ABSTRACT
The beneficial effects of edible mushrooms for improving chronic intractable diseases have been documented. However, the antiatherogenic activity of the new medicinal mushroom Grifola gargal is unknown. Therefore, we evaluated whether Grifola gargal can prevent or delay the progression of atherosclerosis. Atherosclerosis was induced in ApoE lipoprotein-deficient mice by subcutaneous infusion of angiotensin II. Grifola gargal extract (GGE) was prepared and intraperitoneally injected. The weight of heart and vessels, dilatation/atheroma formation of thoracic and abdominal aorta, the percentage of peripheral granulocytes, and the blood concentration of MCP-1/CCL2 were significantly reduced in mice treated with GGE compared to untreated mice. By contrast, the percentage of regulatory T cells and the plasma concentration of SDF-1/CXCL12 were significantly increased in mice treated with the mushroom extract compared to untreated mice. In vitro, GGE significantly increased the secretion of SDF-1/CXCL12, VEGF, and TGF-β1 from fibroblasts compared to control. This study demonstrated for the first time that Grifola gargal therapy can enhance regulatory T cells and ameliorate atherosclerosis in mice.
Key Words: : atheroma, chemokines, cytokines, mushrooms, progenitor cells, remodeling, regulatory T cells, vasculitis
Introduction
Despite a decrease in prevalence, atherosclerosis-associated cardiovascular disease is still the leading cause of death in developed countries according to the World Health Organization.1–3 Atherosclerosis is a chronic disease of the vascular walls characterized by endothelial dysfunction, lipid deposition, infiltration of inflammatory cells, proliferation of smooth muscle cells, intravascular wall neoangiogenesis, and plaque formation.4,5 The pathogenesis of atherosclerosis is not completely clear, but the underlying chronic inflammation of the vessel walls is triggered and sustained by multiple factors, including procoagulant proteins, proinflammatory cytokines and chemokines, metalloproteinases, and growth factors.6,7 Arterial hypertension, diabetes mellitus, dyslipidemia, obesity, and smoking are well-recognized risk factors that contribute to the pathogenesis by aggravating the vascular wall inflammation.6,8
Leukocytes and several cells from the vascular structure, including fibroblasts, endothelial cells, and smooth muscle cells, are involved in the pathogenesis of atherosclerosis.9,10
The value of edible mushrooms as nutritional and medical resources has been recognized since ancient times.11 Mushrooms are rich in minerals, essential amino acids, vitamins, and fiber; some species contain biologically active substances with potential beneficial effects in atherosclerosis, cancer, diabetes mellitus, and infectious diseases.11–14 The edible mushroom Grifola gargal has recently been the focus of attention because of its potential use as medicinal mushroom.15 Grifola gargal belongs to the Polyporaceae family of mushrooms and it was described for the first time by Rolf Singer in 196916; it grows on fallen tree trunks forming multipetaloid fruit bodies with a characteristic almond flavor and is commonly found in the forests of southern regions of Argentina and Chile.17 Among the 11 species of edible Grifola mushrooms, Grifola gargal was found to be the mushroom with the highest content of ergothioneine, which is a well-recognized antioxidant and anti-inflammatory agent.18 In addition, three novel sterols (gargalols) and a new sphingosine were recently isolated from the fruit body of Grifola gargal and found to exert a significant osteoclast-forming suppressive activity in vitro.19,20 However, the effects of this mushroom have not yet been evaluated using in vivo models.
Some edible mushrooms have been shown to inhibit the atherosclerotic process, while others have been found to worsen the disease.21–23 The aim of this study was to clarify the effect of Grifola gargal on atherosclerosis using a mouse model of the disease.
Materials and Methods
Reagents
Dulbecco's modified Eagle's medium (DMEM) was from Sigma (St Louis, MO, USA), fetal bovine serum (FBS) from Bio Whittaker (Walkersville, MD, USA), and the antibiotics penicillin and streptomycin were from Nacalai Tesque (Kyoto, Japan). All other reagents were of the best quality commercially available. Grifola gargal Singer was provided by the Iwade Research Institute of Mycology Corporation that has previously characterized it.24 The data base of the mushroom is currently available online in MycoBank (www.mycobank.org/Biolomics.aspx?Table=Mycobank&MycoBankNr_=331520; deposition number MB#331520) as well as in the DNA Data Bank of Japan (www.ddbj.nig.ac.jp; No AB539700).
Cell culture
Mouse lung fibroblasts were isolated from the lungs of C57BL/6 mice by collagenase digestion; the cells were then cultured in DMEM supplement with 10% fetal calf serum supplemented with 20% heat-inactivated FBS, 50 μg/mL penicillin and 50 μg/mL streptomycin. The cells were cultured in 5% CO2 and 95% air.
Preparation of Grifola gargal extract
The powder of Grifola gargal fruit body was dissolved in water and then incubated at 90–95°C for 6 h. Only extract fractions of the mushroom with molecular weight (MW) less than 6000 Da were used because previous studies have shown the beneficial effect of this fraction.18–20 After Celite filtration, components with MW of more than 6000 Da were excluded by using an ultrafiltration membrane, and fractions containing only components with MW of less than 6000 Da were separated and concentrated. Thereafter, the 6000 MW fraction concentrate of Grifola gargal extract (GGE) was sterilized at 121°C and lyophilized before using in the experiments. The composition of the extract per 100 g is described in Table 1. The purity of the GGE (35 mg) was analyzed by Japan Analytical Industry Corporation (Tokyo, Japan) using high-performance liquid chromatography. The powders of the fruit body of other types of mushrooms (Coprinus comatus, Grifola frondosa, Agaricus blazei) were also prepared by dissolving in water and then incubating at 90–95°C for 6 h; the complete fraction was used in the assays. The amino acid analysis of the mushrooms was performed at the Japan Food Research Laboratories (www.jfrl.or.jp/e/index.html; Tokyo, Japan) using an automated amino acid analyzer according to company standard protocol.
Table 1.
Composition of Grifola gargal Extract Per 100 g
| Components | Amount | Unit | Method of analysis |
|---|---|---|---|
| Moisture | 82.1 | g | Ordinary pressure ustulation method |
| Protein | 9.9 | g | Kjeldahl method |
| Lipid | 0.1 | g | Acidolysis method |
| Carbohydrate | 5.1 | g | 100−(water+protein+lipid+ash)* |
| Dietary fiber | 0.3 | g | Enzymatic-gravimetric method |
| Ash | 2.6 | g | Direct ashing method |
| Sodium | 49.6 | mg | Atomic absorption spectrophotometry |
| Phosphorus | 301.0 | mg | Inductively coupled plasma analysis |
| Iron | 1.1 | mg | Inductively coupled plasma analysis |
| Calcium | 5.9 | mg | Inductively coupled plasma analysis |
| Potassium | 1.2 | g | Atomic absorption spectrophotometry |
| Magnesium | 72.5 | mg | Inductively coupled plasma analysis |
| Energy | 61.0 | kcal | 4×carbohydrate+4×protein+9×fat |
Percentages of water, protein, lipid and ash.
