Abstract
A wide variety of membrane proteins induce membrane curvature for function, thus it is important to develop new methods to simultaneously determine membrane curvature and protein binding sites in membranes with multiple curvatures. We introduce solid-state NMR methods based on magnetically oriented bicelles and off-magic-angle spinning (OMAS) to measure membrane curvature and the binding site of proteins in mixed-curvature membranes. We demonstrate these methods on the influenza virus M2 protein, which not only acts as a proton channel but also mediates virus assembly and membrane scission. An M2 peptide encompassing the transmembrane (TM) domain and an amphipathic helix, M2(21-61), was studied and compared with the TM peptide (M2TM). Static 31P NMR spectra of magnetically oriented DMPC/DHPC bicelles exhibit a temperature-independent isotropic chemical shift in the presence of M2(21-61) but not M2TM, indicating that the amphipathic helix confers the peptide with the ability to generate a high-curvature phase. 2D 31P spectra indicate that this high-curvature phase is associated with the DHPC bicelle edges, suggestive of the structure of budding viruses from the host cell. 31P- and 13C-detected 1H relaxation times of the lipids indicate that the majority of M2(21-61) is bound to the high-curvature phase. Using OMAS experiments, we resolved the 31P signals of lipids with identical headgroups based on their distinct chemical shift anisotropies. Based on this resolution, 2D 1H-31P correlation spectra show that the amide protons in M2(21-61) correlate with the DMPC but not the DHPC 31P signal of the bicelle, indicating that a small percentage of M2(21-61) partitions into the planar region of the bicelles. These results show that the M2 amphipathic helix induces high membrane curvature and localizes the protein to this phase, in excellent agreement with the membrane-scission function of the protein. These bicelle-based relaxation and OMAS solid-state NMR techniques are generally applicable to curvature-inducing membrane proteins such as those involved in membrane trafficking, membrane fusion, and cell division.
Introduction
Many membrane proteins cause membrane curvature to carry out their functions such as membrane trafficking 1, endocytosis 2, virus-cell fusion 3–5 and virus budding 6, 7. Membrane curvature has so far been mainly characterized using electron microscopy 6, 8 and small-angle X-ray scattering (SAXS) 9. However, these techniques do not have the ability to determine the high-resolution structure of the proteins involved in curvature induction. Solid-state NMR (SSNMR) spectroscopy can in principle simultaneously reveal membrane curvature, protein conformation and protein-lipid interactions 5, 10–12. The protein and lipid NMR signals can be readily distinguished by detecting 13C and 15N signals for the former and 31P and 2H signals for the latter, and correlation of the two gives information about protein-lipid interactions 10, 11, 13. It is therefore of interest to develop new SSNMR techniques to determine membrane curvature, protein structure and protein-lipid interactions.
To investigate curvature-dependent protein-lipid interactions, it is desirable to use a lipid membrane with defined curvatures and lower complexity than the cell membrane and virus envelope, since the full complement of lipids that commonly exists in eukaryotic membranes may obscure the effects of protein interactions with a small subset of lipids to cause curvature. Bicelles present an appealing membrane-mimetic system for elucidating the curvature-inducing propensity of membrane proteins. As bilayered discoidal aggregates formed by mixtures of long-chain and short-chain lipids, bicelles have been widely used in solution and solid-state NMR studies of protein structure 14–19. By varying the ratios of long-chain and short-chain lipids, one can produce either weakly aligned isotropic bicelles or strongly aligned anisotropic bicelles to obtain orientational constraints of proteins that are either distributed between bicelles or embedded within bicelles. For SSNMR studies, anisotropic bicelles are usually used. These bicelles are aligned with the planes parallel to the external magnetic field 15 because of their negative anisotropy of magnetic susceptibility. When paramagnetic lanthanide ions 20 or lipids with phenyl rings 21 are added, the bicelle planes can be flipped to be perpendicular to the magnetic field. Compared to mechanically aligned membranes, the magnetically aligned bicelles are easy to prepare, hydrate and stabilize for SSNMR structural studies. The morphology of magnetically oriented bicelles has been extensively studied and variously described as disc shaped 22, perforated lamellar bilayers 23, or worm-like micelles 24. All these models agree about the coexistence of low- and high-curvature domains in the bicelles, with the planar surface dominated by long-chain lipids whereas round edges composed of short-chain lipids. Moreover, the short-chain lipids can exchange between these two domains 25.
The M2 protein of influenza A viruses is a multi-functional protein that acts at several stages of the virus lifecycle. M2 has so far been predominantly studied as a drug-targeted proton channel 26–30, which manifests its activity after the virus is endocytosed into the host cell, where the low pH of the endosome opens the channel to acidify the virion and cause virus uncoating 31. Amantadine and rimantadine inhibit the channel by binding to the transmembrane (TM) pore 27, 32. In a later stage of the virus lifecycle, when a newly assembled virus is ready to be released from the host cell, M2 is recruited to the neck of the budding virus and mediates membrane scission. Electron microscopy data indicate that this second function is carried out by an amphipathic helix (AH) in the cytoplasmic domain, which causes high membrane curvature 6: a peptide corresponding to the AH domain is sufficient to bud into giant unilamellar vesicles, and mutations of the AH domain in full-length M2 inhibits vesicle budding in vitro and prevents virus release in vivo. SAXS data further showed that AH-containing M2 peptides incur bicontinuous lipid cubic phases in phosphoethanolamine-rich lipid membranes 7, consistent with the curvature-inducing function.
In this work, we explore oriented bicelles as a medium to develop new SSNMR methods for investigating the preferential localization of proteins in membrane domains with distinct curvatures. Using the influenza M2 protein, we show that it is possible to measure the protein binding site in low- versus high-curvature membranes by detecting lipid dynamics in the vicinity of the protein and by 2D 31P-1H correlation experiments. Since magic-angle-spinning (MAS) NMR spectra cannot resolve the 31P isotropic chemical shifts of lipids with the same headgroup structure, we introduce off-magic-angle spinning (OMAS) experiments to resolve 31P signals based on the motionally averaged 31P chemical shift anisotropies (CSAs) of long-chain and short-chain lipids. We find that an AH-containing M2 peptide both induces and partitions into a high-curvature membrane phase, thus providing nanometer-scale structural evidence of the membrane scission function of this protein.
Experimental Section
Membrane sample preparation
Two M2 peptides, M2(22-46) and M2(21-61), were synthesized using Fmoc chemistry and purified by PrimmBiotech (Cambridge, MA). M2(21-61) contains both the TM domain (residues 22-46) and the AH domain (residues 47–61). Uniformly 13C, 15N-labeled residues were incorporated at L26, V27, A30, G34 and I35 in M2(21-61). For simplicity, we interchangeably denote M2(22-46) as M2TM and M2(21-61) as M2TM-AH in this paper.
1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) and 1,2-dihexanoyl-sn-glycero-3-phosphocholine (DHPC) were used to prepare most of the oriented bicelle samples in this study. Following the literature 33, we dissolved DHPC in ~45 μL of pH 7.5 Tris buffer and pipetted the solution to a dry powder of the peptide. The mixture was vortexed and then added to the desired amount of DMPC powder. This ternary mixture was cooled and heated between 0°C and 42°C 5 – 7 times until the sample became uniform. The final solution was viscous at room temperature but fluid at 4°C. For peptide-free control samples, the DHPC solution was directly added to the DMPC powder and subjected to the same cool/heat cycles. The bicelles samples were transferred to 4 mm MAS rotors for SSNMR experiments. All samples were hydrated to ~65 wt%, and the molar ratio of DMPC : DHPC : peptide was 48 : 16 : 1. d22-DHPC was incorporated into some of the bicelles to investigate the effect of the peptides on lipid chain order. The M2-containing bicelles were stable for about a month, while the peptide-free bicelles were stable for many months. A second bicelle system consisting of DMPC and 1,2-dihexyl-snglycero-3-phosphocholine (6-O-PC) was also prepared to assess the generality of curvature induction by M2TM-AH.
Solid-state NMR spectroscopy
SSNMR experiments were conducted on Bruker wide-bore 400 MHz and 600 MHz spectrometers using 4-mm MAS probes. The oriented bicelles were measured under either the static or the spinning condition, and the angle of the rotation axis was varied by ~7° around the magic angle. Typical radiofrequency (rf) field strengths were 40–50 kHz for 31P and 13C pulses and ~30 kHz for 1H decoupling. The weak 1H decoupling field was chosen to avoid overheating and dehydrating the bicelles, which would destroy the orientational alignment. For the same reason, most experiments used long recycle delays of 4.5 – 6.0 s and a slow spinning frequency of 3.5 kHz. All 31P chemical shifts were referenced to the 31P peak of hydroxyapatite at 2.73 ppm while all 13C chemical shifts were externally referenced to the adamantine CH2 signal at 38.48 ppm on the TMS scale.
