Abstract
Background
A transdisciplinary approach incorporating biological, psychological, behavioral, and genetic factors was taken to better identify proposed moderators of the effectiveness of an intervention to increase physical activity. This paper illustrates how theory-based individual difference variables can be integrated into a complex randomized controlled trial. The transdisciplinary framework guiding the selection of moderators, the COSTRIDE intervention study and sample, and the relationships among baseline variables are provided.
Methods
Participants were non-active individuals randomly assigned to either the STRIDE exercise or health-and-wellness contact control condition.
Results
Structural equation modeling was utilized to demonstrate that relationships among baseline variables confirm hypothesized relationships in the transdisciplinary framework.
Conclusions
Preliminary data from COSTRIDE suggest that interventions among sedentary individuals may be more effective if a broader range of factors influencing physical activity are considered.
Trial Registration
clinicaltrials.gov NCT01091857
Keywords: Exercise, Intervention Study, Transdisciplinary, Physiology, Mood
Background
Although the importance of physical activity is well known, many individuals remain sedentary (Pleis et al., 2009). Physical inactivity increases the risk of numerous deleterious physical and mental health outcomes (Penedo & Dahn, 2005), and sedentary behavior has been identified as one of the leading preventable causes of death in the United States (Mokdad et al., 2004). Current recommendations are that able individuals engage in no less than 30 minutes of moderate-intensity physical activity (e.g., walking at a brisk pace) on most days of the week (Haskell et al., 2007; USDHHS, 2008), yet according to the National Health Interview Survey, only 32.4% of adults in the United States are active at this level (Pleis et al., 2009). Clearly, it is extremely important to develop successful interventions to increase physical activity among sedentary adults.
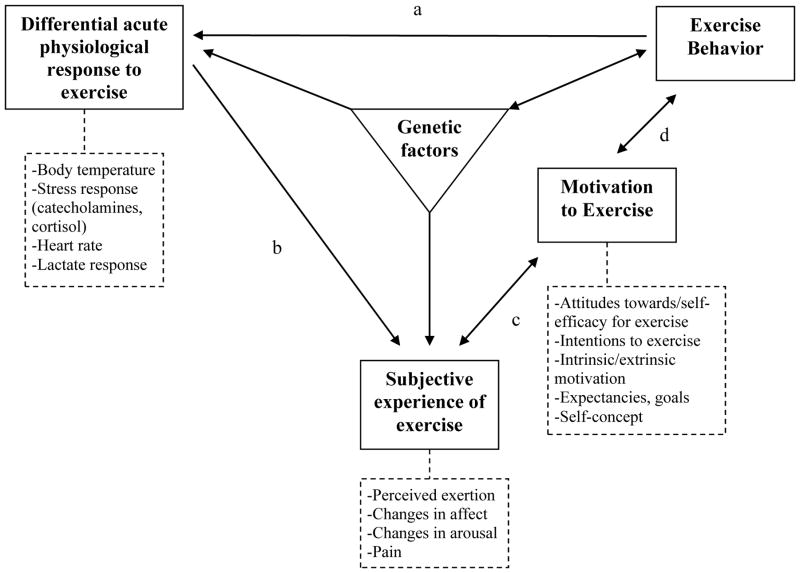
The delineation of individual differences influencing exercise behavior is complex and likely includes social-cognitive, genetic, physiological, and affective factors (Bryan et al., 2007; 2011). One potential way of organizing individual differences important to understanding who is most likely to initiate exercise behavior is a transdisciplinary framework we recently proposed (see Figure 1; Bryan et al., 2007; 2011). In this framework, genetic factors influence both an individual’s physiological responses to exercise (e.g., body-temperature regulation), their subjective experience of exercise (e.g., affective response, perceived exertion), and potentially directly influence exercise behavior. Physiological responses may also influence one’s subjective experiences, (path b) and those subjective experiences will influence motivation to exercise (path c, e.g., attitudes towards exercise, self-efficacy for exercise, intentions to exercise; Kwan & Bryan, 2010a). Importantly, the subjective experiences of exercise include interpretations of physiological changes occurring during exercise (e.g., perceived pain due to increased lactate). Motivation, in turn, influences exercise behavior (pathway d) and increased exercise behavior (i.e., more minutes of physical activity per week) recapitulates the model, affecting both physiological responses to exercise (path a), and, potentially, gene expression (Booth & Neufer, 2006). Therefore, the framework is circular and dynamic. The framework also proposes two bidirectional paths (c and d) which highlight the complexity of the interplay between physiological, behavioral, and cognitive factors. Path c proposes that not only will affective responses to exercise lead to increased motivation to exercise (Bryan et al., 2011; Kwan & Bryan, 2010b), but increased motivation can also influence affective responses to exercise (Annesi, 2005). Path d proposes that motivation will not only lead to more sustained exercise over time, but exercise itself will increase motivation. For example, in a prospective study among older adults, engaging in exercise predicted self-efficacy towards exercise (McAuley et al., 2003).
Figure 1. Transdisciplinary Framework for Exercise Behavior.
Note. Paths: aFitness to physiological exercise response, bPhysiological exercise response to affective exercise response, cAffective exercise response to exercise motivation, and dExercise motivation to exercise behavior.
For the current illustration of this framework, motivational constructs were derived from multiple motivational perspectives in order to illustrate that the motivational constructs can be captured using any theory-based theoretical motivational construct depending on the goals of the individual research study. In this study we used a range of motivational constructs including attitudes, norms, and intentions regarding the behavior from the Theory of Planned Behavior (Ajzen, 1991), which has been used to predict a wide range of health behaviors, including physical activity (Armitage, 2005). We also measured self-efficacy from Social-Cognitive Theory (Bandura, 1986), a construct which has consistently predicted exercise behavior in the literature (e.g., Bryan et al., 2007; Focht et al., 2007; Jerome et al., 2002; Kwan & Bryan, 2010b; McAuley et al., 2003), and intrinsic motivation from Self-Determination Theory (SDT; Deci & Ryan, 1985), a construct which is strongly predictive of physical activity (Caldwell Hooper & Bryan, 2011; Ryan et al., 1997).
The framework implies that the ability to physiologically and psychologically adapt to and cope with physical activity will partially determine whether an individual responds to an intervention to increase exercise behavior. For example, an individual who is better able to modulate body temperature may experience more positive immediate benefits of exercise (e.g., increase in positive affect) resulting in greater motivation to exercise. Importantly, a positive immediate experience of exercise translates into more stable intentions to engage in exercise (Kwan & Bryan, 2010b) and higher levels of future exercise behavior (Kwan & Bryan, 2010a; Williams et al., 2008). Exercise promotion interventions are generally modestly successful at best, and interventions work better for some than for others (Marcus et al., 2000; Rothman et al., 2004). The set of constructs and relationships defined in our framework suggest a way of understanding who is more or less likely to initiate exercise behavior, who is more likely to respond successfully to an exercise intervention, and long-term, may provide a better understanding of who is more likely to maintain exercise behavior. This latter point holds particular importance because exercise interventions have been general success in motivating adoption of physical activity; however, maintenance of physical activity resulting from these interventions has been much more elusive (Bock et al., 2001; Marcus et al, 2000; Rothman et al, 2004).
A framework such as this can be used to test moderation hypotheses in the context of the implementation of previously successful interventions to increase physical activity. In one investigation using this type of intervention (STRIDE; Marcus et al., 2007a; 2007b), Marcus and colleagues (Marcus et al., 2007a) compared the effects of two 12-month long tailored non face-to-face interventions (print and telephone) with an exposure-matched health education control intervention on physical activity. At a 12-month follow-up, participants in the print-based intervention reported significantly more physical activity than either the phone-based or control interventions, suggesting that print-based methods may be more effective at increasing physical activity (Sevick et al., 2007). This same print-based method is the approach we used here.