Animals
Wild-type C57BL/6 mice were purchased from Nihon SLC (Hamamatsu, Japan). Apolipoprotein(Apo)-E deficient mice (B6; 129P2-ApoE<tm1Bal>/J) on C57BL/6 background were from Jackson Laboratory (Sacramento, CA, USA). Twelve-week-old male mice weighing 22–24 g were used in the experiments. The animals were maintained in a specific pathogen-free environment, fed a normal laboratory diet, and subjected to a 12-h light/ 12-h dark cycle in the animal house of Mie University. The Ethics Committee for Animal Investigation from Mie University approved the experimental protocol (Approval No. 24–50, date: February 28, 2014).
Determination of GGE optimal dose
Wild-type (C57BL/6) mice were treated with several doses of GGE to determine the optimal dose of GGE for in vivo studies. Mice were categorized into four treatment groups: one group of mice was treated with saline alone (saline group), and the other groups with 10 (GGE-10), 50 (GGE-50), or 100 (GGE-100) mg/kg of GGE by intraperitoneal injection (300 μL) twice a week for 4 consecutive weeks. Blood was sampled on the fifth week, and the plasma fraction was separated to measure the concentration of stromal cell-derived factor 1/C-X-C motif chemokine 12 (SDF-1/CXCL12).
Atherosclerosis mouse model
The protocol used for induction of atherosclerosis in mice was previously described.5 Briefly, the mice were infused angiotensin II (Ang II; 1.5 mg/kg/day) or saline through osmotic minipumps (Alzet, model 2004, Palo Alto, CA, USA) implanted subcutaneously in the back of the ApoE-deficient mice. The infusion of Ang II or saline was performed continuously through the osmotic minipumps at a constant rate for 28 days. The mice were randomly assigned in the following three groups: (1) Sal/Sal group received sterile saline by osmotic minipump and saline by intraperitoneal (i.p.) injection, (2) Ang II/Sal group received Ang II by osmotic minipump and saline by i.p. injection every day, and (3) Ang II/GGE group received Ang II by osmotic minipump and a dose of intraperitoneal GGE (10 mg/kg of body weight) two times per week. All groups were monitored for 28 days. Mice were treated with GGE or saline by i.p. injection to assure that a constant amount was administered. The objective of the present study was to clarify only the effect of Grifola gargal on atherosclerosis, thus a group of mice treated with other edible mushrooms was not included in the study design.
Sacrifice of animals
Mice were sacrificed on day 29 after an overdose i.p. injection of pentobarbital and then samples for biochemical and histopathological examination were taken. Blood was drawn by cardiac puncture into sterilized tubes containing heparin, centrifuged, and the collected plasma was stored at −80°C until analysis.
Histopathological examination
Laparotomy was performed and the aorta was visualized and perfused with saline and then with warmed 1.6% solution of a low-melting point agarose (SeaPlaque GTG Agarose; Lonza, Rockland, ME, USA) at a constant pressure of 100 mmHg. The complete abdominal aorta was dissected from the surrounding tissues after solidification of the gel, and then it was excised and fixed in 10% formalin. Digital images of the arterial surface area were acquired using an Olympus SZ61 microscope combined with an Olympus DP25 Digital Camera (Tokyo, Japan) after longitudinal excision and expansion of the aorta walls. The inner surface area of the abdominal aorta was measured using the WinRoof image processing software (Mitani Corp, Fukui, Japan) for Windows, as previously described.5 The samples were randomized and the observers were blinded to the names of the samples. The dilation index was defined as the total inner surface area of the aorta divided by the total artery length.
Quantification of atherosclerotic plaques
The tissue surrounding the aorta was removed, and the aorta was longitudinally opened and immersed in Sudan III solution for 40 min (0.2 g Sudan III, 70 mL absolute ethanol, 30 mL distilled water), washed in 2-propanol (99.5%) for 5 min, and then mounted for photography. Digital images were taken and quantification of atheromatous lesions was carried out with the WinRoof image processing software as previously described.5
Flow cytometry analysis
The antibodies against Gr-1 (rat IgG2bκ, clone: RB6-8C5), F4/80 (rat IgG2aκ, clone: BM8), CD3 (Armenian hamster IgG, clone: 145-2C11), CD4 (rat IgG2bκ, clone: GK1.5), and CD25 (rat IgG1λ, clone: PC61) were purchased from Bio Legend (San Diego, CA, USA) and antibody against CD26/32 (clone: 246G2) from eBioscience (San Diego, CA, USA). To evaluate the number of CD4+CD25+ lymphocytes, peripheral blood cells were incubated with anti-CD16/32 for 30 min before treating with the following specific mAbs: fluorescein (FITC)-conjugated anti-CD25, phycoerythrin (PE)-conjugated anti-CD3, and PE-Cy5-conjugated anti-CD4 antibody. To evaluate the number of granulocytes, peripheral blood cells were stained with FITC-conjugated anti-Gr-1 and PE-conjugated anti-F4/80. Granulocytes were identified as Gr-1(high), F4/80(dull) population.
Biochemical analysis
The concentrations of cytokines in plasma were measured with commercial immunoassay kits specific for mouse cytokines. The immunoassay kit for measuring CCL2/MCP-1 was purchased from BD Pharmingen (San Jose, CA, USA), and the EIA kits for measuring stromal cell-derived factor 1/C-X-C motif chemokine 12 (SDF-1/CXCL12), vascular endothelial growth factor (VEGF), and transforming growth factor (TGF)-β1 were from R&D Systems (Minneapolis, MN, USA). All cytokines were measured according to the manufacturer's instructions.
Stimulation of fibroblasts with mushroom extracts
Fibroblasts from murine lungs were employed in the in vitro experiments because they secrete a high level of chemokines, including SDF-1. After culture of fibroblasts in 12-well plates for 48 h, the cells were washed with a medium containing 1% FBS and cell culture was continued in the same medium for 24 h. After appropriate washing, the cells were treated with 100 μg/mL GGE for 24 h, and the cell supernatants were collected and stored at −80°C until analysis. For comparative analysis between mushrooms, fibroblasts were cultured in a similar manner in 12-well plates for 48 h and then stimulated for 24 h in the presence of 100 μg/mL of each mushroom type. The cells were used for RNA extraction and supernatants for ELISA. Lipopolysaccharide (100 μg/mL) was used as positive control and saline as negative control.