Static 31P direct-polarization (DP) spectra were measured as a function of temperature from 283 K to 312 K. To obtain stable alignment, we kept the bicelles in the magnetic field at 312 K for an hour and further stabilized them for 30 min at each temperature before measurements. All reported temperatures were thermocouple values calibrated using the 207Pb signal of lead nitrate. Static 2D 31P-31P exchange spectra were measured using long mixing times of 0.5 to 1.0 s. Bicelle samples with and without M2TM-AH were measured at 297 K and 306 K, respectively. These temperatures were chosen so that the two samples have the same DHPC 31P chemical shift.
31P and 13C-detected 1H T2 relaxation times were measured using a Hahn echo sequence on the 1H channel, followed by 1H spin diffusion and cross polarization (CP) to the heteronucleus 34. Increasing the spin diffusion mixing time (tm) allows us to detect protons further away from the observed 31P site. To exclude the peptide 1H contribution to the 13C-detected 1H T2 data, we additionally carried out an experiment in which a 13C-1H dipolar filter of 100 μs was added before the 1H Hahn-echo period. This dipolar dephasing period suppresses the 1H magnetization of the rigid peptide while retaining the 1H magnetization of the mobile lipids and water. The lipid and water 1H T2 relaxation times were also measured by direct 1H detection at 302 K. 2H quadrupolar echo experiments were conducted on d22-DHPC-containing bicelles to investigate DHPC dynamics in different membranes. A 2H rf field strength of 50 kHz and a recycle delay of 0.8 s were used for these quadrupolar echo experiments.
OMAS experiments were conducted to resolve the 31P chemical shifts of different membrane domains and to obtain high-sensitivity and high-resolution 2D 1H-31P correlation spectra. A spinning frequency of 3.5 kHz was used. This frequency was small enough to avoid perturbing the bicelle morphology but high enough to avoid 31P spinning sidebands 35. The 2D 1H-31P correlation spectra 13 were measured with MREV-8 1H homonuclear decoupling during the t1 evolution period. The 1H 105° pulse length in the MREV-8 pulse train 36 was 6 μs. The 1H chemical shift was calibrated using N-formyl-Met-Leu-Phe-OH (MLF), whose 1H chemical shifts have been reported 37.
Results
Temperature-dependent spectra of peptide-free DMPC/DHPC bicelles
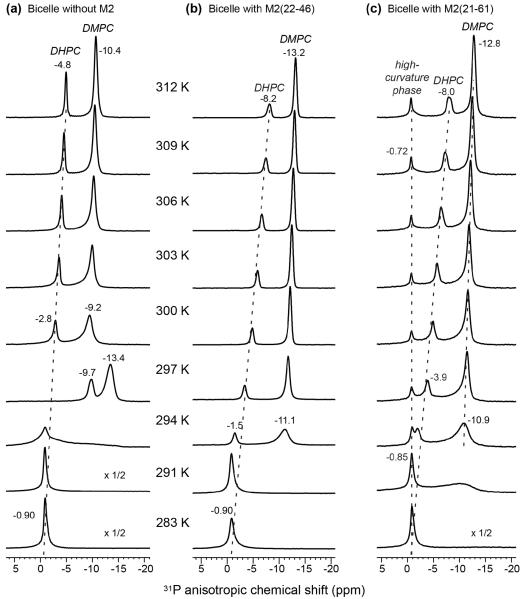
31P NMR is a useful probe of membrane morphology and has been extensively used for studying bicelles. The magnetic alignment of bicelles leads to well-resolved 31P anisotropic chemical shifts under the static condition. From 300 and 312 K, the peptide-free DMPC/DHPC bicelles exhibit two 31P chemical shifts (Fig. 1a). The upfield signal at about −10 ppm can be assigned to DMPC while the downfield peak can be assigned to DHPC. With increasing temperature, both 31P signals move upfield, but the DHPC chemical shift change (from −2.8 ppm to −4.8 ppm) is larger, making it approach the DMPC signal at high temperature. The DMPC versus DHPC chemical shift difference is 6.4 ppm at 300 K and decreases to 5.6 ppm at 312 K. The larger upfield movement of the DHPC 31P signal results from increased exchange of DHPC between the planar and curved regions of bicelles at higher temperature 25, 38.
Figure 1.
Variable-temperature 31P spectra of static DMPC/DHPC (3 : 1) bicelles with and without M2. (a) Bicelles without protein. (b) Bicelle with M2TM. (c) Bicelle with M2TM-AH. M2TM-AH creates a temperature-independent isotropic 31P peak, indicating the formation of a high-curvature membrane phase. The other 31P chemical shifts are anisotropic and change with temperature.
At 283 – 291 K, below the phase transition temperature of DMPC, the 31P spectra exhibit a single peak at the isotropic chemical shift of −0.9 ppm, indicating the formation of a micellar phase or isotropic bicelles 25, 39. At the intermediate temperature of 294 K, a residual power pattern is observed in addition to the isotropic peak, indicating the coexistence of lamellar and isotropic phases. At 297 K, the 31P chemical shifts are discontinuous from the high-temperature values: both DMPC and DHPC peaks moved significantly upfield, to −13.4 ppm and −9.7 ppm, respectively. The DMPC chemical shift is similar to that of glass-plate aligned membranes, suggesting that at this temperature the bicelles are either aligned closer to the 90° angle from the magnetic field or have less internal motion and hence higher order parameters. At this temperature, the DHPC 31P peak is only 3.7 ppm downfield from the DMPC peak, indicating that DHPC undergoes significant exchange between the edge and planar regions of the bicelles.
The M2 amphipathic helix generates high membrane curvature
The addition of M2TM did not change the qualitative trend of the 31P NMR spectra. Two 31P peaks are observed that move upfield with increasing temperatures (Fig. 1b). Thus, M2TM does not perturb the bicelle morphology. Above 300 K, the 31P chemical shifts are about 3 ppm upfield for M2TM-containing bicelles than for peptide-free bicelles. This may be caused by reduced motional amplitudes and thus larger 31P CSAs of the lipids in the presence of M2TM. Between 294 and 297 K, the chemical shift discontinuity and the lamellar phase disappeared, supporting the notion that M2TM stabilizes the bicelles. Both peptide-free and peptide-bound 31P spectra in Fig. 1a, b exhibit DMPC : DHPC 31P area ratios of 3.0 ± 0.3 : 1, which is the same, within experimental uncertainty, as the molar ratio of the lipids in the bicelles. But the M2TM-containing samples show lower height and broader linewidths for the DHPC peak, suggesting that M2TM may have slowed down DHPC motion.
Interestingly, binding of the AH-containing M2(21-61) peptide caused qualitatively different 31P spectra. In addition to the two anisotropic 31P chemical shifts, a temperature-independent 31P peak at the isotropic frequency of −0.8 ppm is observed from 294 to 312 K (Fig. 1c). In general, for non-spinning membrane samples, the 31P CSA is determined by both the chemical structure of the headgroup and lipid reorientational motions. Immobilized lipid headgroups at low temperature give a rigid-limit 31P chemical shift span of ~190 ppm 40. Fast uniaxial rotational diffusion and wobble of the molecular axis in liquid-crystalline lamellar bilayers average this CSA to ~45 ppm, and the maximum intensity occurs at the upfield edge of the power pattern. This uniaxial 31P chemical shift lineshape is further averaged when lipid lateral diffusion and whole-body tumbling are sufficiently fast on the NMR timescale, which occur when the radius of curvature of the membrane is small. Micelles, small vesicles and bicontinuous cubic phases typically have radii of curvature of 5–30 nm, and possess sufficiently high symmetry such that lateral diffusion and tumbling average the 31P CSA to the isotropic value. Thus, the isotropic 31P peak in Fig. 1c indicates that M2(21-61) induces a high-curvature membrane phase, which can be micelles, small vesicles, or bicontinuous cubic phases. The temperature independence of this peak frequency is consistent with the symmetry of these phases.
The 31P isotropic peak represents 10–20% of the total 31P intensities (Fig. 1c). The lipid composition of the high-curvature phase can be estimated from the 31P peak intensities and the 3 : 1 total molar ratio of DMPC/DHPC. The bicelle DMPC and DHPC peaks in Fig. 1c have intensity ratios of 3.0 ± 0.1 : 1, thus the high-curvature phase has a similar lipid composition (3 : 1) as the average composition of this sample. Other bicelle samples prepared in this study had 31P intensity ratios of 2.2 : 1 to 3.5 : 1 (data not shown), suggesting that the composition of the high-curvature phase can vary from predominantly DMPC to ~50% DMPC. Since M2TM does not generate this peak, the amphipathic helix is responsible for causing this high-curvature phase. This isotropic peak was also observed in DMPC/6-O-PC bicelles with bound M2TM-AH (Fig. S1), indicating that curvature induction is an inherent property of the AH-containing M2 and is independent of the exact composition of the bicelle.