Investigations like STRIDE are extremely complex, and it is difficult to include a broad range of individual-difference variables suggested by various disciplines (genetics, physiological and psychological factors) into the same study both because of logistical complexity for the investigator as well as subject burden. Thus, consistent with other similarly complex physical activity randomized clinical trials that publish baseline data and methods (e.g. Bock et al., 2010; Brown et al., 2009; Jung et al., 2010; Maddison et al., 2010; Marcus et al., 1997; 2007b), the purpose of this paper is to present the design, methods, and baseline data from the Colorado STRIDE (COSTRIDE) intervention trial in order to illustrate how theory-based individual difference variables can be integrated into a randomized controlled exercise intervention trial. Specifically, we will (1) describe the study and rationale of COSTRIDE; (2) describe our sample; and (3) provide a preliminary examination of the relationships between variables at baseline. We will fully describe the methods involved in our multi-session 12-month long intervention trial involving genetic, physiological, and psychological moderators.
Methods
COSTRIDE was a 12-month randomized controlled trial (RCT) conducted at the University of Colorado at Boulder (CU). Participants were randomized into either the STRIDE exercise intervention condition (herein referred to as COSTRIDE) or a health-and-wellness contact control condition. Participants completed three baseline sessions (orientation, fitness assessment, and submaximal exercise session), and follow-up assessments at 3, 6, 9, and 12 months following randomization. Participants were compensated up to $300 for completing the study – receiving increments for completion of each phase. This project was approved by CU’s Human Research Committee, the Scientific Advisory Committee of CU’s General Clinical Research Center (GCRC), the University of New Mexico’s Human Research Review Committee for the protection of human participants in research, the Institutional Review Board of the Miriam Hospital and the Institutional Review Board of Brown University.
Participants
Participants were inactive, but otherwise healthy, men and women (ages 18–45). All participants were recruited from the Denver-metro area and the CU community (including students). Participants were primarily recruited through electronic ads placed in campus bulletins, on Craig’s list, and on social-networking websites. Flyers were posted throughout the CU campus, local churches, community centers, and recreation centers. Participants were also recruited at local festivals, farmer’s markets, and campus events. Advertisements and flyers provided a basic description of the study including the time commitment, primary inclusion and exclusion criteria, and compensation.
Individuals were excluded if they smoked cigarettes, were on a restricted diet, taking psychotropic medications, receiving treatment for any psychiatric disorder, diabetic, had a history of cardiovascular or respiratory disease, had the flu or illness in the previous month, or were pregnant (if female). During the recruitment process, inactive was defined as less than 90 minutes of voluntary moderate- or vigorous- intensity physical activity per week for the past three months. All participants were required to have a body mass index (BMI) between 18 and 37.5, be physically capable of engaging in moderate-intensity physical activity, have a regular menstrual cycle (if female), be willing to be randomly chosen for one of the two interventions, and give informed consent.
Three hundred thirty-eight individuals were recruited for this study; thirty-eight were dropped prior to randomization for not meeting inclusion criteria, twelve were not able to complete the submax test, thirty-one could not be reached after the submax test, and nineteen declined to continue participation. A total of 238 individuals (189 female, 49 male) completed baseline assessments and were randomly assigned to either the exercise (n=123) or health-and-wellness (n=115) condition.
Procedure
Following baseline sessions, participants met privately with a trained health educator to be randomized (by coin flip) and learn about their program. Participants in the COSTRIDE exercise intervention were instructed in basic physical activity information (including various moderate-intensity activities), goal setting, and physical activity tracking using provided logs. They were told their goal was to increase their moderate-intensity physical activity to at least five days a week for 30 minutes a day.
COSTRIDE participants received mailings with individually-tailored messages and information based on their currently salient barriers to and level of motivation for increasing physical activity at 14 time points: weekly during Month 1, biweekly during Months 2 and 3, monthly during Months 4 through 6, and bimonthly during Months 7 through 12. Details regarding the development and administration of the COSTRIDE intervention, as well as the assessments used to develop intervention material are available elsewhere (Marcus et al., 2007b). Briefly, participants in the COSTRIDE intervention completed a series of theory-based questionnaires via mail. Based on the responses to these questionnaires, participants were mailed a printed report of the feedback generated by a computer expert system, a self-help manual matched to their stage of motivational readiness for physical activity adoption, and a series of tip sheets. The printed report was a series of individually-tailored messages based on the Stages of Motivational Readiness Model (Prochaska & DiClemente, 1983) and Social Cognitive Theory (Bandura, 1986) that were generated by a computer-based expert system. Depending on the constellation of responses to each of the selected questionnaire items, the computer expert system extracted an appropriate paragraph containing a physical activity counseling message relevant for the participant, thereby mimicking the types of responses that would be delivered in a face-to-face counseling session with a health educator. The content of the expert system paragraphs covered two broad domains: 1) an assessment of the individual’s current stage of motivational readiness for physical activity adoption (motivational feedback), and 2) an assessment of the individual’s self efficacy, decisional balance, and use of cognitive and behavioral processes associated with physical activity adoption (construct feedback). Table 1 presents the schedule of mailings and content for the COSTRIDE intervention.
Table 1.
Schedule and Content of Mailings for the COSTRIDE Intervention.
| Month | # of mailings/month | Week | Content |
|---|---|---|---|
| 1 | 4 | 1 (baseline) 2–4 |
Stage-matched manual Expert system report, tip sheet on barriers |
| 2 | 2 | 5–6 7–8 |
Expert system report Manual and tip sheet on identified barriers |
| 3 | 2 | 9–10 11–12 |
Expert system report Manual and tip sheet on identified barriers |
| 4 | 1 | 13–16 | Expert system report, information in manual, tip sheet on identified barriers |
| 5 | 1 | 17–20 | Expert system report, information in manual, tip sheet on identified barriers Expert system report, information in manual, tip sheet on identified barriers |
| 6 | 1 | 21–24 | Expert system report, information in manual, tip sheet on identified barriers |
| 7 | 0 | 25–28 | |
| 8 | 1 | 29–32 | Expert system report, information in manual, tip sheet on identified barriers |
| 9 | 0 | 33–36 | |
| 10 | 1 | 37–40 | Expert system report, information in manual, tip sheet on identified barriers |
| 11 | 0 | 41–44 | |
| 12 | 1 | 45–48 | Expert system report, information in manual, tip sheet on identified barriers |
| Total | 14 mailings |
Participants in the health-and-wellness contact control were provided with printed materials informing them about various topics including healthy cooking, stress management, and quality sleep and were told their goal was to increase overall health and well-being. They received (non-tailored) uniform printed mailings at the same 14 time-points as individuals in the COSTRIDE intervention.
Measures
Questionnaire packets completed by participants at baseline, three, six, nine, and twelve months measured psychosocial motivational constructs and self-reported levels of voluntary physical activity. The baseline measures included a wide range of demographic variables, including past exercise experience. Specifically, participants were asked to indicate whether they had ever exercised regularly for six months or more and then stopped for an extended period of time (three months or more).
Before answering questions, participants were reminded that the definition of aerobic activity in the current context was “any activity that uses large muscle groups, is done for at least 20 minutes each time, and is done at a level that causes your breathing to be heavy and your heart to beat faster.” Table 2 describes each measure assessed at all sessions and follow-ups.
Table 2.