Stimulation of bone marrow-derived dendritic cells with GGE
Bone marrow cells from C57BL/6 mice were cultured for 7 days in the presence of Flt3 ligand. On day 4, 0, 10, and 100 μg/mL of GGE were added to the culture. Dendritic cells were stimulated with 100 μg/mL of LPS for 24 h. CD4 T cells were purified from OT-II mice and stimulated with OVA peptide-loaded bone marrow-derived dendritic cells for 3 days. Regulatory CD+ T cells were detected by flow cytometry as CD4+CD25+Foxp3+ cells.
Reverse transcription–polymerase chain reaction
Total RNA was extracted from cells by the guanidine isothiocyanate procedure using the Trizol Reagent (Invitrogen, Carlsbad, CA, USA). Total RNA was reverse-transcribed using oligo-dT primers and then the DNA was amplified by PCR. Reverse transcription–polymerase chain reaction (RT-PCR) was performed using the Superscript Preamplification system kit (Invitrogen). The sequences of the primers were as follows: mouse glyceraldehyde 3-phosphate dehydrogenase (GAPDH) sense, CCCTTATTGACCTCAACTACATGGT and anti-sense, GAGGGGCCATCCACAGTCTTCTG; mouse SDF-1/CXCL12 sense GCTCTGCATCAGTGACGGTA and anti-sense ATTTCGGGTCAATGCACACT; mouse VEGF sense ATCTTCAAGCCGTCCTGTGTG and anti-sense GCAGGAACATTTACACGTCTG. PCR was performed with 35 cycles, denaturation at 94°C for 1 min, annealing at 56°C for 1 min, and elongation at 72°C for 1 min; at the end of these cycles, a further extension was carried out at 72°C for 5 min. The PCR products were separated on a 2% agarose gel containing 0.01% ethidium bromide, and the intensities of the stained bands were quantitated by densitometry using the public domain NIH image program (Wayne Rasband, NIH, Research Service Branch). The amount of mRNA was normalized against the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA expression.
Statistical analysis
Data are expressed as the mean±standard error of the mean (SEM). The statistical difference was calculated by analysis of variance with post hoc analysis using Fisher's predicted least significant difference test. All statistics were performed using the StatView 5.0 package (Abacus Concepts, Berkeley, CA, USA). P<.05 was considered as statistically different.
Results
Changes in plasma levels of SDF-1 after GGE therapy in normal mice
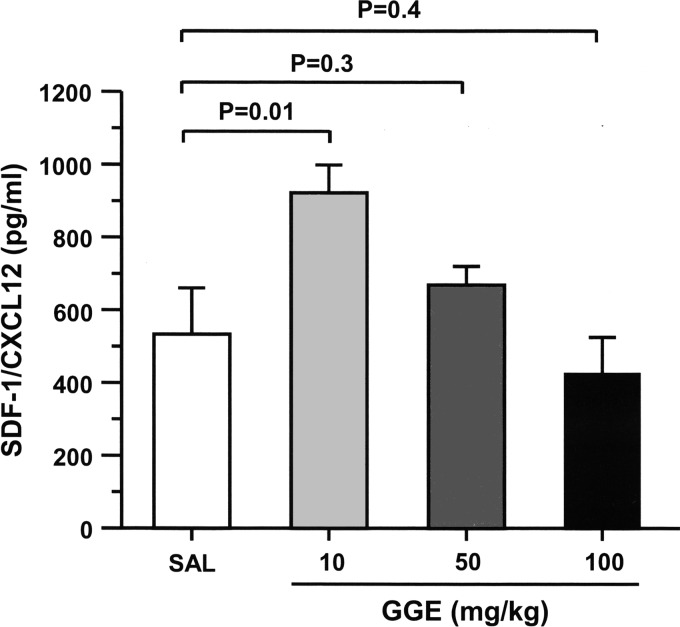
Increasing doses of GGE was administered by intraperitoneal injection twice a week during 4 weeks in normal mice. The plasma level of SDF-1/CXCL12 was significantly increased in mice treated with 10 mg/kg of GGE but not in those treated with much higher doses (Fig. 1).
FIG. 1.
Plasma levels of SDF-1/CXCL12 after Grifola gargal extract (GGE) administration in normal mice. Normal mice received varying doses of GGE by intraperitoneal injection twice a week for 4 weeks. The plasma level of SDF-1/CXCL12 was significantly increased only in mice treated with 10 mg/kg of GGE. Bars indicate the mean±SEM. Statistical analysis was performed by ANOVA with post hoc analysis using Fisher's predicted least significant difference test.
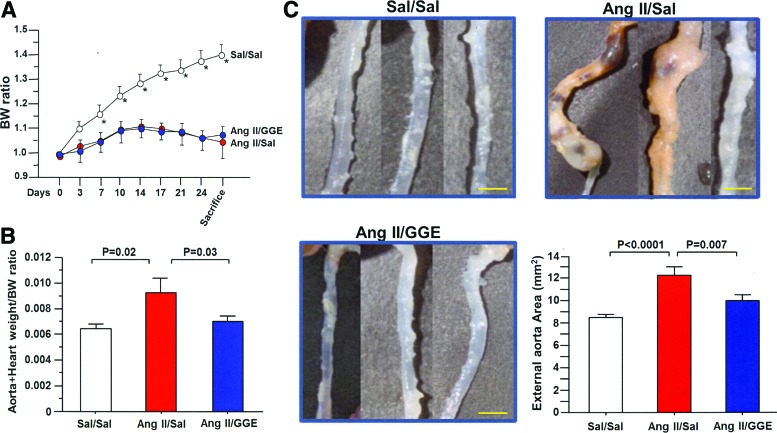
Mouse weight loss in mice with atherosclerosis
The mouse body weight of each group was measured and compared (Fig. 2A). Significant weight loss was observed in Ang II/Sal and Ang II/GGE groups from the seventh day of the experiment compared to the Sa/Sal group. No significant difference in weight loss was observed between the Ang II/Sal and Ang II/GGE groups during the entire experiment. The ratio of the total weight of the heart plus aorta to body weight was also compared between groups and found to be significantly increased in the Ang II/Sal group compared to the Sal/Sal and Ang II/GGE treatment groups (Fig. 2B).