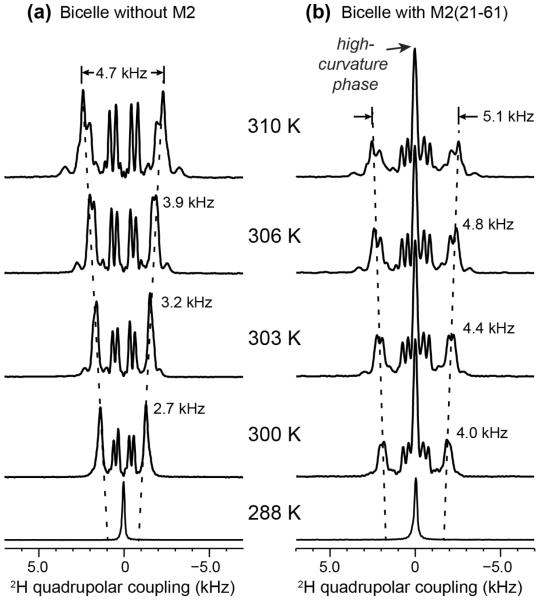
To confirm that the M2(21-61)-induced isotropic 31P peak indeed results from the high curvature of the membrane rather than an accidental magic-angle orientation of the lipid headgroups, we measured the 2H spectra of d22-DHPC, which report lipid chain dynamics. Both bicelles without and with M2TM-AH were measured. Fig. 2 shows that the peptide-free bicelles do not exhibit any zero-frequency peak while the M2TM-AH bound bicelles do, thus the isotropic 31P peak reflects the curvature of the entire membrane. Consistent with the 31P spectra, the 2H quadrupolar splittings are slightly larger in the M2-containing bicelles than in the peptide-free bicelles, indicating that either the lipid order parameters are larger or the bicelles align closer to 90° from the magnetic field.
Figure 2.
Static 2H spectra of d22-DHPC in DMPC/DHPC bicelles. (a) Bicelles without the peptide. (b) Bicelles with M2TM-AH. The peptide-bound membrane shows a zero-frequency peak, indicating the presence of an isotropic membrane domain or a bicontinuous cubic phase.
To investigate whether the high-curvature membrane phase is in spatial contact with the bicelles, we measured 2D 31P-31P exchange spectra of the M2(21-61)-containing bicelle. At mixing times of 0.5 – 1.0 s, the spectra exhibit a cross peak between the isotropic 31P peak and the DHPC peak (Fig. 3a, b), indicating that the high-curvature phase is in physical contact with the round edges of the bicelle. At 1.0 s, the cross peak accounts for 20–30% of the diagonal intensity of the high-curvature phase. No cross peak is observed between DHPC and DMPC. Lipid lateral diffusion over the nanometer dimension of the bicelles occurs on the microsecond timescale, which is sufficiently fast to average out the 31P CSA 41 but too short for the millisecond timescale of 2D exchange NMR experiments, thus the lack of an DMPC/DHPC cross peak in the 2D spectra is understandable. In a recent study of lipid lateral diffusion using 31P exchange NMR 42, 200 mM sucrose was added to the membrane to slow down the diffusion rate and to enable the detection of lipid reorientations on the millisecond timescale. Similar strategies may be applicable for studying exchange between the edge and planar regions of bicelles, if small-molecule additives that do not affect bicelle alignment can be found.
Figure 3.
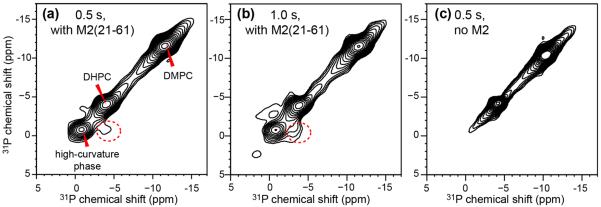
2D 31P-31P exchange spectra of static DMPC/DHPC bicelles. (a) With M2TM-AH and 0.5 s spin diffusion. (b) With M2TM-AH and 1.0 s spin diffusion. (c) Without M2 and with 0.5 s spin diffusion. The spectra were measured at 297 K for (a, b) and 306 K for (c) to obtain the same DHPC 31P chemical shift. Cross peaks between the isotropic peak and the DHPC peak (dotted circle) are observed for the protein-bound bicelles in (a) and (b).
13C spectra of M2-containing DMPC/DHPC bicelles
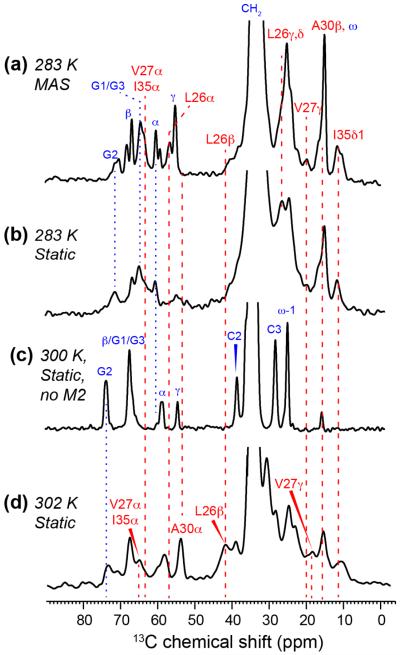
Any indirect detection methods to determine the site of protein binding in the mixed-curvature oriented bicelles require the ability to resolve the peptide 13C signals under the static condition. While 13C MAS spectra readily provide this site resolution in terms of isotropic chemical shifts, the resolution of static 13C spectra of membrane peptides with uniformly 13C-labeled residues can be much lower due to 13C-13C dipolar coupling. We thus measured the static and MAS 13C spectra of DMPC/DHPC bicelles with and without M2TM-AH to identify and assign the peptide signals. Comparison of the static and MAS spectra allows us to distinguish anisotropic and isotropic chemical shifts, while comparison of peptide-bound and peptide-free bicelles allows us to identify the peptide 13C signals. Temperature variation provides additional clues for resonance assignment by changing the membrane phase and alignment.
Many lipid signals such as glycerol G2 and G1/G3 are detected in the 13C spectra (Fig. 4). Their chemical shifts change between 283 K, where the bicelles are in an isotropic phase, and 300 K or higher, where the bicelles are oriented. Thus the high-temperature chemical shifts are anisotropic while the low-temperature shifts are isotropic. This is confirmed by the fact that the 283 K static spectrum matches the MAS spectrum (albeit with broader linewidths) while the static high-temperature spectra differ from the MAS spectra. Two peaks are observed for some of the lipid functional groups in the static spectra, which result from the presence of two different headgroup conformations in phosphocholine and the known conformational differences between the sn-1 and sn-2 carboxyl carbons 43. In addition to these lipid signals, the static 13C spectra also exhibit peptide signals such as I35/V27 Cα (65.3 ppm), L26 Cβ (42.2 ppm), V27 Cγ (18.9 ppm) and I35 Cδ1 (10.9 ppm). These anisotropic chemical shifts do not deviate significantly from the isotropic chemical shifts, indicating that whole-body reorientation of the bicelle significantly scales the 13C CSAs. The L26 Cβ and I35 Cδ1 signals are particularly unambiguous and well resolved, thus they are used as probes of M2 partitioning in mixed-curvature membranes. To verify that the TM domain retains the same α-helical conformation in bicelles as in multilamellar liposomes, we measured a double-quantum filtered 13C spectrum of the sample, where the natural-abundance lipid 13C signals are suppressed (Fig. S2). The spectrum shows the same α-helical chemical shifts for the labeled residues as reported before for multilamellar liposome samples 32, 44.
Figure 4.
1D 13C CP spectra of DMPC/DHPC bicelles with and without M2TM-AH under static and MAS conditions. (a) 13C CP spectrum of peptide-bound bicelles at 283 K under MAS, showing resolved protein (red) and lipid (blue) signals at isotropic 13C chemical shifts. (b) Static 13C spectrum of peptide-bound bicelles at 283 K. The bicelle is in the isotropic phase at this temperature, thus the chemical shifts are the same as in the MAS spectrum but the linewidths are broader. (c) 13C static spectrum of oriented bicelles without M2 at 300 K. The lipid chemical shifts differ from those in the MAS spectrum in (a) due to the presence of CSA under the oriented condition. (d) Static 13C spectrum of M2-bound oriented bicelles at 302 K. The peptide 13C chemical shifts are slightly different from the isotropic shifts in (a) and (b) due to the presence of CSA. Blue and red dashed lines guide the eye for chemical shift changes under different experimental conditions.