Measures Assessed at Each Time Point.
| Month | Assessment | Measures |
|---|---|---|
| 0 | Orientation | Preliminary assessment of eligibility, 3-day PAR |
| Baseline Assessment of Self-Reported Outcomes | Demographics, TPB: attitudes, intentions, norms, SCT: self-efficacy, SDT: intrinsic motivation; self-reported exercise. | |
| 1 | Fitness Assessment | VO2max testing, 7-day PAR, SCID, DNA collection, BMI, resting SBP, DBP and heart rate |
| Submaximal Exercise Session | PAAS, perceived exertion, Tympanic temperature; lactate, 65% VO2max, HRmax, FS, FAS | |
| 3 | Assessment of Self- reported Outcomes | TPB: attitudes, intentions, norms, SCT: self- efficacy, SDT: intrinsic motivation; self- reported exercise |
| 6 | Assessment of Self- reported Outcomes | TPB: attitudes, intentions, norms, self- efficacy; 7-day PAR; self-reported exercise |
| 9 | Assessment of Self- reported Outcomes | TPB: attitudes, intentions, norms, SCT: self- efficacy, SDT: intrinsic motivation; self- reported exercise |
| 12 | Assessment of Self-reported Outcomes | TPB: attitudes, intentions, norms, SCT: self- efficacy, SDT: intrinsic motivation; 7-day PAR; self-reported exercise |
| Assessment of Objective Outcomes: Fitness Assessment | VO2max testing; DNA collection; BMI; resting SBP, DBP; heart rate |
Primary Outcome Measures
Physical Activity
Physical activity was measured in two ways. The 7-Day Physical Activity Recall (PAR; Blair et al., 1985), a researcher-administered interview, assessed minutes and intensity of physical activity including voluntary aerobic exercise, work-related activity, leisure-time physical activity, and walking over the previous seven days. Trained interviewers walked each participant through every day of the past week in order to get detailed information about their physical activity. This measure is widely used in exercise research, has demonstrated reliability and validity (Dishman et al., 2001; Pereira et al., 1997), and is sensitive to changes in moderate-intensity physical activity (Dunn et al., 1999; Marcus, Napolitano et al., 2007a). The 7-day PAR was administered at baseline, 6- and 12-month follow-ups. Self-reported levels of voluntary exercise were assessed with three questions specifically targeting voluntary aerobic exercise (Bryan & Rocheleau, 2002). Participants indicated how often they had engaged in aerobic activity in the past three months (1=never to 7=often) and the average number of days per week they engaged in aerobic exercise for at least 20 minutes over the past three months and the past week. These items were standardized and averaged, α=.75. Initially, self-report measures were validated on a subset of participants by accelerometers and were found to provide information equivalent to the PAR (Kwan et al., 2007). However, due to higher than expected costs, subject burden involved in returning the units, the ability to only sample a subset of participants, and the lack of added gain in measurement, their use was discontinued in the early stages of the project.
Fitness Test
Cardiorespiratory fitness was assessed by measuring oxygen uptake using a Balke protocol (a graded maximal exercise test) on a motorized treadmill. Consistent with established procedures (Christou et al., 2005), maximal oxygen capacity (VO2max) was assessed via online computer-assisted open-circuit spirometry using the Medgraphics Cardi02/CP system (St. Paul, MN) during incremental treadmill exercise (Trackmaster 425 treadmill, Newton, KS). Participants warmed up for 2 to 5 minutes to determine a speed corresponding to 70 to 80 percent of age-predicted maximal heart rate. Participants ran/walked at this speed, while the grade increased by 2.0% (or increased by 2.5% if the speed was greater than 6.0mph) every 2 minutes until volitional exhaustion. Valid measurement of VO2max required three of four criteria be met as outlined in previous work (Evans et al., 1995; Pimentel et al., 2003).
Weight and height measurements for calculation of body mass index (BMI) were measured and saliva samples (5ml) were collected for DNA extraction before the fitness test.
Exercise Motivation
To illustrate the flexibility of the framework to incorporate a wide range of motivational perspectives, we included motivational measures drawn from a number of motivational theoretical perspectives. We examined attitudes, norms, and intentions from the Theory of Planned Behavior (Ajzen & Madden, 1986), self-efficacy from Social-Cognitive Theory (Bandura, 1986), and intrinsic motivation from Self-Determination Theory (Deci & Ryan, 1985) as our operationalization of the motivational construct in the transdisciplinary framework. Behavioral beliefs representing attitudes towards exercise were measured using the physical activity enjoyment scale (PACES; Kendzierski & DeCarlo, 1991), α=.91. The PACES utilizes 18 bi-polar statements (e.g., I like it/I hate it) describing how an individual feels about exercise. For each statement, participants rated how they felt “at the moment” about physical activity on a 7-point bi-polar scale. The measurement of social norms and intentions were drawn from our previous work applying TPB constructs to physical activity (Bryan & Rocheleau, 2002; Bryan et al., 2007). Perceived social norms about exercise were measured with nine items assessing how important others view exercise (e.g., “Most people who are important to me think I should do aerobic exercise.”), α=.86. Intention to engage in aerobic exercise was measured with four items assessing the perceived likelihood of exercise behavior in the next three months (e.g., “How likely is it that you will go to a recreation center or health club to do aerobic exercise in the next three months?”), α=.68. Responses for perceived norms and intentions were made on 7-point scales (1=disagree strongly, 7=agree strongly). Intrinsic Motivation for physical activity was measured using the 21-item Intrinsic Motivation Index for Physical Activity (IMI; Lee & DiClemente, 2001) adapted from McAuley and colleagues’ Intrinsic Motivation Index (McAuley et al., 1989; McAuley et al., 1991). Participants indicated how much they agreed or disagreed with each statement about motivations for engaging in exercise (e.g., “I enjoy participating in physical activity very much.”) on a 7-point scale (1=strongly disagree, 7=strongly agree), α=.68. Scores can range from 21 to 147. This measure has good internal consistency and validity (Buckworth et al., 2007; Vallerand, & Fortier, 1998). Self-efficacy was measured with nine items assessing one’s perceived confidence in their ability to engage in aerobic exercise (e.g., “I feel confident that I could do aerobic exercise for at least 90 minutes per week.”) and to engage in aerobic exercise in the face of barriers (e.g., “I feel confident that I could do aerobic exercise even if the weather was bad.”). Responses were made on a 7-point scale (1=strongly disagree, 7=strongly agree), α = .86 (Bryan & Rocheleau, 2002; Bryan et al., 2007). Specific details and instructions for measurement of these motivational constructs are available in Online Resource 1.
Submaximal Exercise Session
Approximately one week after the fitness test, participants completed a 30-minute bout of physical activity on the treadmill at 65% of their previously estimated VO2max (assessed during the fitness test). Prior to beginning activity, resting heart rate and blood pressure measures were taken and a nurse inserted an intravenous catheter to collect blood samples during the bout. Intensity was maintained by measuring oxygen uptake and expired CO2 for two to three minutes at the beginning of exercise and at 10 and 20 minutes during exercise. All participants were able to maintain the submaximal workload in terms of exercise intensity; however, 12 individuals were dropped due to problems with the IV placement and blood draws (e.g., discomfort, light headedness, dizziness).
Affective Response
The following items were measured at six points during the submaximal exercise session: five minutes prior to activity, immediately before activity began, 10, 20, and 30 minutes into activity (directly before completion of the session), and five minutes post activity. The Physical Activity Affect Scale (PAAS) is a 12-item scale assessing exercise-induced feeling states (positive affect, negative affect, tranquility, and exhaustion). Participants rated their current state for each item (enthusiastic, energetic, upbeat, miserable, discouraged, crummy, calm, relaxed, peaceful, fatigued, tired, worn-out) using a 5-point scale (0=do not feel to 4=feel very strongly). The PAAS shows adequate internal consistency and discriminate validity among the factors (Lox et al., 2000). Rating of Perceived Exertion (RPE) is a single-item 15-point subjective measure of exertion ranging from 6 to 20 (6=no exertion at all to 20=maximal exertion), has adequate reliability and validity (Borg, 1998) and is frequently used in laboratory studies of exercise (e.g., Petruzzello et al., 2001; Williams et al., 2008). Perceived pain experienced during exercise was assessed using a single-item 12-point Borg CR10 scale (Borg, 1998) (0=no pain at all to 10=extremely intense pain). The Feeling Scale (FS; Hardy & Rejeski, 1989) is a single-item, 11-point (−5=very bad to +5=very good) measure that corresponds with the valence component of Russell’s circumplex model of affect (Russell, 1980). It has been used as a measure of general affect during exercise (Hall et al., 2002) and has shown reliability and discriminant validity from the RPE (Hardy & Rejeski, 1989). The Felt Arousal Scale (FAS) is a 6-point single-item subscale of the Telic State Measure assessing perceived activation during exercise (1=low arousal to 6=high arousal; Svebak & Murgatroyd, 1985).
Physiological Response
Blood samples were collected to measure lactate concentration and catecholamine levels (epinephrine and norepinephrine) immediately before activity began (11.5ml), and 10 (5.5ml) and 30 (11.5ml) minutes into activity. Tympanic temperature was measured by taking an average of 2–3 temperature readings before activity, three times during activity, and once post-activity.
