Abstract
Background
The Alaska Native (AN) population experiences twice the incidence and mortality of colorectal cancer (CRC) as does the U.S. white population. CRC screening allows early detection and prevention of cancer.
Objective
We describe pilot projects conducted from 2005 to 2010 to increase CRC screening rates among AN populations living in rural and remote Alaska.
Design
Projects included training rural mid-level providers in flexible sigmoidoscopy, provision of itinerant endoscopy services at rural tribal health facilities, the creation and use of a CRC first-degree relative database to identify and screen individuals at increased risk, and support and implementation of screening navigator services.
Setting
Alaska Tribal Health System.
Patients
AN population.
Interventions
Itinerant endoscopy, patient navigation.
Main Outcome Measurements
AN patients screened for CRC, colonoscopy quality measures.
Results
As a result of these ongoing efforts, statewide AN CRC screening rates increased from 29% in 2000 to 41% in 2005 before the initiation of these projects and increased to 55% in 2010. The provision of itinerant CRC screening clinics increased rural screening rates, as did outreach to average-risk and increased-risk (family history) ANs by patient navigators. However, health care system barriers were identified as major obstacles to screening completion, even in the presence of dedicated patient navigators.
Limitations
Continuing challenges include geography, limited health system capacity, high staff turnover, and difficulty getting patients to screening appointments.
Conclusions
The projects described here aimed to increase CRC screening rates in an innovative and sustainable fashion. The issues and solutions described may provide insight for others working to increase screening rates among geographically dispersed and diverse populations.
Among the Alaska Native (AN) population, cancer is the leading cause of death, and colorectal cancer (CRC) is the second leading cause of cancer death.1 For the period 2004 to 2008, the AN age-adjusted CRC mortality and incidence rates were about twice those of the U.S. white population.2,3 The AN population also have the highest CRC incidence of all Native American groups, with a CRC incidence that is nearly 5 times higher than that of American Indians living in the U.S. Indian Health Services Southwest Region.4 The reasons for these regional disparities are unclear; nonetheless, the morbidity and mortality of CRC can be reduced in all regions by population-based screening and surveillance programs that include endoscopy (colonoscopy and flexible sigmoidoscopy) and fecal occult blood tests. Screening can detect advanced neoplasia (polyps and cancer) and, in the case of endoscopy, can even prevent cancer by removing precancerous polyps.5,6
BACKGROUND
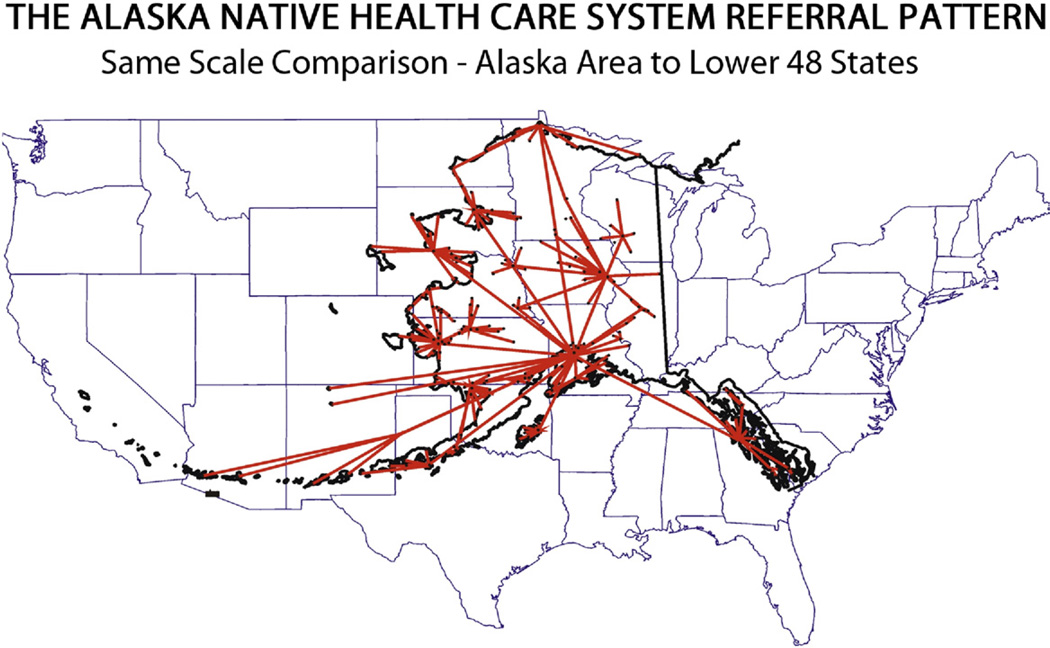
The Alaska Tribal Health System (ATHS) provides health care for the AN population, who belong to 3 major ethnic groups: Eskimo, Aleut, and American Indian. Approximately half of the 125,000 AN people in the state live in rural and remote communities that are off the road system and are accessible only by small aircraft, snowmobiles, or boats.7,8 The ATHS is a spoke-and-hub system consisting of small tribal village clinics, subregional clinics, and regional hospitals, with 1 tertiary care hospital (Alaska Native Medical Center [ANMC]) in