1H T2 relaxation times indicate preferential localization of M2TM-AH in the high-curvature membrane
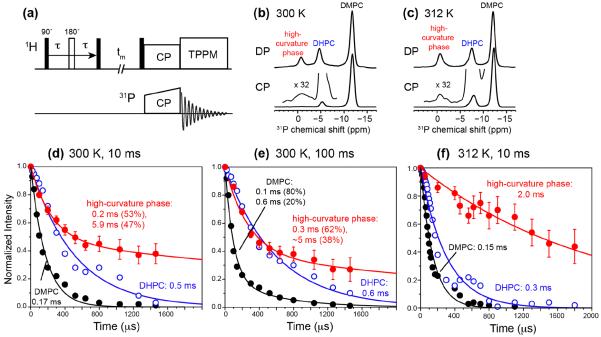
With resolved lipid and peptide 13C signals in the static spectra of the oriented bicelles, we can measure the 1H T2 relaxation times of lipids with 31P and 13C detection (Fig. 5a). 31P detection allows us to investigate the dynamics of DMPC, DHPC, and the high-curvature lipids, while 13C detection allows us to detect the dynamics of lipids in the vicinity of the peptide, thus revealing the membrane environment in which M2 resides 13.
Figure 5.
31P-detected 1H T2 relaxation data of DMPC/DHPC bicelles containing M2TM-AH under the static condition. (a) Pulse sequence of the indirectly detected 1H T2 experiment. (b) Representative 31P DP and CP spectra with 1H spin diffusion mixing at 300 K. (c) Representative 31P DP and CP spectra with 1H spin diffusion at 312 K. The 31P chemical shifts differ between 300 K and 312 K for DMPC and DHPC peaks due to temperature-dependent exchange of DHPC between the planar and edge regions of the bicelle. (d) 1H T2 relaxation decays at 300 K after 10 ms 1H spin diffusion. (e) 1H T2 relaxation decays at 300 K after 100 ms 1H spin diffusion. (f) 1H T2 relaxation decays at 312 K after 10 ms spin diffusion. The DMPC, DHPC and high-curvature phase data are shown in black, blue and red, respectively.
Fig. 5 shows 31P-detected 1H T2 relaxation data and representative 31P spectra. We measured the 1H T2 at 300 K using 10 ms and 100 ms mixing, to probe the dynamics of lipids in small and large domains. Among the three resolved membrane phases, DMPC undergoes the fastest relaxation with a 1H T2 of 0.1-0.2 ms, while the high-curvature phase has the slowest relaxation: apart from an initial decay of 0.1-0.2 ms, 40–50% of the intensity exhibits a T2 of 5–6 ms (Fig. 5d, e). The bicelle-edge DHPC lipids exhibit an intermediate 1H T2 of ~0.5 ms. The slow relaxation of the high-curvature phase is understandable, because the lipids in this phase undergo not only uniaxial rotational diffusion around the local membrane normal but also isotropic tumbling and lateral diffusion over the highly curved membrane surface. Increasing the mixing time from 10 ms to 100 ms did not change the relaxation rates of DMPC and DHPC but increased the relaxation rates of the high-curvature phase, and the initial decay now overlaps with the initial DHPC decay. This convergence is in good agreement with the observed 31P cross peak between DHPC and the high-curvature lipids, further supporting the conclusion that the high-curvature phase is associated with the bicelle-edge DHPC lipids.
Increasing the temperature to 312 K brought interesting counter-directional changes to the relaxation rates of DHPC and the high-curvature lipids: the high-curvature lipids relax more slowly, whereas the DHPC relaxation rates increased. The former indicates faster reorientations of the high-curvature lipids at high temperature, while the latter can be attributed to increased diffusion of DHPC between the planar and edge regions of the bicelle. The upfield 31P chemical shift of −7.6 ppm at 312 K, compared to the value of −4.8 ppm at 300 K, is consistent with this increased exchange.
To determine whether the indirectly measured 1H relaxation times of lipids differ from the directly measured values, we measured the T2 of bicelles containing M2TM-AH by direct 1H detection (Fig. 6a). The acyl-chain CH2 and CH3 signals and the headgroup Hγ peak all exhibit biexponential decays, with a fast component of 0.4–0.7 ms and a slow component of ~10 ms for the chain protons and ~50 ms for the headgroup trimethylamine. For acyl-chain protons, we assign the short T2 component to the oriented bicelle phase and the long T2 to the dynamic high-curvature phase. The long-component T2 values are larger than the T2's measured by 31P detection, likely because direct detection favors the detection of highly dynamic protons while indirect detection by 1H-31P CP preferentially enhances the signals of the more immobile protons. Compared to the lipid 1H T2's, the water T2 is much longer, ~93 ms, consistent with the presence of bulk water in these highly hydrated (65 wt%) samples.
Figure 6.
Directly detected 1H T2 relaxation data of DMPC/DHPC bicelles containing M2TM-AH under the static condition at 302 K. (a) Representative 1H spectra with different echo delays. (b) 1H T2 relaxation curves with single or double exponential fits. The decay constants and the corresponding fractions are listed. The lipid relaxation times range from 0.4 to 50 ms, whereas the water 1H T2 relaxation time is much longer, 93 ms.
Switching to 13C detection for the 1H T2 measurement allowed us to probe the dynamics of lipids in the vicinity of M2TM-AH. At 302 K and with a short spin diffusion time of 10 ms, fast T2 relaxation times of 50–110 μs were observed, indicating that only the relatively immobile peptide protons were detected (Fig. 7e). Increasing the mixing time to 100 ms dramatically slowed down the T2 relaxation: both I35 and L26 sidechains now exhibit biexponential decays with a slow component of 7 and 15 ms, respectively (Fig. 7f). The initial fast decay is dominant for the L26 Cβ signal and accounts for ~30% of the I35 Cδ1 signal. To verify that this initial fast decay results from peptide protons instead of reflecting an immobile pool of lipids, we added a 13C-1H dipolar filter of 100 μs before the echo period (Figs. 7a, S3) to suppress the signals of the rigid peptide. Since the lipids are not 13C labeled, the dipolar filter does not impact the lipid signals. Fig. 7g shows that this dipolar filter removed most of the fast initial decay and dramatically increased the L26 Cβ intensity, thus confirming that the lipids near the peptide have long 1H T2 relaxation times of ~7 ms. This T2 value is closest to the 31P-detected 1H T2 of the high-curvature lipids, but an order of magnitude longer than the relaxation times of bicelle lipids. Therefore, these 13C- and 31P-detected 1H T2 data indicate that M2TM-AH is preferentially bound to the high-curvature domain.
Figure 7.
13C-detected 1H T2 relaxation data of M2TM-AH in oriented bicelles at 302 K under the static condition. (a) Pulse sequence of the dipolar-dephased 1H T2 relaxation experiment used to obtain the data in (g). (b) Representative 13C spectra without dipolar dephasing, measured with 10 ms 1H spin diffusion. (c) Representative 13C spectra without dipolar dephasing, measured with 100 ms 1H spin diffusion. (d) Representative 13C spectra with dipolar dephasing and 100 ms 1H spin diffusion. The echo delay is 0 for all the spectra in (b–d). (e) 13C-detected 1H T2 relaxation curves with 10 ms spin diffusion. The schematic illustration below indicates that 1H magnetization transfer is mostly within the peptide at this mixing time. (f) 13C-detected 1H T2 relaxation curves after 100 ms spin diffusion. At this mixing time, lipid 1H magnetization from the surrounding environment is transferred to the peptide, thus giving much slower relaxation. A fast initial decay is especially pronounced for the L26 Cb signal. (g) 1H T2 relaxation curves measured with 100 μs C-H dipolar dephasing and 100 ms spin diffusion, to selectively detect the T2 relaxation of lipids near the peptide. The disappearance of the fast initial decay of the L26 Cb data verifies that the origin of the fast initial decay is the rigid peptide protons.
OMAS 2D 1H-31P correlation NMR for detecting M2(21-61) partitioning in bicelles
We measured 2D 31P-1H correlation spectra to detect lipid 31P – protein HN correlation signals directly. Although the 31P signals of the three membrane components are well resolved under the static condition, the collective protein HN signals are expected to be much broader than the lipid 1H signals under the static oriented condition, because membrane-bound proteins usually exhibit larger orientational disorder and slower motional rates than lipids, thus giving rise to larger residual 1H-1H dipolar couplings. Therefore, we chose to spin the bicelle to obtain the necessary sensitivity and resolution. Since all three lipid components have the same 31P isotropic chemical shift, they are unresolved in the 31P MAS spectrum (Fig. 8a), making it necessary to conduct OMAS experiments. Deviating from the magic angle by a small degree already retrieved sufficient 31P CSAs and site resolution, and retained reasonable 1H linewidth simultaneously. In the spectrum shown in Fig. 8b, all three lipid signals are resolved and readily assigned based on their relative intensities and the effective 31P CSAs. The most downfield and highest peak can be assigned to DMPC while the most upfield and weakest peak can be assigned to the high-curvature membrane domain. Changing the sample axis to the other side of the magic angle caused the opposite frequency changes, with DMPC giving the most upfield chemical shift (Fig. 8c).