FIG. 2.
Weight loss, amelioration of heart-aorta weight, and aorta dilation. Mice with accelerated atherosclerosis (Ang II/Sal & Ang II/GGE) showed significant weight loss compared to control mice (A). The weight of the heart vessels and aorta was significantly increased in the Ang II/Sal compared to both the control and Ang II/GGE groups (B). The BW ratio was obtained by dividing the body weights on day 0 and the weight on each subsequent day. The external morphology of the aorta of Ang II/Sal mice showed aneurism-like dilation compared to other two groups of mice (C). The aorta showed a significant increased area in the aorta from Ang II/GGE mice compared to Sal/Sal and Ang II/GGE groups. Bars indicate the mean±SEM. Scale bars indicate 2 mm. Statistical analysis was performed by ANOVA with post hoc analysis using Fisher's predicted least significant difference test. Ang II, angiotensin II; Sal, saline. *p<0.05, compared to Ang II/Sal and Ang II/GGE groups. Color images available online at www.liebertpub.com/jmf
Morphological parameters
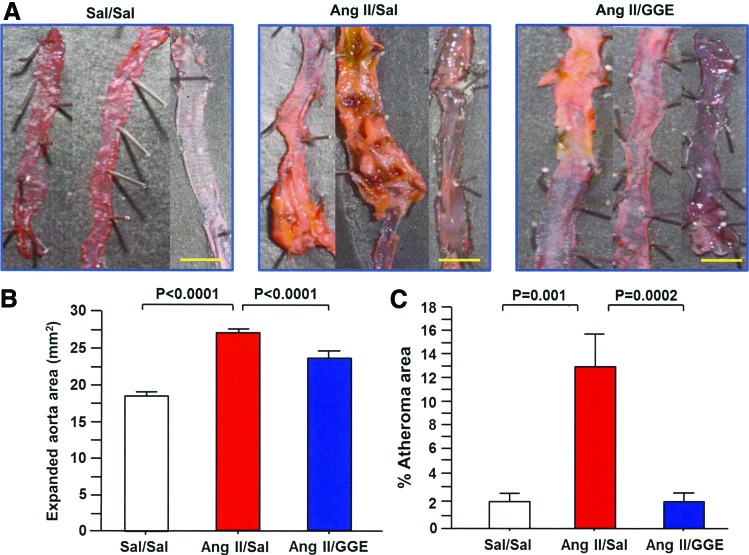
The external surface area of thoracic and abdominal aorta before longitudinal excision was compared and found to be significantly enhanced in the Ang II/Sal group compared to both the Sal/Sal and Ang II/GGE groups (Fig. 2C). Similar results were found when the aortas were excised and expanded; the complete surface areas of the aortas were significantly increased in the Ang II/Sal group compared to both Sal/Sal and Ang II/GGE groups (Fig. 3A, B). To measure the area of atheromatous lesions, the aortas were stained with Sudan III and the staining showed a significantly higher percentage of atheroma areas in the Ang II/Sal group than in the Sal/Sal and Ang II/GGE groups (Fig. 3C).
FIG. 3.
GGE treatment decreases atherosclerotic changes. The total wall area of the aorta after longitudinal excision was significantly expanded in the Ang II/Sal group compared to both the control and Ang II/GGE groups (A, B). The total area with atherosclerotic lesions was measured using the WinRoof image processing software, and it was found to be significantly increased in Ang II/Sal mice compared to control and Ang II/GGE groups (C). Bars indicate the mean±SEM. Scale bars indicate 2 mm. Statistical analysis was performed by ANOVA with post hoc analysis using Fisher's predicted least significant difference test. Color images available online at www.liebertpub.com/jmf
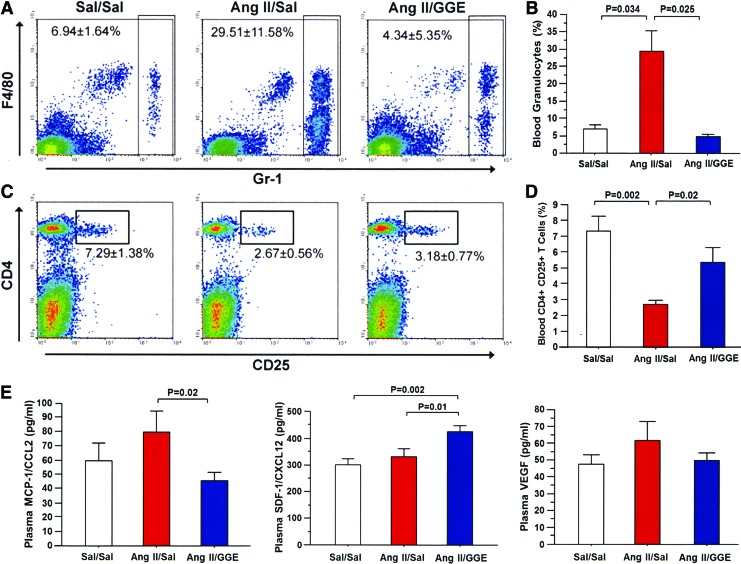
Peripheral blood granulocytes and lymphocytes
The percentage of leukocytes subsets was evaluated by flow cytometry in the peripheral blood of each group of mice. As expected, there was a significant increase in the percentage of granulocytes in the Ang II/Sal mice compared to the control group; however, the percentage of granulocytes was significantly decreased in mice treated with GGE (Ang II/GGE) compared to the Ang II/Sal group (Fig. 4A, B). In addition, the percentage of CD4+CD25+ T cells, which are mainly comprised by regulatory T cells, was significantly lower in the Ang II/Sal group than in the control group, but it was significantly higher in the Ang II/GGE mice than in the Ang II/Sal group (Fig. 4C, D). No difference was found in the total CD4+ and CD8+ T-cell subsets.
FIG. 4.
Changes in the number of granulocytes and CD4+CD25+ T cells and in the concentration of chemokines in mice treated with GGE. The number of granulocytes in the peripheral blood was significantly increased in the Ang II/Sal group compared to the control and Ang II/GGE groups (A, B). The number of CD4+CD25+ T cells in the peripheral blood was significantly decreased in the Ang II/Sal mice compared to the control and Ang II/GGE groups (C, D). The concentration of MCP-1/CCL2 was decreased in mice treated with GGE (Ang II/GGE) compared to untreated mice (Ang II/Sal) (E). The concentration of SDF-1/CXCL12 was increased in GGE-treated mice compared to untreated mice (E). No difference was found in the concentration of vascular endothelial growth factor (VEGF). Bars indicate the mean±SEM. Statistical analysis was performed by ANOVA with post hoc analysis using Fisher's predicted least significant difference test. Color images available online at www.liebertpub.com/jmf
Circulating chemokines
The effect of GGE treatment on circulating levels of inflammatory cytokines was also evaluated. The plasma concentration of MCP-1/CCL2 was significantly decreased in mice treated with GGE (Ang II/GGE) compared with the untreated group (Ang II/Sal) (Fig. 4E). By contrast, the plasma concentration of the chemokine SDF-1/CXCL12 was significantly increased in mice treated with GGE (Ang II/GGE) compared with the untreated group (Ang II/Sal); no differences in VEGF levels were found among the groups (Fig. 4E).