Anchorage. The tribal village clinics are staffed by community health aides/practitioners who are laypersons trained as first responders in emergencies and who provide basic primary and preventive health care. Subregional clinics are staffed by community health aides/practitioners, physician assistants, and nurse practitioners. Regional hospitals provide inpatient, outpatient, and emergency services and are staffed by physician assistants, nurse practitioners, and physicians.8 CRC screening in Alaska is challenging because of the unique geography and climate, which often necessitate long-distance, high-cost air travel to access endoscopy services (Fig. 1).
Figure 1.
Alaska Native health care referral pattern distances compared with United States land mass.
Formed in 1997, the Alaska Native Tribal Health Consortium (ANTHC) is a statewide nonprofit health services organization owned and operated by the AN people to provide health services to members of the 229 tribes throughout Alaska and to support the tribal health organizations that comprise the ATHS. In 1994, Alaska Tribes and Tribal representatives signed the Alaska Tribal Health Compact with the U.S. Department of Health And Human Services Indian Health Service for management of all statewide health services formerly provided by that agency for the AN population. In the late 1990s, ANTHC began endoscopy training for nurse practitioners and physician assistants to improve access to screening flexible sigmoidoscopy and colonoscopy. These initial efforts led to the collaboration in 2004 of the ANTHC Alaska Native Epidemiology Center and the Centers for Disease Control and Prevention Division of Cancer Prevention and Control.
This article was reviewed and approved by ANTHC tribal review committee on behalf of the ANTHC Board of Directors. It was also approved for clearance by the Centers for Disease Control and Prevention, which funded the pilot projects. This article describes the implementation and outcome of 3 pilot projects to improve CRC screening rates among the AN population in the ATHS. We describe facilitators and barriers to CRC screening and highlight considerations for others working to increase screening rates among geographically and ethnically diverse populations.
PILOT PROJECT DESCRIPTIONS
Endoscopy
Fecal occult blood tests are not recommended for use in the AN population because of a high prevalence of Helicobacter pylori–associated hemorrhagic gastritis, which results in high false-positive rates,9,10 making lower endoscopy (flexible sigmoidoscopy and colonoscopy) the preferred screening method for CRC. However, endoscopy is routinely available in Alaska only in Anchorage and 2 of the 7 regional hospitals. For patients living in areas not directly served at regional centers, access to endoscopic services are limited to biyearly specialty field clinics staffed by Anchorage-based providers or travel to the ANMC in Anchorage. Because of the limited time frame of these field clinics, patients in need of diagnostic examinations are given priority over screening procedures. Although endoscopic equipment is available in other regional hospitals, providers there are either untrained in the procedures or are not allotted time to conduct them because of competing clinical responsibilities for providers and support staff.11 Therefore, endoscopy at the regional level prioritizes symptomatic patients ahead of screening patients.