Genetic Factors
Our prior work took a single nucleotide polymorphism (SNP) approach and focused on a SNP in the Brain Derived Neurotrophic Factor (BDNF) gene as a proposed genetic factor involved in voluntary exercise. The BDNF gene was chosen due to evidence linking the neurotrophic factor it codes for to exercise in animal models (Adlard et al., 2005; Donovan et al., 2000; Johnson & Mitchell, 2003; Olson et al., 2006). Although we focus on the BDNF SNP here, it is just one of many possible genetic candidates (others include the mu opioid receptor SNP OPRM1), and additional exploration is needed to expand our knowledge of genetic impacts on physical activity. With regard to BDNF in these baseline data, any naturally occurring relationships between BDNF and exercise response are likely to be attenuated given that sedentary participants were specifically selected. Nevertheless, we have shown some marginal associations of BDNF with diastolic blood pressure response (r=−.13, p=.05) and pain response (r=.13, p=.06), indicating, along with our prior work with regular exercisers, that BDNF is a good candidate for examination as a moderator of intervention response. Preliminary genome wide association analyses conducted on physiological and affective changes during the submaximal session have resulted in more than 7400 significant associations between variables in the framework and various genetic markers (Bryan et al., unpublished data). Thus, describing the full extent of the complex relationships between experiences during exercise and genetic markers is beyond the scope of this paper.
Statistical Analysis
Statistical analyses for this investigation were completed using SPSS 15.0 for Windows and the EQS Structural Equation Modeling program version 6.1. Descriptive analyses examining differences between intervention conditions were performed using t-tests for continuous variables and chi-square analyses for categorical variables. We first computed difference scores for variables assessed during the submaximal exercise session which were measured directly before starting and 30 minutes into the session (directly before finishing exercise). Then, all variables were checked for normality. With the exception of epinephrine, all variables were normally distributed; therefore, epinephrine difference scores were transformed to approximate normality using a rank-normalizing procedure (Blom, 1958). Because analyses were conducted on a prioi hypothesized relationships, we maintained a significance level of p<.05 (Shadish et al., 2002). Based on examination of relationships in the baseline data, we constructed and tested an exploratory structural equation model in which latent variables were estimated for physiological response to exercise, subjective experience of exercise, and motivation to exercise in the future. Because we had almost no variability on current exercise behavior at baseline (all participants were sedentary) this variable was not included in the model.
Results
Demographics
Table 3 shows the baseline characteristics for participants in the control and exercise interventions. As seen in the table, our randomization procedure created equivalent groups. On average, the sample was 28.18 years of age (SD=7.87). The majority were White (68.9%), followed by Asian (11.3%), Hispanic (10.9%), Black (3.4%), American Indian/Alaskan Native (2.5%), and multi-racial/other (2.5%). Participants were predominantly female (79.4%), had an average of 15.81 years of education (SD=2.60), and more than half had a total household income of $50,000 or more (55.8%).
Table 3.
Baseline Characteristics by Intervention Group.
| Health & Wellness | COSTRIDE | |||||
|---|---|---|---|---|---|---|
| Female (n=92) | Male (n=23) | Total (n = 115) | Female (n=97) | Male (n=26) | Total (n = 123) | |
| Demographics | ||||||
| Ethnicity (% White) | 67.4 | 82.6 | 70.4 | 68.0 | 65.4 | 67.5 |
| Age | 28.23 (8.09) | 24.00 (5.54) | 27.38 (7.81) | 30.01 (8.05) | 24.88 (5.72) | 28.93 (7.88) |
| Past Regular Exercise (% yes) | 65.2 | 78.3 | 67.8 | 61.9 | 65.4 | 62.6 |
| Proximal Outcomes | ||||||
| Exercise Attitudes | 78.93 (8.09) | 75.26 (14.57) | 78.20 (15.92) | 81.04 (16.28) | 83.12 (16.10) | 81.48 (16.20) |
| Intentions | 4.60 (1.07) | 4.27 (.83) | 4.54 (1.03) | 4.63 (1.07) | 4.37 (.94) | 4.58 (1.05) |
| Norms | 4.76 (1.01) | 4.55 (.74) | 4.71 (.97) | 4.89 (.99) | 4.11 (1.05) | 4.72 (1.05) |
| Self-Efficacy | 4.84 (1.11) | 4.86 (.91) | 4.85 (1.07) | 4.82 (1.07) | 5.08 (1.07) | 4.88 (1.07) |
| Intrinsic Motivation | 84.65 (18.52) | 88.04 (13.98) | 85.33 (17.70) | 88.04 (16.15) | 90.23 (20.81) | 88.50 (17.17) |
| Physical Activity Behavior Outcomes | ||||||
| Self-report activity (standardized) | .03 (.86) | .25 (.85) | .07 (.86) | −.04 (.78) | −.22 (.73) | −.08 (.77) |
| Exercise in past 3 months±,a | 2.59 (1.14) | 2.65 (1.03) | 2.60 (1.11) | 2.64 (1.17) | 2.19 (.98) | 2.55 (1.14) |
| # days/week in past 3 monthsa | 1.15 (1.01) | 1.48 (1.16) | 1.22 (1.04) | .98 (.91) | .77 (.77) | .93 (.89) |
| # days in past weeka | .88 (1.11) | 1.17 (1.27) | .94 (1.14) | .82 (.91) | .88 (1.03) | .84 (.93) |
| Voluntary exercise minutes (7-day PAR) | 23.12 (39.87) | 56.09 (96.17) | 29.71 (56.83) | 26.26 (44.83) | 33.85 (47.34) | 27.86 (45.28) |
| Fitness | ||||||
| BMI | 25.31 (5.13) | 25.60 (4.43) | 25.36 (4.98) | 25.20 (4.61) | 23.69 (3.67) | 24.88 (4.46) |
| Underweight (%) | 2.2 | 4.3 | 2.6 | 2.1 | 0.0 | 1.7 |
| Normal (%) | 47.8 | 34.8 | 45.2 | 51.6 | 76.0 | 56.7 |
| Overweight (%) | 28.3 | 43.5 | 31.3 | 28.4 | 16.0 | 25.8 |
| Obese (%) | 21.7 | 17.3 | 20.8 | 17.9 | 8.0 | 15.8 |
| Resting HR | 74.68 (12.08) | 71.61 (11.98) | 74.07 (12.07) | 74.64 (12.22) | 70.92 (11.89) | 73.82 (12.19) |
| Resting SBP | 113.31 (10.95) | 120.87 (12.82) | 114.85 (11.70) | 112.60 (13.84) | 117.35 (12.32) | 113.66 (13.61) |
| Resting DBP | 68.08 (7.78) | 71.78 (9.36) | 68.83 (8.22) | 68.22 (9.40) | 66.81 (8.62) | 67.91 (9.22) |
| VO2max (ml/kg/min) | 31.70 (6.14) | 41.37 (8.19) | 33.63 (7.63) | 31.67 (5.87) | 44.21 (7.52) | 34.32 (8.07) |
Note. No significance differences were found between intervention conditions. Five significant gender effects were found. Woman were older (p<.001), had higher exercise norms (p=.002), lower resting SBP (p=.003), lower VO2max (p<.001), and reported less voluntary moderate-intensity physical activity (p=.02) than men.
1=never to 7=often.
For descriptive purposes, the individual self-report physical activity items are included in this table; however, analyses are only conducted on the standardized combined measure.
Baseline Activity and Physiological Measures
On average, participants reported engaging in 28.76 minutes (SD=51.08) of at least moderate-intensity voluntary exercise over the past week, with a majority (55.5%) reporting being completely sedentary (i.e., 0 minutes), and 65% reporting having been physically active in the past. In addition, they reported engaging in aerobic exercise 1.07 days (SD=.97) per week over the past three months and less than one day (M=.89, SD=1.04) in the past week. Participants’ average estimated VO2max was 33.99ml/kg/min (SD=7.85) and they had an average BMI of 25.09 (SD=4.72) indicating that on average they were overweight. Participants also had normal resting heart rates (M=73.89 bpm, SD=12.10), systolic blood pressure (SBP; M=114.22, SD=12.68), and diastolic blood pressure (DBP; M=68.4, SD=8.74).