GGE regulates the secretion chemokines and cytokines
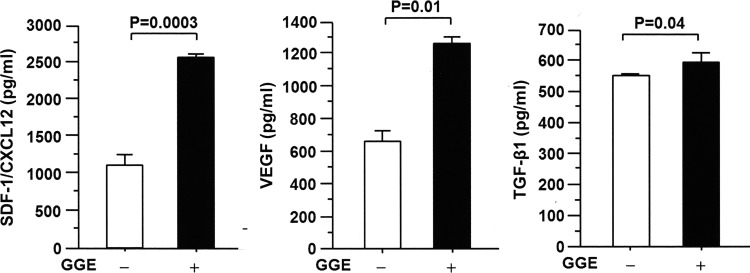
Lung fibroblasts were cultured in vitro and stimulated in the presence or absence of GGE, and the secretion of chemokines and cytokines was evaluated. GGE significantly stimulated the secretion of SDF-1/CXCL12, VEGF, and TGF-β1 in the cell supernatants compared to control cells cultured in the absence of GGE (Fig. 5).
FIG. 5.
GGE increases the secretion of chemokines and transforming growth factor β1 (TGF-β1) in vitro. Mouse fibroblasts were cultured in vitro and stimulated in the presence or absence of GGE (100 μg/mL), and the concentrations of SDF-1/CXCL12, VEGF, and TGF-β1 were measured by EIA. The concentrations of SDF-1/CXCL12, VEGF, and TGF-β1 were increased in the supernatants of cells treated with GGE compared to untreated cells. Bars indicate the mean±SEM. Statistical analysis was performed by ANOVA with post hoc analysis using Fisher's predicted least significant difference test.
GGE is a potent stimulator compared to extracts from other mushrooms
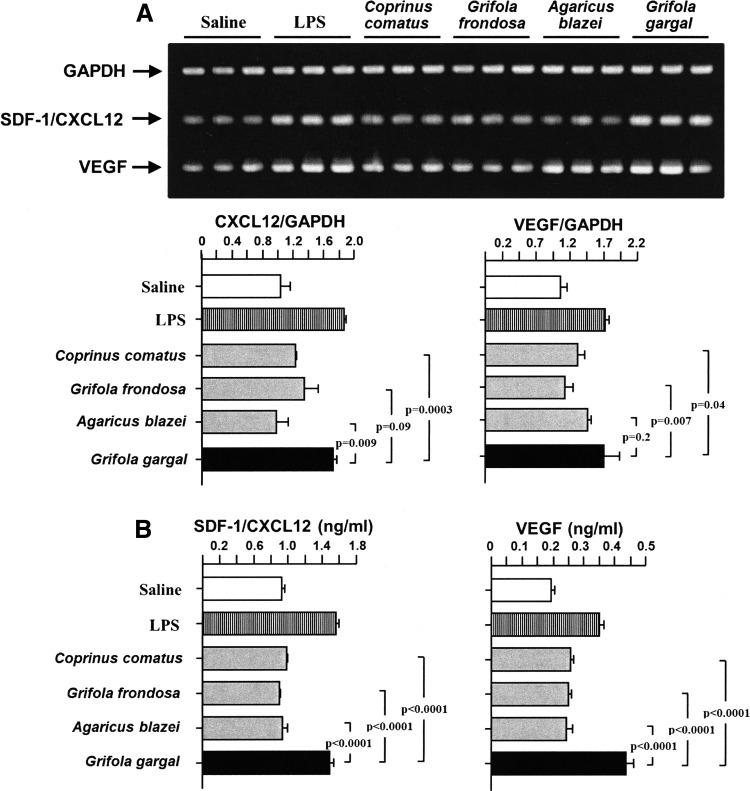
The stimulatory effect of GGE on the expression of SDF-1/CXCL12 and VEGF from fibroblasts was compared with the extracts from other mushrooms. The RNA and protein expression of SDF-1/CXCL12 and VEGF was significantly enhanced by GGE compared to the extracts from other types of mushrooms (Fig. 6).
FIG. 6.
Grifola gargal is a potent chemokine stimulator compared to other mushrooms. Cultured fibroblasts were stimulated in the presence or absence of each mushroom extract for 24 h and the concentrations of SDF-1/CXCL12 and VEGF were measured by RT-PCR (A) and EIA (B). The RNA expressions of SDF-1/CXCL12 and VEGF were significantly increased by GGE compared to the extracts of most mushrooms, while the concentration of the chemokine protein was significantly increased compared to all mushrooms. Bars indicate the mean±SEM. Lipopolysaccharide (LPS) was used as a positive control and saline as a negative control. Statistical analysis was performed by ANOVA with post hoc analysis using Fisher's predicted least significant difference test.
Amino acid composition
As shown in Table 2, Grifola gargal is very rich in arginine, histidine, phenylalanine, leucine, isoleucine, methionine, glycine, proline, and aspartic acid compared to Coprinus comatus, Grifola frondosa, and Agaricus blazei.
Table 2.