We previously described efforts to increase flexible sigmoidoscopy screening services in rural areas by training mid-level health care providers.11 In the late 1990s, an internal review of medical records at the ANMC found that only 10% of the age-eligible AN population served by the ANMC had been screened for CRC. To address this issue, the ANMC Surgery Department trained a physician assistant to perform screening with flexible sigmoidoscopy. In 2000, CRC screening services were expanded by recruiting and training a nurse practitioner for a dedicated screening flexible sigmoidoscopy clinic at the ANMC. CRC screening rates in the Anchorage area improved dramatically with the initiation of this screening clinic. As a result of these combined efforts, the percentage of age-eligible ANs in the Anchorage area screened for CRC increased rapidly to 47% by December 2003, representing a fivefold increase.12 Based on this success, we then developed a flexible sigmoidoscopy training program for nurse practitioners and physician assistants from regional hub communities across the state. The curriculum included didactic and clinical skills components. Seven providers were trained from 2005 to 2009, and, as a result, 205 rural patients underwent screening flexible sigmoidoscopies. However, by the end of 2009, only 1 trainee continued to perform screening examinations. Factors affecting attrition included staff turnover, competing clinical priorities, and a general shift to colonoscopy as the preferred screening modality for this high-risk population.11
As a result of the limited success of the flexible sigmoidoscopy training program for nonphysicians, ANTHC shifted focus in 2007 to supporting itinerant screening colonoscopy field clinics. In this model, an endoscopist from the ANMC travels to remote areas of Alaska to conduct CRC screenings at 3 regional hospitals with clinic space available for endoscopy. Priority is given to patients who have never been screened and to those individuals who have a family history of CRC or adenomatous polyps in 1 or more first-degree relatives. Patients in whom CRC is found are referred to the ANMC in Anchorage for further care.
Two models of financial support for colonoscopy screening were piloted from 2007 to 2009: (1) a capitated model in which payment was determined by the number of colonoscopies performed, and (2) a flat day-rate model in which funding was based on a certain number of days of endoscopist time, regardless of the number of screenings completed in that period. Each facility was required to report data on patient demographics, number of patients screened, cancellation and “no-show” rates, screening history, personal and/or family history of CRC and/or colonic polyps, and pathology outcomes. In 2008, we added quality of care measures to the data-reporting requirements using national quality standards.13,14 These measures included the cecal intubation rate, adenoma detection rate, and colonoscopy complication rate. Colonoscope withdrawal time was not added until the end of the project and is not reported here.
Approximately 3828 AN patients who received care through the regional tribal health organizations were due for colonoscopy screening at the beginning of the project period. As a result of the itinerant clinic project, a total of 290 AN individuals were screened for CRC by colonoscopy at 3 different rural sites (Table 1). Each of the 3 regions comprised remote AN communities scattered over an area ranging in size from 36,000 to 98,000 square miles, including many communities that could only be reached by small aircraft. Cancellation rates ranged from 25% to 54% (mean 37%) with an additional “no-show” rate of 4% to 10% (mean 7%). The proportion of cancellations that were rescheduled and completed was not recorded. The most common reasons for patient cancellation were weather (19%), acute medical issues (13%), and work constraints (13%). Other reasons given (37%) for cancellation were lack of child care, other competing priorities, or a desire to get the screening examination done in Anchorage instead of at the regional health facility. The proportion of men (48%) and women (52%) screened was about equal. The majority of patients (70%) came from very small communities (average size of 400 persons) surrounding the regional hub community where the screening procedures were completed.
TABLE 1.