Relationships between Physiological and Affective Response
Correlations between physiological and affective responses to exercise are presented in Table 4. With the exception of DBP, physiological responses experienced during the exercise bout were consistently associated with affective responses. Greater increases in lactate were associated with smaller increases in positive affect, tranquility, and positive feelings, and greater increases in negative affect and pain. Greater increases in both epinephrine and norepinephrine were both associated with greater increases in pain and RPE. Greater increases in epinephrine were also associated with greater increases in negative affect and smaller decreases in tranquility.
Table 4.
Relationships between Baseline Fitness, Physiological Response, and Affective Response (n=238).
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. BMI | -- | ||||||||||||||||
| 2. VO2max | −.57*** | -- | |||||||||||||||
| 3. Lactate | −.04 | .13* | -- | ||||||||||||||
| 4. Epinephrine | .01 | .23*** | .08 | -- | |||||||||||||
| 5. Norepinephrine | .01 | .37*** | .16* | .39*** | -- | ||||||||||||
| 6. Temperature | .05 | .01 | .22*** | .10 | .20** | -- | |||||||||||
| 7. HR | −.22*** | .44*** | .37*** | .19** | .43*** | .19** | -- | ||||||||||
| 8. SBP | .11 | .19** | .19** | .17* | .30*** | .08 | .17* | -- | |||||||||
| 9. DBP | −.07 | −.02 | −.02 | −.06 | −.11 | .04 | −.15* | −.17** | -- | ||||||||
| 10. RPE | −.10 | .19*** | .05 | .23*** | .13* | −.01 | .25*** | −.03 | −.01 | -- | |||||||
| 11. PA | −.02 | −.04 | −.12 a | −.02 | .08 | .04 | −.07 | .01 | −.04 | −.08 | -- | ||||||
| 12. NA | .06 | .03 | .21** | .15* | .07 | −.04 | .13* | .18** | −.09 | −.01 | −.09 | -- | |||||
| 13. Exhaustion | −.10 | .11b | .10 | .06 | .03 | −.02 | .15* | .00 | −.04 | .27*** | −.31*** | .30*** | -- | ||||
| 14. Tranquility | .06 | −.16** | −.13* | −.15* | −.10 | .04 | −.19** | −.03 | .03 | −.18** | .18** | −.13* | −.06 | -- | |||
| 15. FS± | −.09 | −.03 | −.17* | .07 | .06 | .11 | −.06 | −.02 | −.07 | −.15* | .46*** | −.20** | −.29*** | .19** | -- | ||
| 16. Arousal | −.06 | .01 | .09 | .00 | .02 | .21* | .19* | .02 | −.01 | .31*** | .56*** | −.07 | −.13 | −.12 | .37*** | -- | |
| 17. Pain | .04 | .00 | .19** | .23** | .19** | .05 | .11 | .10 | .06 | .23*** | −.08 | .22*** | .29*** | −.12a | −.36*** | −.06 | -- |
Note.
p<.05,
p<.01,
p<.001
p=.07,
p=.08.
Higher numbers indicate a more positive feeling-state.
Relationship between Affective Response and Motivation
Correlations between affective responses during physical activity and motivation measures are presented in Table 5. Greater increases in tranquility, positive feelings (as measured by the Feeling Scale), arousal, and smaller increases in pain were associated with more positive attitudes towards exercise. Smaller increases in exhaustion and greater increases in tranquility were associated with higher intentions to exercise. Greater increases in RPE during exercise were associated with higher exercise self-efficacy. Finally, smaller increases in arousal and pain, and greater increases in RPE were associated with more intrinsic motivation.
Table 5.
Correlations between Proximal Outcome Measures with Affective Response to Exercise (n=238)
| Attitudes | Norms | Intentions | Self-Efficacy | Intrinsic Motivation | |
|---|---|---|---|---|---|
| Positive Affect | .06 | .12a | .03 | −.01 | .08 |
| Negative Affect | −.10 | −.10 | −.06 | −.04 | −.06 |
| Exhaustion | −.05 | −.11c | −.17** | −.09 | −.02 |
| Tranquility | .12 a | .05 | .12b | .10 | .09 |
| Feeling Scale | .15* | −.02 | .06 | .03 | .09 |
| Arousal | .18 a | .04 | .08 | −.09 | −.16* |
| Pain | −.18** | −.06 | .03 | .02 | −.18** |
| RPE | .08 | −.02 | .10 | .14* | .15* |
Note.
p<.05,
p<.01,
<.001,
p=.06,
p=.07,
p=.08.
Exploratory Structural Equation Model
The goal of the final analysis was to conduct a multivariate, path analytic test of the relationships proposed in the transdisciplinary framework (see Figure 2). We focused here on the links proposed between physiological response to exercise, subjective experience of exercise, and motivation to exercise in the future. We first examined the correlations among variables hypothesized to capture each of these domains, and empirically selected those that displayed at least moderate correlation with each other, and moderate correlation with variables in other domains. For example, as presented in Table 4, change in lactate is empirically associated both with other physiological response variables (change in temperature, change in heart rate), and also with subjective experience of exercise variables (perceptions of pain and affect). Thus, although the domains characterizing the latent constructs are based on a priori theory, our selection of variables that comprise the latent constructs are post hoc and based on empirical associations in the data. The latent variable for physiological response to exercise was formed from five measured indicator variables: temperature change in response to exercise (Temp Δ), lactate change in response to exercise (Lactate Δ), systolic blood pressure change in response to exercise (SBP Δ), norepinephrine change in response to exercise (NorEpi Δ), and heart rate change in response to exercise (HR Δ). The latent variable for subjective response to exercise was formed from two measured indicator variables: perceived pain and feelings in response to exercise (as measured by the Feeling Scale). Note that both scales are coded such that a positive loading indicates a more negative response (i.e., more pain, more negative feelings). The motivation latent variable was formed from three measured indicator variables: attitudes towards exercise, self-efficacy for exercise, and intrinsic motivation to exercise. The framework proposes that there is variability in physiological responses to exercise that partially explains an individual’s subjective experience of exercise, and those subjective experiences of exercise then influence motivation to exercise. This was the causal structure imposed on the model. Due to some missing data on various indicator variables, we utilized the full information maximum likelihood estimation option within EQS to account for missing data (Schafer & Graham, 2002). Accordingly, we utilized robust estimation of standard errors, and report the Yuan-Bentler rescaled χ2 for use with robust estimation (Yuan & Bentler, 2000). The adequacy of the model is assessed both in terms of the significance of the estimated loadings and hypothesized structural paths, as well as the estimation of overall measures of goodness-of-fit of the data to the model (c.f., Bryan, Schmiege, & Broaddus, 2007). Goodness-of-fit was determined based on an evaluation of the root mean square error of approximation (RMSEA; Steiger, 2007; Steiger & Lind, 1980) and the Comparative Fit Index (CFI; Bentler, 1990). Guidelines for cut-off points suggest that values close to or above .90 for the CFI, and .07 or lower for the RMSEA are indicative of good fit. According to these criteria, this model was an adequate fit to the data, Yuan-Bentler scaled χ2 (33, n=238) = 75.42, p<.001, CFI=.889, RMSEA=.069. All indicators had significant loadings on their hypothesized latent variables, and both structural paths in the model were significant. Standardized parameter estimates along with significance values can be seen in Figure 2. The findings indicate that a more negative physiological response to exercise (e.g., greater increase in lactate, greater increase in norepinephrine, greater increase in heart rate) is associated with a more negative subjective experience of exercise (e.g., more pain, relatively more negative affect). In turn, a more negative subjective experience of exercise is associated with less motivation to exercise in the future. Because the exact indicators of the latent variables in the model were empirically derived, this exploratory model requires replication in future studies, but provides excellent preliminary support for the relationships hypothesized in the transdisciplinary framework.
Figure 2.
