of HIV-1 resistance to short peptide fusion inhibitors targeting the Gp41 pocket. J Virol [Epub ahead of print]; DOI: 10.1128/JVI.00373-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Deval J., Hong J., Dyatkina N., Prhavc M., Taylor J., et al. (2015). Discovery of 4'-Chloromethyl-2'-deoxy-3',5'-di-O-isobutyryl-2'-fluorocytidine (ALS-8176), A first-in-class RSV polymerase inhibitor for treatment of human respiratory syncytial virus infection. J Med Chem 58, 1862–1878 [DOI] [PubMed] [Google Scholar]
- Weiner L.B., Masaquel A.S., Polak M.J., and Mahadevia P.J. (2012). Cost-effectiveness analysis of palivizumab among pre-term infant populations covered by Medicaid in the United States. J Med Econ 15, 997–1018 [DOI] [PubMed] [Google Scholar]
- Wyde P.R., Chetty S.N., Timmerman P., Gilbert B.E., and Andries K. (2003). Short duration aerosols of JNJ 2408068 (R170591) administered prophylactically or therapeutically protect cotton rats from experimental respiratory syncytial virus infection. Antivir Res 60, 221–231 [DOI] [PubMed] [Google Scholar]
- Wyde P.R., Moore-Poveda D.K., O'Hara B., Ding W.D., Mitsner B., and Gilbert B.E. (1998). CL387626 exhibits marked and unusual antiviral activity against respiratory syncytial virus in tissue culture and in cotton rats. Antivir Res 38, 31–42 [DOI] [PubMed] [Google Scholar]
- Yan D., Lee S., Thakkar V.D., Luo M., Moore M.L., and Plemper R.K. (2014). Cross-resistance mechanism of respiratory syncytial virus against structurally diverse entry inhibitors. Proc Natl Acad Sci U S A. 111, E3441–E3449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H.S., Wen X., Paterson R.G., Lamb R.A., and Jardetzky T.S. (2006). Structure of the parainfluenza virus 5 F protein in its metastable, prefusion conformation. Nature 439, 38–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K.L., Zhang Y., Civiello R.L., Kadow K.F., Cianci C., Krystal M., et al. (2003). Fundamental structure-activity relationships associated with a new structural class of respiratory syncytial virus inhibitor. Bioorg Med Chem Lett 13, 2141–2144 [DOI] [PubMed] [Google Scholar]