Itinerant colonoscopy screening pilot project outcomes among Alaska Native people, 2007–2009
| Site 1 | Site 2 | Site 3 | Total | |||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |
| Screening | ||||||||
| Scheduled | 142 | 100 | 316 | 100 | 60 | 100 | 518 | 100 |
| No show | 6 | 4 | 22 | 7 | 6 | 10 | 34 | 7 |
| Cancelled | 76 | 54 | 103 | 33 | 15 | 25 | 194 | 37 |
| Reasons for cancellation | ||||||||
| Weather | 21 | 28 | 15 | 15 | n/a | 36 | 19 | |
| Work | 7 | 9 | 19 | 18 | n/a | 26 | 13 | |
| Medical | 9 | 12 | 16 | 16 | n/a | 25 | 13 | |
| Other | 39 | 51 | 33 | 32 | n/a | 72 | 37 | |
| Completed | 60 | 42 | 191 | 60 | 39 | 65 | 290 | 56 |
| Demographics | ||||||||
| Male | 26 | 43 | 73 | 48 | 22 | 56 | 121 | 48 |
| Female | 34 | 57 | 79 | 52 | 17 | 44 | 130 | 52 |
| Large community (average population >400)* | 9 | 15 | 58 | 38 | 9 | 23 | 76 | 30 |
| Small community (average population ≥400)* | 51 | 85 | 94 | 62 | 30 | 77 | 175 | 70 |
| Never screened previously | 54 | 90 | 109 | 57 | 14 | 36 | 177 | 61 |
| History of polyps | ||||||||
| Familial (first-degree) | 6 | 10 | 38 | 20 | 4 | 10 | 48 | 17 |
| Personal | 6 | 10 | 33 | 17 | 18 | 46 | 57 | 20 |
| Pathology (≥1 tubular adenoma found) | 24 | 40 | 62 | 32 | 16 | 41 | 102 | 35 |
| Quality of care* | ||||||||
| No. of patients seen | n/a | 64 | 100 | 39 | 100 | 103 | 100 | |
| Cecal intubation rate | n/a | 61 | 95 | 35 | 90 | 96 | 93 | |
| Adenoma detection rate (tubular or worse)† | n/a | 20 | 31 | 16 | 41 | 36 | 35 | |
| Complication rate (perforation) | n/a | 1 | 2 | 0 | 0 | 1 | 1 | |
n/a, Not available.
Quality of care measures added in December 2008. Data for these measures are missing for a total of 187 patients.
Adenoma detection rate was calculated as the total number of colonoscopies in which a tubular or more severe polyp was found, including procedures done on patients who had a history of polyps or CRC.
From 36% to 90% (mean 61%) of patients had never been screened previously for CRC. A family history of CRC was reported by 10% to 20% (mean 17%) of patients, whereas a personal history of CRC or polyps was reported by 10% to 46% (mean 20%) of patients. From 32% to 41% (mean 35%) of patients had at least 1 adenoma found on the screening examination. Colonoscopy quality was high; the cecal intubation rate was 93% and the average adenoma detection rate was 35%. The complication rate was 1% because of a single perforation that did not require hospitalization.
First-degree relative outreach
Family history is a critical component of CRC risk stratification.6,15 In 2007, the ANTHC began systematically asking CRC patients seen at the ANMC for permission to contact their first-degree relatives. If they agree, CRC patients are approached for a contact list of relatives at one of the following times: while still in the hospital, when coming back for follow-up appointments, or via a form sent with a business reply envelope to their home. Since the project began, only 3 of 588 patients (0.5%) have declined to give lists of their first-degree relatives. The contact information is entered into a computerized database. A CRC screening patient navigator then uses this information to provide direct outreach to AN family members to encourage them to get screened for CRC as appropriate. Contact of first-degree relatives is conducted by telephone and mailed reminder letters. Lists of first-degree relatives in need of screening are also sent annually to regional tribal medical and clinical directors for outreach by their facility. As of March 1, 2011, there were 588 CRC cases and 1444 first-degree relatives in the database. On average, about 3 first-degree relatives were identified per CRC case. A total of 661 first-degree relatives were due for screening; 465 were screened, 194 were not due yet (too young), and 124 were missing contact information such that they could not be identified for outreach. A total of 221 relatives included in the database were screened before the initiation of the Patient Navigator project. From the initiation of outreach efforts in January, 2007, to March 1, 2011, a total of 600 persons were reached out to and 254 first-degree relatives were screened for CRC as a result of the outreach efforts. Of the 254 persons screened, 58% had normal findings on examinations, 22% had tubular adenomas, 4% had tubulovillous or villous adenomas, and 5% had adenocarcinomas. The results from 26 individuals (5%) were unable to be ascertained from the medical record.
Patient navigation
To build on the success of navigation among individuals with a family history of CRC, from April 2009 through March 2010, the ANTHC partnered with a regional tribal health organization to implement the CRC Screening Patient Navigator Demonstration Project. A patient navigator was hired to guide average-risk asymptomatic patients through the screening process by encouraging them to obtain screening appointments, called patients to remind them about upcoming appointments, ensured that they had a transportation plan, answered questions about bowel preparation, and tracked screening results to ensure that appropriate follow-up was completed.