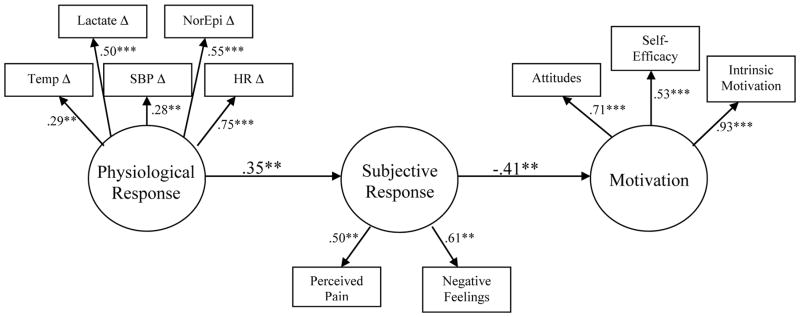
Exploratory Structural Equation Model of Relationships Proposed in the Transdisciplinary Framework.
Discussion
Exercise behavior is multifaceted and likely influenced at the individual level by biological, psychological, behavioral, and genetic factors. Obtaining a better understanding of how these multiple individual difference factors work together to influence the initiation of physical activity is important as it can provide researchers and clinicians with information that can be used to develop effective targeted interventions for both initiating and, ultimately, maintaining physical activity. Our goal here was to demonstrate how these multiple factors of influence (derived from various areas of research) can be incorporated into a single study. Specifically, we focused on how a transdisciplinary framework (Figure 1) can guide the selection of important individual-difference variables. Comprehensive studies that explore relationships among individual-difference variables have the potential to identify moderators of the effectiveness of interventions and, ultimately, inform the design, targeting, and tailoring of more effective physical-activity interventions.
To provide preliminary support for our chosen moderators, we assessed the relationship between physiological responses and affective responses to exercise and we found these factors to be associated with each other in ways that are consistent with our framework (Table 4). Specifically, changes in lactate were associated with changes in negative affect, tranquility, and positive feelings (and moderately associated with positive affect); changes in epinephrine were associated with changes in negative affect, tranquility, perceived pain, and perceived exertion; changes in norepinephrine were associated with changes in pain and perceived exertion; and changes in heart rate were associated with changes in negative affect, exhaustion, tranquility, arousal, and perceived exertion. Importantly, perceived exertion was associated with greater increases in exhaustion, pain, and arousal, and with lesser increases in tranquility and positive feelings suggesting that the interpretation of one’s physiological response to exercise is related to how he or she affectively responds. Taken together, these relationships suggest that how one physiologically responds to exercise may be translated into one’s subjective and affective experience of exercise, a finding consistent with the transdisciplinary framework.
Although the relationships were not large, there were significant associations between affective response and motivational variables, consistent with the notion that one’s affective response to exercise is associated with motivation to exercise (Bryan et al., 2011). Specifically, a greater change in tranquility and positive feelings, and smaller change in arousal and pain were associated with more positive attitudes towards exercise, while smaller change in exhaustion and higher tranquility were associated with greater intentions to exercise in the future, and less change in arousal and pain were associated with greater intrinsic motivation. RPE was also associated with self-efficacy and intrinsic motivation. The perception of working hard during exercise seems to have been associated with greater confidence in abilities to exert oneself during exercise in the future. This finding is especially important among sedentary individuals who likely have less experience with exercise than their more active counterparts. That is, they may not know they are capable of working hard, but once they try it, they discover confidence in their abilities to continue to exercise. Thus, the subjective experience of exercise was associated with motivation.
Finally, we tested an exploratory structural equation model indentifying latent variables that characterize the physiological response to exercise, the subjective experience of exercise, and the motivation to engage in exercise behavior. As predicted, a greater responsivity to acute exercise (e.g., greater increases in temperature, Norepinephrine, systolic blood pressure) was associated with a more negative subjective experience of exercise (i.e., greater perceived pain and more negative feelings in response to exercise). The more negative one’s subjective experience of exercise, the weaker was their motivation to engage in exercise behavior. These relationships support the overall structure of the transdisciplinary framework, and illustrate the flexibility of the framework to incorporate a range of biological, subjective, and psychological constructs.
The advantage of the type of multifaceted approach we have outlined here is its novelty and the extent to which the focus is on the overarching “big picture” of a complex behavior. The framework we utilize is useful as a foundation for stimulating and encouraging innovative research. Additionally, this approach highlights the idea that by better understanding a broad range of factors involved in exercise initiation and maintenance, including social-cognitive, genetic, physiological, and affective factors, researchers will be better able to design and implement efficacious interventions for this purpose.
One drawback is that such an approach cannot have the specificity or detail in any one domain. For example, there is extensive work on affective response to exercise with the conclusion that, in general, exercise leads to improvement in mood (Reed, 2005), although this finding is by no means universal (Focht et al., 2007) suggesting that individual differences play a role in affective responses to exercise. Another drawback is that we focus entirely on individual difference variables, and certainly do not provide full coverage of all possible important individual difference variables. Ultimately, beyond the domain of individual difference variables, there are numerous social and environmental factors that are also crucially important to fully understanding exercise behavior and its maintenance. Thus, use of the PACES with sedentary individuals may be an issue because people who have not experienced physical activity are not in a position to evaluate it. However, it is extremely unlikely that any person has not physically exerted themselves in some way in the past. In fact, 65% of the current sample acknowledged being physically active for at least six months in the past, and no significant differences were found for attitudes towards exercise, intentions to exercise in the future, self-efficacy towards exercise, or intrinsic motivation about exercise between past regular exercises and past non-exercisers. Importantly, although participants indicated whether or not they had been previously active for an extended period of time using a dichotomous response choice, we do not know the nature of the activities during these periods, the intensity of these activities, the duration of the active period, or how long ago the periods occurred. Thus, we cannot determine conclusively the effect of past regular exercise on these motivational constructs in the current paper. We also rely on difference scores to capture the essence of change in physiological and affective factors experienced during the submax session. Using difference scores could result in misleading interpretations (Edwards et al., 2001), although there is not agreement about this point and some routinely recommend their use (Judd & McClelland, 1989). Another concern with the current study may be the lack of objective measurement of self-reported physical activity. As mentioned previously, self-reported physical activity during each 3-month follow-up period was originally intended to be validated using accelerometry; however, due to subject burden, cost, and lack of added measurement gain, use of accelerometers was discontinued. Nevertheless, self-reported physical activity was not validated in this study and therefore there is a possibility of individuals reporting in a socially desirable manner (e.g., reporting more physical activity than they actually did) or inaccurately reporting physical activity due to retrospection. Finally, the sample used in the current study was drawn from a community which tends to be highly active and offers many resources for living an active lifestyle. Thus, the results from the current investigation may not be generalizble to other populations with fewer available resources. Relatedly, given the exploratory nature of our structural equation model, these findings require replication in general, and replication in different populations, in particular.
Conclusion
The purpose of this paper was to illustrate how theory-based individual difference measures can be incorporated into a large-scale exercise intervention trial. A particular strength of this research is its focus on sedentary individuals, a group for whom testing of relationships among genetic factors, physiological indicators, and affective responses to exercise is rare, but for whom the data are crucially important. Despite the difficulty and complexity of these types of studies, it is highly likely that exercise interventions among sedentary individuals may be more efficacious if a broader range of individual difference factors influencing physical activity and the relationships among them are understood. Given extremely high rates of sedentary lifestyle, and the enormous benefits to be gained in terms of the decrease of incidence of cancer, cardiovascular disease, hypertension, and Type II diabetes, it is of crucial importance to gain information that will aid in the development of effective and targeted interventions to increase regular physical activity among those who do not currently exercise.
Supplementary Material
Acknowledgments
The research was supported by grants awarded to Angela Bryan from the National Cancer Institute (RO1 CA109858), and the General Clinical Research Center Program of the National Center for Research Resources, National Institutes of Health (M01-RR00051) – now the Colorado Clinical and Translational Sciences Institute (UL1-RR025780).