Amino Acid Content of Mushrooms
| Mushroom (g/100 g dry powder) | ||||
|---|---|---|---|---|
| Amino acid | Coprinus comatus | Grifola frondosa | Agaricus blazei | Grifola gargal |
| Arginine | 0.92 | 1.39 | 1.33 | 1.41 |
| Lysine | 1.06 | 1.44 | 1.44 | 1.41 |
| Histidine | 0.37 | 0.81 | 0.57 | 0.60 |
| Phenylalanine | 0.77 | 0.85 | 1.03 | 1.08 |
| Tyrosine | 0.56 | 0.65 | 0.76 | 0.71 |
| Leucine | 1.33 | 0.88 | 1.63 | 1.69 |
| Isoleucine | 0.82 | 0.26 | 0.92 | 1.06 |
| Methionine | 0.25 | 0.28 | 0.34 | 0.35 |
| Valine | 0.97 | 1.82 | 1.17 | 1.37 |
| Alanine | 1.23 | 1.97 | 1.50 | 1.49 |
| Glycine | 0.86 | 0.98 | 1.11 | 1.27 |
| Proline | 0.82 | 0.94 | 1.12 | 1.12 |
| Glutamic acid | 2.98 | 3.71 | 5.35 | 4.59 |
| Serine | 0.92 | 2.28 | 1.13 | 1.34 |
| Threonine | 0.93 | 1.89 | 1.17 | 1.38 |
| Aspartic acid | 1.90 | 1.28 | 2.10 | 2.50 |
| Tryptophan | 0.24 | 0.45 | 0.38 | 0.39 |
| Cystine | 0.16 | 0.33 | 0.29 | 0.32 |
GGE promotes dendritic cell-induced differentiation of regulatory T cells
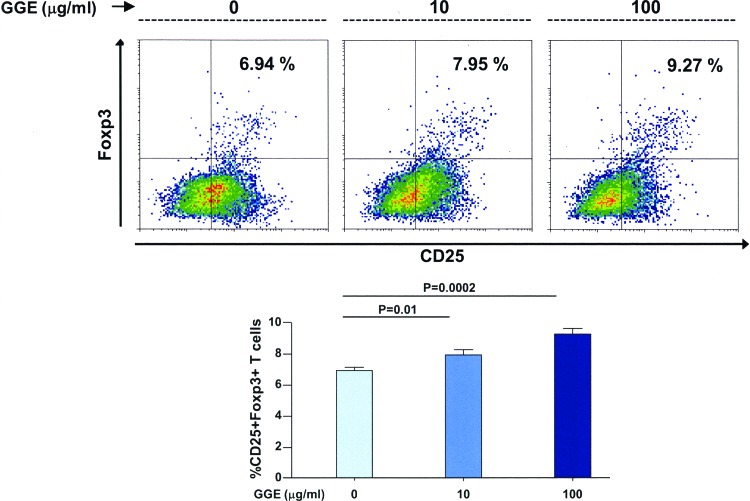
Bone marrow cells were differentiated to dendritic cells in the presence of GGE and then cultured in the presence of ovalbumin-specific naive T cells, and the percentage of regulatory T cells was assessed. The percentage of CD25+ Foxp3+ T cells were significantly increased in the presence of increasing concentrations of GGE compared to cells cultured in the absence of the extract (Fig. 7).
FIG. 7.
GGE promotes dendritic cell-induced differentiation of regulatory T cells in vitro. Bone marrow cells from mice were cultured in the presence of Flt3 ligand and then in the presence of GGE and LPS; ovalbumin-specific CD4 T cells were then added to the culture. There was a significantly higher percentage of CD25+ Foxp3 T cells in the presence of increasing concentrations of GGE than in the absence of the extract. Bars indicate the mean±SEM. Statistical analysis was performed by ANOVA with post hoc analysis using Fisher's predicted least significant difference test. Color images available online at www.liebertpub.com/jmf
Discussion
The main finding of this study was that administration of GGE inhibits the progression of atherosclerosis.
Inflammation in atherosclerosis
Hyperlipidemia triggers vascular wall inflammation.2,4,25 High circulating levels of lipoproteins accelerate intracellular lipid peroxidation causing increased formation of lipoperoxides, which are toxic to plasma membranes.4 Damage of the vascular endothelial lining is associated with enhanced local concentrations of adhesion molecules and proinflammatory chemokines, including MCP-1/CCL2, which promote the recruitment and passage of monocytes and T lymphocytes into the inner structures of vessel walls.25 Macrophages ingest oxidized lipoproteins becoming foam cells.26,27 Leukocytosis is a common clinical observation in atherosclerosis and foam cells are abundant in atheromatous vessels.28–30 Consistent with previous studies, blood granulocytosis was observed in the angiotensin II-induced atherosclerosis model used in the present study; we examined the anti-inflammatory activity of Grifola gargal in this mouse model. The optimal dose of the mushroom extract, as determined in a preliminary experiment, was given by intraperitoneal route to assure a full dose administration and the therapy was continued for the total period (28 days) of angiotensin II infusion. At the end of the experiment, mice treated with the GGE showed a significant inhibition of atheroma formation and less dilation of the aorta in association with a dramatic decrease in the percentage of circulating granulocytes, suggesting that the extract inhibits atherosclerotic inflammation. It is worth noting that because the absorption rate of the low MW GGE fraction is expected to be high in the digestive tract, we believe that the beneficial effect of the mushroom by oral administration would be similar to that observed by the intraperitoneal route; for the same reason, a relatively high concentration of the mushroom extract was used for the in vitro experiments.
Adaptive immunity and atherosclerosis
In addition to cells of the innate immune system, the adaptive immune response has also been implicated in the pathogenesis of atherosclerosis.28 Substantial numbers of T lymphocytes can be found in tissues surrounding atheromatous lesions.28 Recognition of atheroma-related antigens leads to clonal expansion of T cells and classically, there is a predominance of CD4+ cells over CD8+ cells.28,31 CD4+ T cells can be differentiated in several subtypes, including Th1, Th2, Th17, and regulatory T cells (Tregs); of these, Th1 and Th17 have been reported to promote proatherogenic effects.32,33 Tregs are CD4+CD25+ cells whose main function is to block the excessive response of the other T-cell subtypes.33 Tregs can inhibit by direct cell-to-cell contact or by secreting anti-inflammatory cytokines, including TGF-β1, which can also stimulate the differentiation of CD4+ cells into Tregs. Previous studies have documented that Tregs ameliorate atherosclerosis, and that under atherosclerotic conditions there is an imbalance between effector T cells and Tregs responses.34–37 The exact mechanism is unclear, but some evidence suggests that Tregs may protect against atherosclerosis directly by inhibiting the activity of effector cells or indirectly by secreting TGF-β1 a/or IL-10.36–39 In the present study, the percentage of Tregs was significantly reduced in mice with accelerated atherosclerosis compared to control mice, but it was significantly restored in mice treated with Grifola gargal. In addition, an in vitro experiment demonstrated that the mushroom extract can stimulate the secretion of TGF-β1 from fibroblasts and promote dendritic cell-mediated differentiation of regulatory T cells. Overall, these observations suggest that Grifola gargal may ameliorate atherosclerotic inflammation by increasing the number of Tregs, at least partially, through TGF-β1 secretion.