Approximately 1431 AN patients who received care through the regional tribal health organization were due for colonoscopy screening at the beginning of the project period. The patient navigator conducted direct outreach to 336 patients eligible for screening, with a total of 1047 contact attempts (average 28–41 per month). More than half (58%) of the outreach was in the form of phone calls, with 36% mailed letters, 2% e-mail, 3% provider contacts, and 2% other methods (eg, personal encounters, chart review). At the end of the project period, 46 patients (14%) completed CRC screening as a result of the patient navigator’s efforts and 22 referrals were still in progress. The majority of the patients screened (89%) had never been screened before, 39% had a family history of CRC, and about half (54%) were men. As a result of the screening, tubular or villous adenomatous polyps were found in 33% of patients, and 2 patients were found to have adenocarcinomas. Of the 2 cancers found, 1 was in a patient who had a history of CRC and the other in an individual who had never been screened before.
Although the patient navigation system was successful at targeting patients who had never been screened or who had a family history of CRC, fewer patients completed screening than was hoped. Health care system barriers were identified as a major obstacle to screening completion. The tribal health organization surgery department required that all patients have a precolonoscopy physical examination, laboratory tests and blood work, and an electrocardiogram. Most of the primary care providers who referred patients did not know all of the medical clearance procedures that patients needed to complete before they could get screened. Additionally, there were long delays in availability of these precolonoscopy appointments. It could take as long as 10 weeks from the time of referral by a primary care provider or outreach call from the patient navigator to the actual screening colonoscopy. The patient navigator spent most of her time helping patients understand and obtain all the necessary appointments in a timely manner, which resulted in less time available for outreach to new patients. The patient navigator assisted with communication between clinical case managers and patients regarding transportation from small communities to the regional health facility.
Since the end of the project, the regional tribal health organization has addressed many of these systems-level barriers to screening. They have now developed a streamlined medical protocol for colonoscopy referrals and a training program for their case managers and primary care providers so that all are aware of the medical clearance requirements at the organization. Additionally, the position of a patient care coordinator is being created; that person would facilitate patient travel, help patients with bowel preparation instruction, and continue outreach to patients.
DISCUSSION
The overall CRC screening rate in the ATHS has improved from 29% of AN patients in 2000 to 41% in 2005 before the initiation of these projects. Screening rates increased to 55% in 2010.16 CRC screening included colonoscopy in the past 10 years or flexible sigmoidoscopy in past 5 years. Although it is widely accepted that CRC screening saves lives and overall AN screening rates have increased, regional rates still range from 23% to 68%, indicating a need for continued targeted screening efforts, especially in rural and remote areas.
The size and geography of Alaska can present formidable barriers to obtaining access to health care, including cancer screening services. This article describes 3 projects in the ATHS to increase CRC screening rates among AN men and women. Our previous project to train mid-level rural providers in flexible sigmoidoscopy was successful in getting providers trained but ultimately unsuccessful in significantly increasing screening rates because of staff turnover, competing clinical priorities, and a general shift to colonoscopy as the preferred screening modality. We found that using itinerant endoscopists to perform periodic screening clinics in regional health facilities appears to be the most effective and sustainable model for screening in rural areas. This is not surprising given that this strategy overcomes many inherent barriers to screening, especially patient travel. From a health system standpoint, it is significantly more cost-effective to bring the provider to the patients than to bring the patients to the provider. All of the organizations involved have continued to use the itinerant endoscopy model to provide screening of their patient population using their own funding sources after the end of the project period. Additionally, this pilot project led to a greater emphasis at the tribal health organizations involved in the project to do health promotion around cancer screening.
The quality of colonoscopic examinations in the itinerant endoscopist model was high. Overall, the cecum was reached in 93% of examinations. Additionally, the adenoma detection rate was 35%, far exceeding the national quality guidelines set for men (≥25%) and women (≥15%).17 Because of the high incidence of cancer in the AN population, the prevalence of H pylori infection, and the high adenoma detection rates documented by these pilot projects, endoscopy remains the preferred test for screening this high-risk population. However, nonendoscopy-based screening methods should be explored to increase the number of AN individuals screened for this disease.