Figure 8.
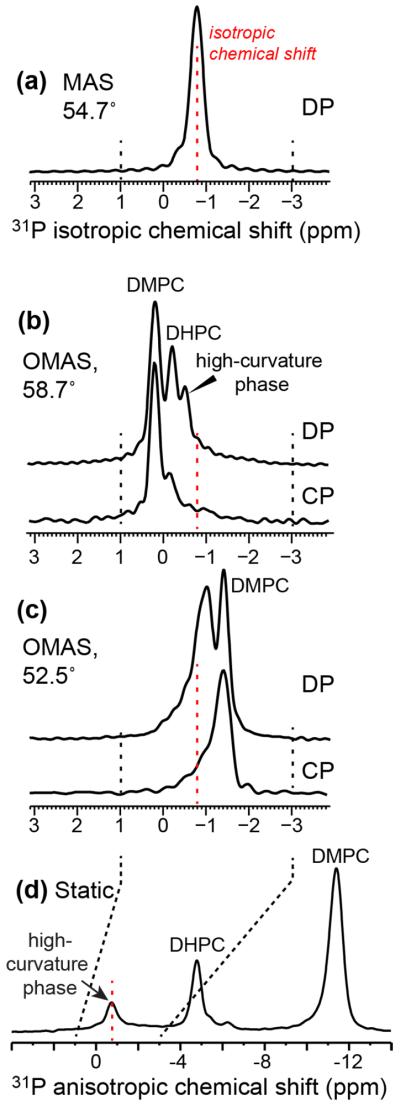
1D 31P spectra of bicelles containing M2TM-AH under 3.5 kHz spinning at 300 K. (a) 31P DP MAS spectrum. (b) 31P DP and CP spectra under OMAS at 58.7°. Three 31P peaks are resolved. (c) 31P DP and CP spectra under OMAS at 52.5°. (d) Comparative static 31P spectrum, showing three well resolved peaks.
The precise angles (θ) of the rotation axis from the magnetic field can be determined from the chemical shift differences among the 31P MAS, OMAS and static spectra. The static spectrum in Fig. 8d is equivalent to a θ = 0° OMAS spectrum with a scaling factor (3cos2θ −1)/2 of 1. The OMAS spectrum of Fig. 8b has the opposite intensity distribution from the static spectrum, indicating that the rotation axis is tilted by an angle larger than 54.7° to cause a negative scaling factor to the 31P CSA. The DMPC 31P chemical shift is 1.0 ppm larger than the isotropic chemical shift in Fig. 8b but 10.6 ppm smaller than the isotropic frequency in the static spectrum (Fig. 8d). Thus the scaling factor is 1/(−10.6) = −0.094, which corresponds to a rotation angle of 58.7°. Similar arguments indicate a rotation angle of 52.5° for the spectrum in Fig. 8c.
Fig. 9 shows the 2D 1H-31P correlation spectra DMPC/DHPC bicelles containing M2TM-AH under 58.7° OMAS. Without 1H spin diffusion, the DMPC and DHPC 31P signals only correlate with lipid protons such as glycerol G2 (5.4 ppm) and G3 and headgroup α/β (4.2 ppm) (Fig. 9b). These anisotropic 1H chemical shifts do not deviate significantly from the isotropic values measured in the MAS spectrum (Fig. 9d, e) because of the small motionally averaged 1H CSAs and the small deviation of the rotation angle from the magic angle. With 0.5 ms 1H spin diffusion, 31P – lipid chain 1H cross peaks were observed. Importantly, broad HN signals at 6 – 9 ppm were detected to correlate with the DMPC 31P peak but not the DHPC peak (Fig. 9c, f), indicating that some M2TM-AH partitions into the planar region of the bicelles. The amide protons that correlate with the DMPC 31P most likely result from the surface-bound amphipathic helix rather than the TM domain 45.
Figure 9.
2D 31P-1H correlation spectra with 1H homonuclear decoupling, measured at 306 K under OMAS at 58.7°. (a) Pulse sequence for the homonuclear-decoupled HETCOR experiment. (b) 2D spectrum without 1H spin diffusion. (c) 2D spectrum with 0.5 ms 1H spin diffusion. (d) 1D 1H MAS spectrum. (e) 1D 1H OMAS spectrum at 58.7°. (f) 1H cross sections of DMPC with 0.5 ms (top) and 0 ms (bottom) spin diffusion, extracted from the 2D spectra in (b) and (c).
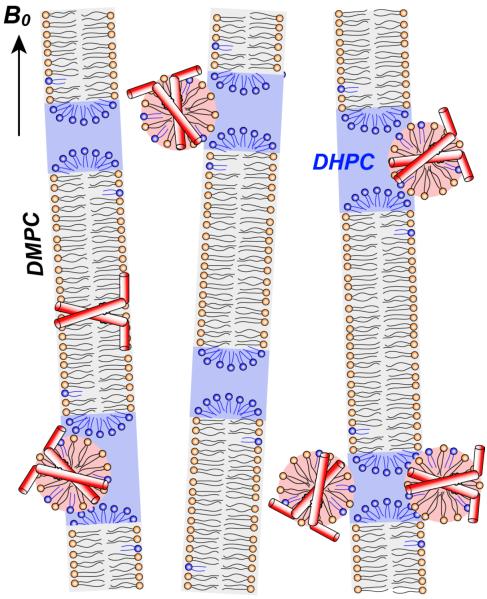
Taken together, the 1D 31P and 2H spectra and the 2D 31P-31P exchange spectra show that the amphipathic helix of M2 generates a high-curvature membrane domain that is associated with the DHPC edges of bicelles, while 1H relaxation data and 2D 1H-31P correlation spectra indicate that the protein predominantly binds the high-curvature domain, followed by a small fraction that partitions into the planar region of the bicelle. These results are depicted in Fig. 10, where the high-curvature phase is represented as micelles, although bicontinuous cubic phase cannot be ruled out.
Figure 10.
Schematic of M2TM-AH binding to membranes with mixed curvatures. The peptide creates a high-curvature phase that is partially associated with the round caps of bicelles. Depicted here are micelles, but bicontinuous cubic phases cannot be ruled out. Most of the peptide binds to this high-curvature phase, while a small fraction resides in the planar region of the bicelle due to its stabilization of the hydrophobic transmembrane helix. For simplicity, only two out of four molecules of the tetrameric protein is represented.
Discussion
So far, bicelles have been mainly used to determine the high-resolution structures of membrane proteins by solution and solid-state NMR spectroscopy 46–49. The data shown here represent a novel application of bicelles as a medium for characterizing protein-induced membrane curvature. The static 31P spectra distinguish the lipid signals in the planar and edge regions of the bicelle versus lipids in high-curvature phases. By comparing lipid 31P-detected and protein 13C-detected lipid 1H relaxation times, we can deduce the dynamic and curvature environment of the protein. By correlating lipid 31P and protein 1H chemical shifts, we can further define the protein binding sites.
The fact that M2(21-61) and not M2(22-46) generates the isotropic 31P chemical shift and a zero-frequency 2H peak in the static spectra indicates that the amphipathic helix is solely responsible for incurring membrane curvature. The high-curvature phase can be micelles, small vesicles, or bicontinuous cubic phases, all of which possess the symmetry to give an isotropic NMR peak. Recent synchrotron SAXS data indicate that both full-length M2 and an M2 peptide encompassing the TM and AH domains generate bicontinuous cubic phases 7. Full-length M2 required >60 mol% phosphatidylethanolamine (PE) in the membrane to induce the cubic phase, and the SAXS spectra index to an Ia3d gyroid phase with lattice parameters of ~20 nm. The TM - AH peptide generated a mixture of Pn3m and Im3m phases in a wider range of lipid compositions. In PE-free DOPC/DOPS membranes, a Pn3m double-diamond phase was observed with lattice parameters of 21–29 nm. These bicontinuous cubic phases have the common characteristics of possessing negative Gaussian curvature, which is the type of curvature present in the neck of a budding virus. Thus their generation by the AH-containing M2 protein is consistent with the membrane scission function of M2 6. Although it is tempting to conclude, on the basis of these SAXS data, that the isotropic 31P and 2H peaks detected here also results from a cubic phase, several factors argue for a micellar interpretation. DMPC and DHPC lipids have significantly stronger positive intrinsic curvature than the unsaturated DOPC, DOPS and DOPE lipids used in the SAXS study, thus a micellar phase with positive curvature is possible. From the biological standpoint, while the bicontinuous cubic phase captures the membrane geometry at the neck of the budding virus 6, the micelle morphology captures the membrane curvature of the virus away from the budding neck. Finally, the exchange peak between DHPC and the isotropic peak in the 2D 31P-31P correlation spectra indicates that the high-curvature phase is partly associated with the bicelle edges (Fig. 3). Since bicontinuous phases have much larger overall dimensions due to their periodically repeating nature 50, 51, they are less likely to form significant contacts with other membrane phases. Thus, the 31P cross peak in the 2D spectra is also in favor of a micellar interpretation of the isotropic peak in the 31P and 2H NMR spectra.