Repair of injured vessels
The repair process in response to vascular injury is also important to prevent the progression of the atherosclerotic disease. Bone marrow-derived vascular endothelial cells and fibroblasts exert antiatherogenic activity by contributing to vascular repair.5,40,41 Consistent with this, chemokines that promote the vascular homing of progenitor cells from bone marrow, including SDF-1/CXCL12 and VEGF, were reported to ameliorate atherosclerosis.42,43 A variety of cells from the vascular walls, including adventitial fibroblasts, may be the source of chemokines.9,44,45 In an attempt to find another mechanism for the beneficial effect of Grifola gargal in atherosclerosis, the effect of its extract on the expression of SDF-1/CXCL12 and VEGF from fibroblasts was evaluated; both chemokines were significantly enhanced by the mushroom extract. In addition, the plasma concentration of SDF-1/CXCL12 was also significantly increased in mice treated with the mushroom extract compared to untreated experimental animals. Overall, these observations suggest that Grifola gargal may also protect against atherosclerosis by inducing the secretions of chemokines that recruit vascular progenitor cells.
In brief, the results of this study demonstrated for the first time that administration of Grifola gargal ameliorates atherosclerosis by at least two mechanisms. First, inhibiting inflammation by expanding the population of regulatory T cells and second, increasing vascular repair by stimulating the secretion of SDF-1/CXCL12. In vivo experiments to compare the antiatherosclerotic activity of Grifola gargal with other edible mushrooms should be performed in future studies.
Acknowledgments
This study was supported, in part, by a grant-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (ECG: Grant Kakenhi No 23591471) and by a grant from the Iwade Research Institute of Mycology. The funders had no role in the study design, data analysis, decision to publish, or preparation of the manuscript.
Author Disclosure Statement
T. Sumiya is the chief executive officer and E. Harada and T. Morizono are employees of Iwade Research Institute of Mycology. T. Sumiya, E. Harada, and T. Morizono have a pending patent (Patent No 2013-123425) on the Grifola gargal extract. The other authors have no conflicts of interest.
References
- 1.Vaccarino V, Badimon L, Corti R, et al. : Ischaemic heart disease in women: are there sex differences in pathophysiology and risk factors? Position paper from the working group on coronary pathophysiology and microcirculation of the European Society of Cardiology. Cardiovasc Res 2011;90:9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Brien EC, Rose KM, Shahar E, Rosamond WD: Stroke Mortality, Clinical Presentation and Day of Arrival: The Atherosclerosis Risk in Communities (ARIC) Study. Stroke Res Treat 2011;2011:383012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Go AS, Mozaffarian D, Roger VL, et al. : Heart Disease and Stroke Statistics—2014 Update: A Report From the American Heart Association. Circulation 2013;127:143–152 [DOI] [PubMed] [Google Scholar]
- 4.Kalanuria AA, Nyquist P, Ling G: The prevention and regression of atherosclerotic plaques: Emerging treatments. Vasc Health Risk Manag 2012;8:549–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gil-Bernabe P, Boveda-Ruiz D, D'Alessandro-Gabazza C, et al. : Atherosclerosis amelioration by moderate alcohol consumption is associated with increased circulating levels of stromal cell-derived factor-1. Circ J 2011;75:2269–2279 [DOI] [PubMed] [Google Scholar]
- 6.Vanhoutte PM: Endothelial dysfunction: The first step toward coronary arteriosclerosis. Circ J 2009;73:595–601 [DOI] [PubMed] [Google Scholar]
- 7.Egashira K: Clinical importance of endothelial function in arteriosclerosis and ischemic heart disease. Circ J 2002;66:529–533 [DOI] [PubMed] [Google Scholar]
- 8.Teramoto T, Sasaki J, Ueshima H, et al. : Risk factors of atherosclerotic diseases. Executive summary of Japan Atherosclerosis Society (JAS) guideline for diagnosis and prevention of atherosclerosis cardiovascular diseases for Japanese. J Atheroscler Thromb 2007;14:267–277 [DOI] [PubMed] [Google Scholar]
- 9.Frostegard J: Immunity, atherosclerosis and cardiovascular disease. BMC Med 2013;11:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keeley EC, Mehrad B, Strieter RM: The role of fibrocytes in fibrotic diseases of the lungs and heart. Fibrogenesis Tissue Repair 2011;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wasser SP: Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl Microbiol Biotechnol 2002;60:258–274 [DOI] [PubMed] [Google Scholar]
- 12.Zaidman BZ, Yassin M, Mahajna J, Wasser SP: Medicinal mushroom modulators of molecular targets as cancer therapeutics. Appl Microbiol Biotechnol 2005;67:453–468 [DOI] [PubMed] [Google Scholar]
- 13.Wasser SP: Current findings, future trends, and unsolved problems in studies of medicinal mushrooms. Appl Microbiol Biotechnol 2011;89:1323–1332 [DOI] [PubMed] [Google Scholar]
- 14.Han SS, Cho CK, Lee YW, Yoo HS: Antimetastatic and immunomodulating effect of water extracts from various mushrooms. J Acupunct Meridian Stud 2009;2:218–227 [DOI] [PubMed] [Google Scholar]
- 15.Postemsky PD, Palermo AM, Curvetto NR: Protective effects of new medicinal mushroom, Grifola gargal singer (higher Basidiomycetes), on induced DNA damage in somatic cells of Drosophila melanogaster. Int J Med Mushrooms 2011;13:583–594 [DOI] [PubMed] [Google Scholar]
- 16.Singer R: Mycoflora Australis. Nova Hedwigia Beiheg 1969;29:1–405 [Google Scholar]
- 17.Bruijn J de LC, Aqueveque P, Canumir J, Cortez M, France A: Antioxidant properties of extracts obtained from Grifola gragal mushrooms. Micologia Aplicada Int 2009;21:11–18 [Google Scholar]
- 18.Ito T KM, Tsuchida H, Harada E, Niwa T, Osawa T: Ergothioneine as an anti-oxidant/anti-inflammatory component in several edible mushrooms. Food Sci Tech Res 2011;17:103–110 [Google Scholar]