References
- Adlard P, Perreau V, Cotman C. The exercise-induced expression of BDNF within the hippocampus varies across life-span. Neurobiology of Aging. 2005;26:511–520. doi: 10.1016/j.neurobiolaging.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Ajzen I. The theory of planned behavior. Organizational Behavior and Human Decision Processes. 1991;50:179–211. [Google Scholar]
- Ajzen I, Madden TJ. Prediction of goal-directed behavior: Attitudes, intentions, and perceived behavioral control. Journal of Experimental Social Psychology. 1986;22:453–474. [Google Scholar]
- Annesi JJ. Relations of self-motivation, perceived physical condition, and exercise induced changes in revitalization and exhaustion with attendance in women initiating a moderate cardiovascular exercise regimen. Women & Health. 2005;42:77–93. doi: 10.1300/j013v42n03_05. [DOI] [PubMed] [Google Scholar]
- Armitage CJ. Can the theory of planned behavior predict the maintenance of physical activity. Health Psychology. 2005;24:235–245. doi: 10.1037/0278-6133.24.3.235. [DOI] [PubMed] [Google Scholar]
- Bandura A. Social foundations of thought and action: A social cognitive theory. Englewood Cliffs, NJ: Prentice-Hall; 1986. [Google Scholar]
- Bentler PM. Comparative fit indexes in structural models. Psychological Bulletin. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Blair SN, Haskell WL, Ho P, Paffenbarger RS, Vranizan KM, Farquhar JW, Wood PD. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. American Journal of \ Epidemiology. 1985;122:794–804. doi: 10.1093/oxfordjournals.aje.a114163. [DOI] [PubMed] [Google Scholar]
- Blom G. Statistical estimates and transformed beta variables. New York: Wiley; 1958. [Google Scholar]
- Bock BC, Marcus BH, Pinto BM, Forsyth LH. Maintenance of physical activity following an individualized motivationally tailored intervention. Annals of Behavioral Medicine. 2001;23(2):79–87. doi: 10.1207/S15324796ABM2302_2. [DOI] [PubMed] [Google Scholar]
- Bock BC, Morrow KM, Becker BM, Williams DM, Tremont G, Gaskins RB, et al. Yogas as a complementary treatment for smoking cessation: Rationale, study design, and participant characteristics of the Quitting-in-Balance study. BMC Complimentary and Alternative Medicine. 2010;10:14. doi: 10.1186/1472-6882-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth FW, Neufer PD. Exercise genomics and proteomics. In: Tipton CM, editor. ACSM’s Advanced Exercise Physiology. Baltimore, MD: Lippincott, Williams, & Wilkins; 2006. pp. 623–651. [Google Scholar]
- Borg G. Borg’s perceived exertion and pain scales. Champaign, IL: Human Kinetics; 1998. pp. 44–52. [Google Scholar]
- Brown RA, Abrantes AM, Read JP, Marcus BH, Jakicic J, Strong DR, et al. Aerobic exercise for alcohol recover: Rationale, program description, and preliminary findings. Behavior Modification. 2009;33:220–249. doi: 10.1177/0145445508329112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan AD, Hutchison KE, Seals DR, Allen DE. A transdisciplinary model integrating genetic, physiological, and psychological correlates of voluntary exercise. Health Psychology. 2007;26:30–39. doi: 10.1037/0278-6133.26.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan AD, Magnan RE, Nilsson R, Marcus BH, Tompkins SA, Hutchison KE. The big picture of individual differences in physical activity behavior change: A transdisciplinary approach. Psychology of Sport and Exercise. 2011;12:20–26. doi: 10.1016/j.psychsport.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan AD, Rocheleau CA. Predicting aerobic versus resistance exercise using the Theory of Planned Behavior. American Journal Health Behavior. 2002;26:83–94. doi: 10.5993/ajhb.26.2.1. [DOI] [PubMed] [Google Scholar]
- Bryan A, Schmiege SJ, Broaddus MR. Mediational analysis in HIV/AIDS research: Estimating multivariate path analytic models in a structural equation modeling framework. AIDS and Behavior. 2007;11:365–383. doi: 10.1007/s10461-006-9150-2. [DOI] [PubMed] [Google Scholar]
- Buckworth J, Lee RE, Regan G, Schneider LK, DiClemente CC. Decomposing intrinsic and extrinsic motivation for exercise: Application to stages of motivational readiness. Psychology of Sport and Exercise. 2007;8:441–461. [Google Scholar]
- Caldwell Hooper AE, Bryan AD. What keeps a body moving? The BDNF SNP and intrinsic motivation to exercise. 2011. Manuscript under review. [DOI] [PubMed] [Google Scholar]
- Christou DD, Gentile CL, DeSouza CA, Seals DR, Gates PE. Fatness is a better predictor of cardiovascular disease risk factor profile than aerobic fitness in healthy men. Circulation. 2005;111:1904–1914. doi: 10.1161/01.CIR.0000161818.28974.1A. [DOI] [PubMed] [Google Scholar]
- Deci EL, Ryan RM. Intrinsic motivation and self-determination in human behavior. New York: Plenum; 1985. [Google Scholar]
- Dishman RK, Washburn RA, Schoeller DA. Measurement of physical activity. Quest. 2001;53:295–309. [Google Scholar]
- Donovan MJ, Lin MI, Wiegn P, Ringstedt T, Kraemer R, Hahn R, et al. Brain derived neurotropic factor is an endothelial cell survival factor required for intramyocardial vessel stabilization. Development. 2000;127:4531–4540. doi: 10.1242/dev.127.21.4531. [DOI] [PubMed] [Google Scholar]
- Dunn AL, Marcus BH, Kampert JB, Garcia ME, Kohl HW, Blair SN. Comparison of lifestyle and structured interventions to increase physical activity and cardiorespiratory fitness: A randomized trial. Journal of the American Medical Association. 1999;281:327–334. doi: 10.1001/jama.281.4.327. [DOI] [PubMed] [Google Scholar]
- Edwards JR. Ten difference score myths. Organizational Research Methods. 2001;4:265–287. [Google Scholar]
- Evans SL, Davy KP, Stevenson ET, Seals DR. Physiological determinants of 10km performance in highly trained female runners of different ages. Journal of Applied Physiology. 1995;78:1931–1941. doi: 10.1152/jappl.1995.78.5.1931. [DOI] [PubMed] [Google Scholar]
- Focht BC, Knapp DJ, Gavin TP, Raedeke TD, Hickner RC. Affective and self-efficacy responses to acute aerobic exercise in sedentary older and younger adults. Journal of Aging and Physical Activity. 2007;15:123–138. doi: 10.1123/japa.15.2.123. [DOI] [PubMed] [Google Scholar]
- Hall EE, Ekkekakis P, Petruzzello SJ. The affective beneficence of vigorous exercise revisited. British Journal of Health Psychology. 2002;7:47–66. doi: 10.1348/135910702169358. [DOI] [PubMed] [Google Scholar]
- Hardy CJ, Rejeski WJ. Not what, but how one feels: The measurement of affect during exercise. Journal of Sport and Exercise Psychology. 1989;11:304–317. [Google Scholar]
- Haskell WA, Lee I, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Medicine & Science in Sports & Exercise. 2007;39:1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- Jerome GJ, Marquez DX, McAuley E, Canaklisova S, Snook E, Vickers M. Self-efficacy effects on feeling states in women. International Journal of Behavioral Medicine. 2002;9:139–154. doi: 10.1207/s15327558ijbm0902_05. [DOI] [PubMed] [Google Scholar]
- Johnson RA, Mitchell GS. Exercise-induced changes in hippocampal brain-derived neurotrophic factor and neuotrophin-3: Effects of rat strain. Brain Research. 2003;983:108–114. doi: 10.1016/s0006-8993(03)03039-7. [DOI] [PubMed] [Google Scholar]
- Judd CM, McClelland GH. Data analysis: A model comparison approach. San Diego, CA: Harcourt, Brace, Jovanovich; 1989. [Google Scholar]
- Jung ME, Fitzgeorge L, Prapavessis H, Faulkner G, Maddison R. The getting physical on cigarettes trial: Rationale and methods. Mental Health and Physical Activity. 2010;3:35–44. [Google Scholar]
- Kendzierski D, DeCarlo KJ. Physical Activity Enjoyment Scale: Two validation studies. Journal of Sport and Exercise Psychology. 1991;13:50–64. [Google Scholar]