Providing targeted outreach to family members of CRC patients was effective at achieving screening uptake among individuals at increased risk of CRC. Patient navigation is a way to improve effectiveness of care and increase screening rates, especially among low-income and minority populations.18–20 We found that patient navigation and targeted outreach were effective at increasing screening rates, but were sometimes hampered by system-level barriers. Health systems need to include efficient ways to provide screening including simplified prescreening procedures for asymptomatic low-risk patients and the use of direct endoscopic referral.21 We recommend that patient navigation programs conduct a screening referral process flow assessment in the start up phase to identify potential barriers before program initiation. Patient navigation programs must also be fully integrated into clinic flow systems.
A limitation of the programs presented here was that data were not collected on resource use and related costs for CRC screening. Therefore, analyses of costs are not possible. Further research on relative costs may help inform the development of organized CRC programs.22 Another limitation of the study is the heterogeneous nature of the ATHS, which includes multiple regional tribal health facilities. This heterogeneity makes it more difficult to draw conclusions as to best practices in promoting CRC screening among all AN men and women. However, we have found that even if screening and/or travel for screening are financially covered, other barriers, such as system-level issues, still exist. Because of this, we have increasingly focused on expanding patient navigation, education, and outreach efforts to ensure greater numbers of patients receive recommended CRC screening. Continued efforts to address and respond to these types of challenges will help to increase CRC screening and ultimately reduce the disparities in CRC morbidity and mortality among the AN population.
Take-home Message.
Increasing colorectal cancer (CRC) screening among rural and remote Alaska Native populations requires exploration of multiple methods, including travel by itinerant endoscopists to regional communities and increased patient navigation services.
By addressing geographic and community-level barriers, the Alaska Tribal Health System was able to successfully increase CRC screening rates among Alaska Native populations.
ACKNOWLEDGMENTS
We acknowledge the contributions and support of the Centers for Disease Control and Prevention Division of Cancer Prevention and Control, the Indian Health Service, and the ANTHC Board of Directors. The findings and conclusions in this report are those of the authors and do not represent the official position of the Centers for Disease Control and Prevention.
Abbreviations
- AN
Alaska Native
- ANMC
Alaska Native Medical Center
- ANTHC
Alaska Native Tribal Health Consortium
- ATHS
Alaska Tribal Health System
- CRC
colorectal cancer
Footnotes
DISCLOSURE: The authors disclosed no financial relationships relevant to this publication.
REFERENCES
- 1.Lanier, Kelly, Maxwell, et al. Cancer in Alaska natives: thirty-five year report 1969–2003. Anchorage, AK: Office of Alaska Native Health Research and Alaska Native Epidemiology Center; 2006. [Google Scholar]
- 2.SEER Stat Database: Mortality-All COD, Aggregated With State, Total U.S. (1990–2007) Katrina/Rita Population Adjustment. [Accessed May 11, 2011];National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch. 2011 Available at: www.seer.cancer.gov. [Google Scholar]
- 3.SEER Stat Database: Incidence-SEER 13 Regs Research Data, Nov 2010 Sub (1992–2008) Katrina/Rita Population Adjustment Linked to County Attributes. Total U.S., 1969–2009 Counties. [Accessed May 11, 2011];National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch. 2011 Available at: www.seer.cancer.gov. [Google Scholar]
- 4.Perdue DG, Perkins C, Jackson-Thompson J, et al. Regional differences in CRC incidence, stage, and subsite among American Indians and Alaska Natives, 1999–2004. Cancer. 2008;113(5 Suppl):1179–1190. doi: 10.1002/cncr.23726. [DOI] [PubMed] [Google Scholar]