Since the high-curvature phase generated by M2TM-AH represents only a small fraction (10–20%) of all lipids in our samples, preferential M2 binding to this domain may appear counter-intuitive. However, consideration of equilibrium indicates that this preferential binding is to be expected, because if the protein only resides in the low-curvature membrane while inducing high-curvature domains, the lamellar domain will be eventually depleted. Therefore, the small fraction of the high-curvature domain must be enriched with the protein at equilibrium. Given the preference of M2TM-AH for the high-curvature phase, a natural question is why a small amount of M2 binds the planar DMPC region rather than the round DHPC edges of the bicelle. We attribute this result to the unfavorable geometry of the bicelle edges. For the amphipathic helix to lie on the round caps formed by DHPC, the TM helix must lie perpendicular to the interior DMPC chains. This orientation is not only sterically unfavorable but also prevents the exposure of the N-terminus of the TM helix to water, which is seen in all high-resolution structures of M2 27, 30, 45, 52. In contrast, M2 binding to the planar DMPC region of the bicelle satisfies hydrophobic interactions of the TM helix with the membrane as well as polar interactions of the terminal residues with water, and is thus energetically favorable. Based on the 13C-detected 1H T2 relaxation data (Fig. 7f), we estimate that ~70% of M2TM-AH binds the high-curvature domain while ~30% binds the lamellar domain.
Several mechanisms exist with which proteins can induce high membrane curvature. First, amphipathic helices in proteins can insert asymmetrically into one leaflet of the membrane to promote local curvature. An example is the fusion peptide of the influenza hemagglutinin 4, 53. Analogously, integral membrane proteins or protein assemblies with intrinsic curvature can induce curvature by inserting into the membrane. Second, peripheral membrane proteins can brace the lipid bilayer like a scaffold to deform them into nonlamellar morphologies. For example, the dynamin family of proteins can bend the membrane into tubules to mediate vesicle invagination, membrane scission and cell division 1. Third, arginine-rich peptides such as antimicrobial peptides and cell-penetrating peptides can induce membrane curvature by electrostatic interactions between the cationic residues and lipid phosphates 12, 40, 54–57. The M2 amphipathic helix, which lies on the membrane surface 45, likely induces membrane curvature through the scaffolding mechanism.
The fact that M2TM-AH generates this high-curvature phase in the simple membrane composition of DMPC/DHPC, without cholesterol, is noteworthy. A previous study of M2TM-AH in a virus-mimetic membrane containing phosphocholine, phosphoethanolamine, sphingomyelin, and cholesterol, also detected a 31P isotropic peak 13. Combined with the recent SAXS results, which were obtained in cholesterol-free membranes 7, these biophysical data together show that M2TM-AH can generate curvature in a variety of membrane compositions, and cholesterol is not a necessary condition for curvature induction. However, the exact type of curvature may differ among different membrane compositions, since the lipid components may affect the depth of the amphipathic helix, the clustering of the M2 tetramers, and the orientation of the TM and amphipathic helices 45, 58–60. Additional experiment will be necessary to determine the types of membrane curvatures induced by M2 in various lipid environments.
The 2D OMAS correlation NMR experiment shown here is analogous to variable-angle spinning, switched-angle spinning, and dynamic-angle spinning experiments pioneered by Terao, Pines and Fung 61–65. Most of these techniques focused on separating anisotropic interactions such as dipolar couplings and quadrupolar couplings in the indirect dimension by isotropic chemical shifts in the direct dimension. The current experiment differs in using anisotropic chemical shifts to resolve different membrane curvatures rather than chemical functional groups. Interestingly, the rotation angle determined from our 31P spectra (Fig. 8) under 3.5 kHz spinning indicates a different bicelle alignment from that predicted by the classical theory of variable-angle spinning of liquid crystals 66. The classical theory states that when the spinning frequency is larger than a critical frequency of several hundred hertz, liquid crystals do not have time to align with the magnetic field and adopt an orientation that minimizes the time-averaged potential energy. For liquid crystals with a negative anisotropy of magnetic susceptibility, Δχ, which is the case for bicelles, the bilayer directors are predicted to be distributed in a plane perpendicular to the rotation axis (β = 90°) when the rotation angle θ is smaller than the magic angle, but the directors would be parallel to the rotation axis (β = 0°) when θ > 54.7° 67, 68. The overall scaling factor, (3cos2θ −1)/2·(3cos2β −1)/2, is thus negative under both conditions, indicating that the anisotropic 31P chemical shift should be smaller than the isotropic shift at all rotation angles. This was observed for spinning frequencies less than 1 kHz 35, 69. But under our spinning frequency of 3.5 kHz, the spectrum in Fig. 8b shows 31P chemical shifts that are larger (i.e. downfield) from the isotropic chemical shift, which translates to a positive overall scaling factor. This indicates that the directors remain perpendicular to the rotation axis even when θ > 54.7°, in contrast to the classical theory. This bicelle orientation under fast spinning has also been reported by Meier and coworkers 35, and suggests that under sufficiently fast spinning, the directors do not have time to change their alignment axis from the non-spinning condition and instead remain perpendicular to the rotation axis.
Relatively high amide HN chemical shift resolution of ~0.4 ppm on bicelle-bound membrane proteins has recently been shown to be achievable under the static condition, without spinning, by using optimized 1H-1H homonuclear decoupling sequences and by correlating HN chemical shifts with 15N chemical shifts 70, 71. The OMAS 1H-31P correlation technique is complementary to that approach. Since HN-31P cross peaks are intermolecular by design, all protein amide HN signals are observed together in the same 31P cross section, thus residue-specific HN resolution is not necessary. In addition, surface-bound membrane protein segments such as the M2 amphipathic helix may be less uniformly oriented than transmembrane segments, thus the OMAS approach, which is independent of the degree of alignment, is more robust for these difficult-to-orient membrane proteins.
Conclusion
We presented two bicelle-based SSNMR methods for determining membrane curvature and the binding site of proteins in mixed-curvature membranes. We demonstrated these methods on the influenza M2 protein, which mediates membrane scission during virus budding in addition to serving as a proton channel for virus uncoating. Our data show that the amphipathic helix in M2 confers the ability to cause high membrane curvature, and directs the protein to this high-curvature domain, while a small fraction of the protein resides in the planar region of the bicelle. The M2-induced high-curvature phase is associated with the round edges of the bicelles, reminiscent of the membrane geometry and deformation in a budding virus. The indirect detection of lipid 1H relaxation NMR experiment and the 2D OMAS heteronuclear correlation technique are generally applicable to a wide range of membrane proteins to determine their binding sites in complex membranes with multiple curvatures.
Supplementary Material
Acknowledgments
Funding Sources This work is supported by National Institutes of Health grant GM088204 to M.H.
Footnotes
Supporting Information Additional NMR spectra are provided. This information is available free of charge via the internet at http://pubs.acs.org.