- Kwan BM, Bryan A. In-task and post-task affective response to exercise: Translating exercise intentions into behavior. British Journal of Health Psychology. 2010a;15:115–131. doi: 10.1348/135910709X433267. [DOI] [PubMed] [Google Scholar]
- Kwan BM, Bryan A. Affective response to exercise as a component of exercise motivation: Self-efficacy, outcome expectations and temporal stability of intentions. Psychology of Sport and Exercise. 2010b;11:71–79. doi: 10.1016/j.psychsport.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan BM, Tompkins SA, Bryan AD, Marcus BH, Ciccolo JT. Physical activity measures in sedentary participants: Comparison of 3 day PAR, 3-item self-report and accelerometer. Poster presented at the American College of Sports Medicine conference; New Orleans, LA. 2007. May, [Google Scholar]
- Lee RE, DiClemente CC. Extrinsic and intrinsic motivation: Which is important for exercise? Medicine and Science in Sports and Exercise. 2001;33:S112. [Google Scholar]
- Lox CL, Jackson S, Tuholski S, et al. Revisiting the measurement of exercise induced feeling states: The Physical Activity Affect Scale (PAAS) Measurement in Physical Education and Exercise Science. 2000;4:79–95. [Google Scholar]
- Maddison R, Roberts V, Bullen C, McRobbie H, Jiang Y, Prapavessis H, et al. Design and conduct of a pragmatic randomized controlled trial to enhance smoking-cessation outcomes with exercise: The Fit2Quit study. Mental Health and Physical Activity. 2010;3:92–101. [Google Scholar]
- Marcus BH, King TK, Albrecht AE, Parisi AF, Abrams DB. Rationale, design, and baseline data for Commit to Quit: An exercise efficacy trail for smoking cessation among women. Preventive Medicine. 1997;26:586–597. doi: 10.1006/pmed.1997.0180. [DOI] [PubMed] [Google Scholar]
- Marcus BH, Napolitano MA, King AC, Lewis BA, Whiteley JA, Albrecht A, et al. Telephone versus print delivery of an individualized motivationally-tailored physical activity intervention: Project STRIDE. Health Psychology 2007. 2007a;26:401–409. doi: 10.1037/0278-6133.26.4.401. [DOI] [PubMed] [Google Scholar]
- Marcus BH, Napolitano MA, King AC, Lewis BA, Whiteley JA, Albrecht A, et al. Examination of print and telephone channels for physical activity promotion: Rationale, design, and baseline data from project STRIDE. Contemporary Clinical Trials. 2007b;28:90–104. doi: 10.1016/j.cct.2006.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus BH, Nigg CR, Riebe D, Forsyth LH. Interactive communication strategies: Implications for population-based physical activity promotions. American Journal of Preventive Medicine. 2000;19:121–126. doi: 10.1016/s0749-3797(00)00186-0. [DOI] [PubMed] [Google Scholar]
- McAuley E, Duncan T, Tammen VV. Psychometric properties of the Intrinsic Motivation Inventory in a competitive sport setting: A confirmatory factor analysis. Research Quarterly for Exercise and Sport. 1989;11:84–93. doi: 10.1080/02701367.1989.10607413. [DOI] [PubMed] [Google Scholar]
- McAuley E, Jerome GJ, Marquez DX, Elavsky S, Blissmer B. Exercise self efficacy in older adults: Social, affective, and behavioral influences. Annals of Behavioral Medicine. 2003;25:1–7. doi: 10.1207/S15324796ABM2501_01. [DOI] [PubMed] [Google Scholar]
- McAuley E, Wraith S, Duncan TE. Self-efficacy, perceptions of success, and intrinsic motivation for exercise. Journal of Applied Social Psychology. 1991;21:139–155. [Google Scholar]
- Olson AK, Eadie BD, Ernst C, Christie BR. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. 2006;16:250–260. doi: 10.1002/hipo.20157. [DOI] [PubMed] [Google Scholar]
- Penedo FJ, Dahn JR. Exercise and well-being: A review of mental and physical health benefits associated with physical activity. Current Opinion in Psychiatry. 2005;18:189–193. doi: 10.1097/00001504-200503000-00013. [DOI] [PubMed] [Google Scholar]
- Pereira MA, FitzerGerald SJ, Gregg EW, Joswiak ML, Ryan WJ, Suminski RR, et al. A collection of physical activity questionnaires for health-related research. Medicine & Science in Sports & Exercise. 1997;29(Suppl 6):S1–205. [PubMed] [Google Scholar]
- Petruzzello SJ, Hall EE, Ekkekakis P. Regional brain activation as a biological marker of affective responsivity to acute exercise: Influences of fitness. Psychophysiology. 2001;38:99–106. [PubMed] [Google Scholar]
- Pimentel AE, Gentile CL, Tanaka H, Seals DR, Gates PE. Greater rate of decline in maximal aerobic capacity with age in endurance-trained than in sedentary men. Journal of Applied Physiology. 2003;94:2406–1243. doi: 10.1152/japplphysiol.00774.2002. [DOI] [PubMed] [Google Scholar]
- Pleis JR, Lucas JW, Ward BW. Summary health statistics for U.S. adults: National Health Interview Survey, 2008. National Center for Health Statistics. Vital Health Statistics. 2009;10:74–75. [PubMed] [Google Scholar]
- Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: Toward an integrative model of change. Journal of Consulting and Clinical Psychology. 1983;51:390–395. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- Reed J. Acute physical activity and self-reported affect: A review. In: Clark AV, editor. Causes, role, and influence of mood states. Hauppauge, NY: Nova Science Publishers; 2005. pp. 91–113. [Google Scholar]
- Rothman AJ, Bladwin AS, Hertel AW. Self-regulation and behavior change. In: Baumeister RR, Vohs KD, editors. Handbook of self regulation: Research, theory and applications. New York: Guilford; 2004. pp. 130–148. [Google Scholar]
- Russell JA. A circumplex model of affect. Journal of Personality and Social Psychology. 1980;39:1161–78. doi: 10.1037//0022-3514.79.2.286. [DOI] [PubMed] [Google Scholar]
- Ryan RM, Frederick CM, Lepes D, Rubio N, Sheldon KM. Intrinsic motivation and exercise adherence. International Journal of Sport Psychology. 1997;28:335–354. [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Shadish WR, Cook TD, Campbell DT. Experimental and quasi-experimental designs for generalized causal inference. Boston, MA: Houghton-Mifflin; 2002. (hereafter, SCC) [Google Scholar]
- Sevick M, Napolitano MA, Papandonatos GD, Gordon AJ, Reiser LM, Marcus BH. Cost-effectiveness of alternative approaches for motivating activity in sedentary adults: Results of Project STRIDE. Preventive Medicine. 2007;45:54–61. doi: 10.1016/j.ypmed.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger JH. Understanding the limitations of global fit assessment in strucutral equation modeling. Personality and Individual Differences. 2007;42:893–898. [Google Scholar]
- Steiger JH, Lind JC. Statistically based tests for the number of factors. Paper presented at the annual spring meeting of the Psychometric Society; Iowa City, IA. 1980. [Google Scholar]
- Svebak E, Murgatroyd S. Metamotivational domincance: A multimethod validation of reversal theory constructs. Journal of Personality and Social Psychology. 1985;48:107–116. [Google Scholar]
- U.S. Department of Health and Human Services. [Accessed November 4, 2008];Physical activity guidelines for Americans. 2008 Available at: http://www.health.gov/PAGuidelines/pdf/paguide.pdf.
- Williams DM, Dunsiger S, Ciccolo JT, Lewis BA, Albrecht AE, Marcus BH. Acute affective response to a moderate-intensity exercise stimulus predicts physical activity participation 6 and 12 months later. Psychology of Sport and Exercise. 2008;9:231–245. doi: 10.1016/j.psychsport.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan K, Bentler PM. Three likelihood-based methods for mean and covariance structure analysis with nonnormal missing data. Sociological Methodology. 2000;30:167–202. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.