- 5.Smith RA, Cokkinides V, Eyre HJ. Cancer screening in the United States, 2007: a review of current guidelines, practices, and prospects. CA Cancer J Clin. 2007;57:90–104. doi: 10.3322/canjclin.57.2.90. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 7.Demographics Unit, Research and Analysis, Alaska Department of Labor and Workforce Development, State of Alaska. Alaska State Race Bridged Series 2001–2009. [Accessed May 13, 2011];Juneau, AK. 2010 Aug 20; 2010. Available at: http://laborstats.alaska.gov. [Google Scholar]
- 8.Lanier AP. Cancer in Circumpolar Inuit. Acta Oncol. 1996;35:523–525. doi: 10.3109/02841869609096982. [DOI] [PubMed] [Google Scholar]
- 9.Parkinson AJ, Gold BD, Bulkow L, et al. High prevalence of Helicobacter pylori in the Alaska native population and association with low serum ferritin levels in young adults. Clin Diagn Lab Immunol. 2000;7:885–888. doi: 10.1128/cdli.7.6.885-888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yip R, Limburg PJ, Ahlquist DA, et al. Pervasive occult gastrointestinal bleeding in an Alaska native population with prevalent iron deficiency. Role of Helicobacter pylori gastritis. JAMA. 1997;277:1135–1139. doi: 10.1001/jama.1997.03540380049030. [DOI] [PubMed] [Google Scholar]
- 11.Redwood D, Joseph DA, Christensen C, et al. Development of a flexible sigmoidoscopy training program for rural nurse practitioners and physician assistants to increase colorectal cancer screening among Alaska Native people. J Health Care Poor Underserved. 2009;20:1041–1048. doi: 10.1353/hpu.0.0223. [DOI] [PubMed] [Google Scholar]
- 12.Alaska Area 2007 Aggregate Report. Anchorage (Alaska): Alaska Area Native Health Service; 2007. Indian Health Service 2007 National Government Performance and Results Act of 1993 Clinical Performance Report CRS. [Google Scholar]
- 13.Rex DK, Bond JH, Winawer S, et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2002;97:1296–1308. doi: 10.1111/j.1572-0241.2002.05812.x. [DOI] [PubMed] [Google Scholar]
- 14.Lieberman D, Nadel M, Smith RA, et al. Standardized colonoscopy reporting and data system: report of the Quality Assurance Task Group of the National Colorectal Cancer Roundtable. Gastrointest Endosc. 2007;65:757–766. doi: 10.1016/j.gie.2006.12.055. [DOI] [PubMed] [Google Scholar]
- 15.Fuchs CS, Giovannucci EL, Colditz GA, et al. Aprospective study of family history and the risk of colorectal cancer. N Engl J Med. 1994;331:1669–1674. doi: 10.1056/NEJM199412223312501. [DOI] [PubMed] [Google Scholar]
- 16.Indian Health Service. Clinical Performance Report CCRS, Version 10.0. Alaska Area 2010 Aggregate Report. Anchorage, Alaska: 2010. Aug 9, 2010 National GPRA (Government Performance and Results Act of 1993) [Google Scholar]
- 17.Rex DK, Petrini JL, Baron TH, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2006;101:873–885. doi: 10.1111/j.1572-0241.2006.00673.x. [DOI] [PubMed] [Google Scholar]
- 18.Jandorf L, Gutierrez Y, Lopez J, et al. Use of a patient navigator to increase colorectal cancer screening in an urban neighborhood health clinic. J Urban Health. 2005;82:216–224. doi: 10.1093/jurban/jti046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Percac-Lima S, Grant RW, Green AR, et al. A culturally tailored navigator program for colorectal cancer screening in a community health center: a randomized, controlled trial. J Gen Intern Med. 2009;24:211–217. doi: 10.1007/s11606-008-0864-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petereit DG, Molloy K, Reiner ML, et al. Establishing a patient navigator program to reduce cancer disparities in the American Indian communities of Western South Dakota: initial observations and results. Cancer Control. 2008;15:254–259. doi: 10.1177/107327480801500309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nash D, Azeez S, Vlahov D, et al. Evaluation of an intervention to increase screening colonoscopy in an urban public hospital setting. J Urban Health. 2006;83:231–243. doi: 10.1007/s11524-006-9029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tangka FK, Subramanian S, Bapat B, et al. Cost of starting colorectal cancer screening programs: results from five federally funded demonstration programs. Prev Chronic Dis. 2008;5:A47. [PMC free article] [PubMed] [Google Scholar]