References
- [1].McMahon HT, Gallop JL. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 2005;438:590–596. doi: 10.1038/nature04396. [DOI] [PubMed] [Google Scholar]
- [2].Mim C, Unger VM. Membrane curvature and its generation by BAR proteins. Trends Biochem. Sci. 2012;37:526–533. doi: 10.1016/j.tibs.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Harrison SC. Viral membrane fusion. Nat Struct Mol Biol. 2008;15:690–698. doi: 10.1038/nsmb.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Han X, Bushweller JH, Cafiso DS, Tamm LK. Membrane structure and fusion-triggering conformational change of the fusion domain from influenza hemagglutinin. Nat Struct Biol. 2001;8:715–720. doi: 10.1038/90434. [DOI] [PubMed] [Google Scholar]
- [5].Yao H, Hong M. Conformation and lipid interaction of the fusion peptide of the paramyxovirus PIV5 in anionic and negative-curvature membranes from solid-state NMR. J Am Chem Soc. 2014;136:2611–2624. doi: 10.1021/ja4121956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rossman JS, Jing X, Leser GP, Lamb RA. Influenza virus M2 protein mediates ESCRT-independent membrane scission. Cell. 2010;142:902–913. doi: 10.1016/j.cell.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Schmidt NW, Mishra A, Wang J, DeGrado WF, Wong GC. Influenza virus A M2 protein generates negative Gaussian membrane curvature necessary for budding and scission. J Am Chem Soc. 2013;135:13710–13719. doi: 10.1021/ja400146z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gidalevitz D, Ishitsuka Y, Muresan AS, Konovalov O, Waring AJ, Lehrer RI, Lee KY. Interaction of antimicrobial peptide protegrin with biomembranes. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:6302–6307. doi: 10.1073/pnas.0934731100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mishra A, Gordon VD, Yang L, Coridan R, Wong GC. HIV TAT forms pores in membranes by inducing saddle-splay curvature: potential role of bidentate hydrogen bonding. Angew Chem Int Ed Engl. 2008;47:2986–2989. doi: 10.1002/anie.200704444. [DOI] [PubMed] [Google Scholar]
- [10].Wang T, Yao H, Hong M. Determining the depth of insertion of dynamically invisible membrane peptides by gel-phase (1)H spin diffusion heteronuclear correlation NMR. J Biomol NMR. 2013;56:139–148. doi: 10.1007/s10858-013-9730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Huster D, Yao X, Hong M. Membrane protein topology probed by (1)H spin diffusion from lipids using solid-state NMR spectroscopy. J Am Chem Soc. 2002;124:874–883. doi: 10.1021/ja017001r. [DOI] [PubMed] [Google Scholar]
- [12].Mani R, Cady SD, Tang M, Waring AJ, Lehrer RI, Hong M. Membrane-dependent oligomeric structure and pore formation of a beta-hairpin antimicrobial peptide in lipid bilayers from solid-state NMR. Proc. Natl. Acad. Sci. USA. 2006;103:16242–16247. doi: 10.1073/pnas.0605079103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang T, Cady SD, Hong M. NMR determination of protein partitioning into membrane domains with different curvatures and application to the influenza M2 peptide. Biophys J. 2012;102:787–794. doi: 10.1016/j.bpj.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sanders CR, Landis GC. Reconstitution of membrane proteins into lipid-rich bilayered mixed micelles for NMR studies. Biochemistry. 1995;34:4030–4040. doi: 10.1021/bi00012a022. [DOI] [PubMed] [Google Scholar]
- [15].Sanders CR, Schwonek JP. Characterization of magnetically orientable bilayers in mixtures of dihexanoylphosphatidylcholine and dimyristoylphosphatidylcholine by solid-state NMR. Biochemistry. 1992;31:8898–8905. doi: 10.1021/bi00152a029. [DOI] [PubMed] [Google Scholar]
- [16].Sanders CR, Hare BJ, Howard KP, Prestegard JH. Magnetically-oriented phospholipid micelles as a tool for the study of membrane-associated molecules. Prog. Nuc. Magn. Reson. Spectros. 1994;26:421–444. [Google Scholar]
- [17].Ottiger M, Bax A. Characterization of magnetically oriented phospholipid micelles for measurement of dipolar couplings in macromolecules. J. Biomol. NMR. 1998;12:361–372. doi: 10.1023/a:1008366116644. [DOI] [PubMed] [Google Scholar]
- [18].Tolman JR, Al-Hashimi HM, Kay LE, Prestegard JH. Structural and dynamic analysis of residual dipolar coupling data for proteins. J. Am. Chem. Soc. 2001;123:1416–1424. doi: 10.1021/ja002500y. [DOI] [PubMed] [Google Scholar]
- [19].Prosser RS, Evanics F, Kitevski JL, Al-Abdul-Wahid MS. Current applications of bicelles in NMR studies of membrane-associated amphiphiles and proteins. Biochemistry. 2006;45:8453–8465. doi: 10.1021/bi060615u. [DOI] [PubMed] [Google Scholar]
- [20].Prosser RS, Hunt SA, DiNatale JA, Vold RR. Magnetically aligned membrane model systems with positive order parameter: Switching the sign of S-zz with paramagnetic ions. Journal of the American Chemical Society. 1996;118:269–270. [Google Scholar]
- [21].Tan CB, Fung BM, Cho GJ. Phospholipid bicelles that align with their normals parallel to the magnetic field. Journal of the American Chemical Society. 2002;124:11827–11832. doi: 10.1021/ja027079n. [DOI] [PubMed] [Google Scholar]
- [22].Glover KJ, Whiles JA, Wu G, Yu N, Deems R, Struppe JO, Stark RE, Komives EA, Vold RR. Structural evaluation of phospholipid bicelles for solution-state studies of membrane-associated biomolecules. Biophys J. 2001;81:2163–2171. doi: 10.1016/s0006-3495(01)75864-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gaemers S, Bax A. Morphology of three lyotropic liquid crystalline biological NMR media studied by translational diffusion anisotropy. J Am Chem Soc. 2001;123:12343–12352. doi: 10.1021/ja011967l. [DOI] [PubMed] [Google Scholar]
- [24].Nieh MP, Raghunathan VA, Glinka CJ, Harroun TA, Pabst G, Katsaras J. Magnetically alignable phase of phospholipid “bicelle” mixtures is a chiral nematic made up of wormlike micelles. Langmuir. 2004;20:7893–7897. doi: 10.1021/la048641l. [DOI] [PubMed] [Google Scholar]
- [25].Triba MN, Warschawski DE, Devaux PF. Reinvestigation by phosphorus NMR of lipid distribution in bicelles. Biophys J. 2005;88:1887–1901. doi: 10.1529/biophysj.104.055061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pinto LH, Holsinger LJ, Lamb RA. Influenza virus M2 protein has ion channel activity. Cell. 1992;69:517–528. doi: 10.1016/0092-8674(92)90452-i. [DOI] [PubMed] [Google Scholar]
- [27].Cady SD, Schmidt-Rohr K, Wang J, Soto CS, Degrado WF, Hong M. Structure of the amantadine binding site of influenza M2 proton channels in lipid bilayers. Nature. 2010;463:689–692. doi: 10.1038/nature08722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cady S, Wang T, Hong M. Membrane-dependent effects of a cytoplasmic helix on the structure and drug binding of the influenza virus M2 protein. J Am Chem Soc. 2011;133:11572–11579. doi: 10.1021/ja202051n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jing X, Ma C, Ohigashi Y, Oliveira FA, Jardetzky TS, Pinto LH, Lamb RA. Functional studies indicate amantadine binds to the pore of the influenza A virus M2 proton-selective ion channel. Proc Natl Acad Sci U S A. 2008;105:10967–10972. doi: 10.1073/pnas.0804958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Stouffer AL, Acharya R, Salom D, Levine AS, Di Costanzo L, Soto CS, Tereshko V, Nanda V, Stayrook S, DeGrado WF. Structural basis for the function and inhibition of an influenza virus proton channel. Nature. 2008;451:596–599. doi: 10.1038/nature06528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hong M, Degrado WF. Structural basis for proton conduction and inhibition by the influenza M2 protein. Protein Sci. 2012;21:1620–1633. doi: 10.1002/pro.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cady SD, Mishanina TV, Hong M. Structure of amantadine-bound M2 transmembrane peptide of influenza A in lipid bilayers from magic-angle-spinning solid-state NMR: the role of Ser31 in amantadine binding. J. Mol. Biol. 2009;385:1127–1141. doi: 10.1016/j.jmb.2008.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].De Angelis AA, Opella SJ. Bicelle samples for solid-state NMR of membrane proteins. Nature Protocols. 2007;2:2332–2338. doi: 10.1038/nprot.2007.329. [DOI] [PubMed] [Google Scholar]
- [34].Williams JK, Hong M. Probing membrane protein structure using water polarization transfer solid-state NMR. J Magn Reson. 2014;247:118–127. doi: 10.1016/j.jmr.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zandomeneghi G, Tomaselli M, Williamson PT, Meier BH. NMR of bicelles: orientation and mosaic spread of the liquid-crystal director under sample rotation. J Biomol NMR. 2003;25:113–123. doi: 10.1023/a:1022236217018. [DOI] [PubMed] [Google Scholar]
- [36].Rhim W-K, Elleman DD, Vaughan RW. Analysis of multiple-pulse NMR in solids. J. Chem. Phys. 1973;59:3740–3749. [Google Scholar]
- [37].Li S, Su Y, Luo W, Hong M. Water-protein interactions of an arginine-rich membrane peptide in lipid bilayers investigated by solid-state nuclear magnetic resonance spectroscopy. J Phys Chem B. 2010;114:4063–4069. doi: 10.1021/jp912283r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Triba MN, Devaux PF, Warschawski DE. Effects of lipid chain length and unsaturation on bicelles stability. A phosphorus NMR study. Biophys J. 2006;91:1357–1367. doi: 10.1529/biophysj.106.085118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sternin E, Nizza D, Gawrisch K. Temperature Dependence of DMPC/DHPC Mixing in a Bicellar Solution and Its Structural Implications. Langmuir. 2001;17:2610–2616. [Google Scholar]
- [40].Tang M, Waring AJ, Hong M. Trehalose-protected lipid membranes for determining membrane protein structure and insertion. J. Magn. Reson. 2007;184:222–227. doi: 10.1016/j.jmr.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Marasinghe PAB, Buffy JJ, Schmidt-Rohr K, Hong M. Membrane curvature change induced by an antimicrobial peptide detected by 31P exchange NMR. J. Phys. Chem. B. 2005;109:22036–22044. doi: 10.1021/jp054396i. [DOI] [PubMed] [Google Scholar]
- [42].Saleem Q, Lai A, Morales HH, Macdonald PM. Lateral diffusion of bilayer lipids measured via (31)P CODEX NMR. Chem. Phys. Lipids. 2012;165:721–730. doi: 10.1016/j.chemphyslip.2012.08.001. [DOI] [PubMed] [Google Scholar]
- [43].Lee M, Hong M. Cryoprotection of lipid membranes for high-resolution solid-state NMR studies of membrane peptides and proteins at low temperature. J. Biomol. NMR. 2014;59:263–277. doi: 10.1007/s10858-014-9845-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Cady SD, Goodman C, Tatko C, DeGrado WF, Hong M. Determining the orientation of uniaxially rotating membrane proteins using unoriented samples: a 2H, 13C, and 15N solid-state NMR investigation of the dynamics and orientation of a transmembrane helical bundle. J. Am. Chem. Soc. 2007;129:5719–5729. doi: 10.1021/ja070305e. [DOI] [PubMed] [Google Scholar]
- [45].Sharma M, Yi M, Dong H, Qin H, Peterson E, Busath D, Zhou HX, Cross TA. Insight into the mechanism of the influenza A proton channel from a structure in a lipid bilayer. Science. 2010;330:509–512. doi: 10.1126/science.1191750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Tjandra N, Bax A. Direct measurement of distances and angles in biomolecules by NMR in a dilute liquid crystalline medium. Science. 1997;278:1111–1114. doi: 10.1126/science.278.5340.1111. [DOI] [PubMed] [Google Scholar]
- [47].Prosser RS, Evanics F, Kitevski JL, Al-Abdul-Wahid MS. Current applications of bicelles in NMR studies of membrane-associated amphiphiles and proteins. Biochemistry. 2006;45:8453–8465. doi: 10.1021/bi060615u. [DOI] [PubMed] [Google Scholar]
- [48].De Angelis AA, Howell SC, Nevzorov AA, Opella SJ. Structure determination of a membrane protein with two transmembrane helices in aligned phospholipid bicelles by solid-state NMR spectroscopy. J. Am. Chem. Soc. 2006;128:12256–12267. doi: 10.1021/ja063640w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Xu J, Durr UH, Im SC, Gan Z, Waskell L, Ramamoorthy A. Bicelle-enabled structural studies on a membrane-associated cytochrome B5 by solid-state MAS NMR spectroscopy. Angew Chem Int Ed Engl. 2008;47:7864–7867. doi: 10.1002/anie.200801338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Luzzati V, Spegt PA. Polymorphism of lipids. Nature. 1967;215:701–704. [Google Scholar]
- [51].Scriven LE. Equilibrium bicontinuous structure. Nature. 1976;263:123–125. [Google Scholar]
- [52].Schnell JR, Chou JJ. Structure and mechanism of the M2 proton channel of influenza A virus. Nature. 2008;451:591–595. doi: 10.1038/nature06531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Tamm LK. Hypothesis: spring-loaded boomerang mechanism of influenza hemagglutinin-mediated membrane fusion. Biochim. Biophys. Acta. 2003;1614:14–23. doi: 10.1016/s0005-2736(03)00159-7. [DOI] [PubMed] [Google Scholar]
- [54].Schmidt NW, Lis M, Zhao K, Lai GH, Alexandrova AN, Tew GN, Wong GCL. Molecular Basis for Nanoscopic Membrane Curvature Generation from Quantum Mechanical Models and Synthetic Transporter Sequences. J. Am. Chem. Soc. 2012;134:19207–19216. doi: 10.1021/ja308459j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Schmidt NW, Mishra A, Lai GH, Davis M, Sanders LK, Tran D, Garcia A, Tai KP, McCray PB, Ouellette AJ, Selsted ME, Wong GC. Criterion for amino acid composition of defensins and antimicrobial peptides based on geometry of membrane destabilization. J Am Chem Soc. 2011;133:6720–6727. doi: 10.1021/ja200079a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Tang M, Hong M. Structure and mechanism of beta-hairpin antimicrobial peptides in lipid bilayers from solid-state NMR spectroscopy. Mol. Biosyst. 2009;5:317–322. doi: 10.1039/b820398a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hong M, Su Y. Structure and dynamics of cationic membrane peptides and proteins: Insights from solid-state NMR. Protein Sci. 2011;20:641–655. doi: 10.1002/pro.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hu F, Luo W, Cady SD, Hong M. Conformational plasticity of the influenza A M2 transmembrane peptide in lipid bilayers under varying pH, drug binding and membrane thickness. Biochim. Biophys. Acta. 2011;1808:415–423. doi: 10.1016/j.bbamem.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Luo W, Cady SD, Hong M. Immobilization of the Influenza A M2 Transmembrane Peptide in Virus-Envelope Mimetic Lipid Membranes: A Solid-State NMR Investigation. Biochemistry. 2009;48:6361–6368. doi: 10.1021/bi900716s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Nguyen PA, Soto CS, Polishchuk A, Caputo GA, Tatko CD, Ma C, Ohigashi Y, Pinto LH, DeGrado WF, Howard KP. pH-induced conformational change of the influenza M2 protein C-terminal domain. Biochemistry. 2008;47:9934–9936. doi: 10.1021/bi801315m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Terao T, Miura H, Saika A. I-S Dipolar Switching-Angle Spinning 2D NMR (SLF) J. Chem. Phys. 1986;85:3816–3826. [Google Scholar]
- [62].Fung BM, Afzal J. C-13 NMR of liquid-crystals - spinning near the magic angle with proton-proton dipolar decoupling. J. Am. Chem. Soc. 1986;108:1107–1108. [Google Scholar]
- [63].Kolbert AC, Grandinetti PJ, Baldwin M, Pruisner SB, Pines A. Measurement of internuclear distances by switched angle spinning NMR. J. Phys. Chem. 1994;98:7936–7938. [Google Scholar]
- [64].Mueller KT, Samoson A, Sun BQ, Chingas GC, Zwanziger JW, Terao T, Pines A. Dynamic-angle spinning of quadrupolar nuclei. J. Magn. Reson. 1990;86:470. doi: 10.1016/j.jmr.2011.08.034. [DOI] [PubMed] [Google Scholar]
- [65].Frydman L, Chingas GC, Lee YK, Grandinetti PJ, Eastman MA, Barrall GA, Pines A. Variable-angle correlation spectroscopy in solid-state nuclear magnetic resonance. J. Chem. Phys. 1992;97:4800. [Google Scholar]
- [66].Courtieu J, Bayle JP, Fung BM. Variable angle sample spinning NMR in liquid crystals. Prog. Nuc. Magn. Reson. Spectros. 1994;26:141–169. [Google Scholar]
- [67].Hong M, Pines A, Caldarelli S. Measurement and Assignment of Long-Range C–H Dipolar Couplings in Liquid Crystals by Two-Dimensional NMR Spectroscopy. The Journal of Physical Chemistry. 1996;100:14815–14822. [Google Scholar]
- [68].Caldarelli S, Hong M, Emsley L, Pines A. Measurement of Carbon–Proton Dipolar Couplings in Liquid Crystals by Local Dipolar Field NMR Spectroscopy. The Journal of Physical Chemistry. 1996;100:18696–18701. [Google Scholar]
- [69].Zandomeneghi G, Williamson PT, Hunkeler A, Meier BH. Switched-angle spinning applied to bicelles containing phospholipid-associated peptides. J Biomol NMR. 2003;25:125–132. doi: 10.1023/a:1022244025351. [DOI] [PubMed] [Google Scholar]
- [70].Lu GJ, Opella SJ. Resonance assignments of a membrane protein in phospholipid bilayers by combining multiple strategies of oriented sample solid-state NMR. J. Biomol. NMR. 2014;58:69–81. doi: 10.1007/s10858-013-9806-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Lu GJ, Park SH, Opella SJ. Improved 1H amide resonance line narrowing in oriented sample solid-state NMR of membrane proteins in phospholipid bilayers. J. Magn. Reson. 2012;220:54–61. doi: 10.1016/j.jmr.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.