- 19.Choi JH YM, Suzuki T, Harada E, Kawade M, Yazawa K, Nishimoto S, Hirai H, Kawagishi H: A novel sphingosine with osteoclast-forming suppressing activity, from the edible mushroom Grifola Gargal. Tetrahedron 2013;69:8609–8611 [Google Scholar]
- 20.Wu J CJ, Yoshida M, Hirai H, Harada E, Masuda K, Koyama T, Yazawa K, Noguchi K, Nagasawa K, Kawagishi H: Osteoclast-forming suppressing compounds,, gargalos A, B, and C, from the edible mushroom Grifola gargal. Tetrahedron 2011;67:6576–6581 [Google Scholar]
- 21.Goncalves JL, Roma EH, Gomes-Santos AC, et al. : Pro-inflammatory effects of the mushroom Agaricus blazei and its consequences on atherosclerosis development. Eur J Nutr 2012;51:927–937 [DOI] [PubMed] [Google Scholar]
- 22.Mori K, Kobayashi C, Tomita T, Inatomi S, Ikeda M: Antiatherosclerotic effect of the edible mushrooms Pleurotus eryngii (Eringi), Grifola frondosa (Maitake), and Hypsizygus marmoreus (Bunashimeji) in apolipoprotein E-deficient mice. Nutr Res 2008;28:335–342 [DOI] [PubMed] [Google Scholar]
- 23.Zhang C, Han C, Zhao B, Yu H: The protective effects of aqueous extracts of wild-growing and fermented Royal Sun mushroom, Agaricus brasiliensis S. Wasser et al. (higher basidiomycetes), in CCl4-induced oxidative damage in rats. Int J Med mushrooms 2012;14:557–561 [DOI] [PubMed] [Google Scholar]
- 24.Harada E, M. K, Matsuda Y, Meguro S: Morphological and molecular characterization of Grifola gargal, an edible mushroom from Chile. Nippon Kingakukai Kaiho (in Japanese) 2007;51:71–76 [Google Scholar]
- 25.Iso H: Lifestyle and cardiovascular disease in Japan. J Atheroscler Thromb 2011;18:83–88 [DOI] [PubMed] [Google Scholar]
- 26.Mallika V, Goswami B, Rajappa M: Atherosclerosis pathophysiology and the role of novel risk factors: A clinicobiochemical perspective. Angiology 2007;58:513–522 [DOI] [PubMed] [Google Scholar]
- 27.Lundberg AM, Hansson GK: Innate immune signals in atherosclerosis. Clin Immunol 2010;134:5–24 [DOI] [PubMed] [Google Scholar]
- 28.Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK: Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis 1986;6:131–138 [DOI] [PubMed] [Google Scholar]
- 29.Elkind MS, Cheng J, Rundek T, Boden-Albala B, Sacco RL: Leukocyte count predicts outcome after ischemic stroke: The Northern Manhattan Stroke Study. J Stroke Cerebrovasc Dis 2004;13:220–227 [DOI] [PubMed] [Google Scholar]
- 30.Elkind MS, Cheng J, Boden-Albala B, Paik MC, Sacco RL, Northern Manhattan Stroke S: Elevated white blood cell count and carotid plaque thickness: The Northern Manhattan Stroke Study. Stroke 2001;32:842–849 [DOI] [PubMed] [Google Scholar]
- 31.Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK: T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci USA 1995;92:3893–3897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Z, Lu F, Pan H, et al. : Correlation of peripheral Th17 cells and Th17-associated cytokines to the severity of carotid artery plaque and its clinical implication. Atherosclerosis 2012;221:232–241 [DOI] [PubMed] [Google Scholar]
- 33.Hansson GK, Hermansson A: The immune system in atherosclerosis. Nat Immunol 2011;12:204–212 [DOI] [PubMed] [Google Scholar]
- 34.Liu ZD, Wang L, Lu FH, et al. : Increased Th17 cell frequency concomitant with decreased Foxp3+Treg cell frequency in the peripheral circulation of patients with carotid artery plaques. Inflamm Res 2012;61:1155–1165 [DOI] [PubMed] [Google Scholar]
- 35.Mor A, Planer D, Luboshits G, et al. : Role of naturally occurring CD4+CD25+regulatory T cells in experimental atherosclerosis. Arterioscler Thromb Vasc Biol 2007;27:893–900 [DOI] [PubMed] [Google Scholar]
- 36.Sasaki N, Yamashita T, Takeda M, Hirata K: Regulatory T cells in atherogenesis. J Atheroscler Thromb 2012;19:503–515 [DOI] [PubMed] [Google Scholar]
- 37.Shimada K: Immune system and atherosclerotic disease: Heterogeneity of leukocyte subsets participating in the pathogenesis of atherosclerosis. Circ J 2009;73:994–1001 [DOI] [PubMed] [Google Scholar]
- 38.He S, Li M, Ma X, Lin J, Li D: CD4+CD25+Foxp3+regulatory T cells protect the proinflammatory activation of human umbilical vein endothelial cells. Arterioscler Thromb Vasc Biol 2010;30:2621–2630 [DOI] [PubMed] [Google Scholar]
- 39.Lin J, Li M, Wang Z, He S, Ma X, Li D: The role of CD4+CD25+regulatory T cells in macrophage-derived foam-cell formation. J Lipid Res 2010;51:1208–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wassmann S, Werner N, Czech T, Nickenig G: Improvement of endothelial function by systemic transfusion of vascular progenitor cells. Circ Res 2006;99:e74–e83 [DOI] [PubMed] [Google Scholar]
- 41.Sata M: Molecular strategies to treat vascular diseases: Circulating vascular progenitor cell as a potential target for prophylactic treatment of atherosclerosis. Circ J 2003;67:983–991 [DOI] [PubMed] [Google Scholar]
- 42.Matsui K, Yoshioka T, Murakami Y, Takahashi M, Shimada K, Ikeda U: Serum concentrations of vascular endothelial growth factor and monocyte-colony stimulating factor in peripheral arterial disease. Circ J 2003;67:660–662 [DOI] [PubMed] [Google Scholar]
- 43.Hiasa K, Ishibashi M, Ohtani K, et al. : Gene transfer of stromal cell-derived factor-1alpha enhances ischemic vasculogenesis and angiogenesis via vascular endothelial growth factor/endothelial nitric oxide synthase-related pathway: Next-generation chemokine therapy for therapeutic neovascularization. Circulation 2004;109:2454–2461 [DOI] [PubMed] [Google Scholar]
- 44.Kim KW, Cho ML, Kim HR, et al. : Up-regulation of stromal cell-derived factor 1 (CXCL12) production in rheumatoid synovial fibroblasts through interactions with T lymphocytes: Role of interleukin-17 and CD40L-CD40 interaction. Arthritis Rheum 2007;56:1076–1086 [DOI] [PubMed] [Google Scholar]
- 45.Schnittker D, Kwofie K, Ashkar A, Trigatti B, Richards CD: Oncostatin M and TLR-4 ligand synergize to induce MCP-1, IL-6, and VEGF in human aortic adventitial fibroblasts and smooth muscle cells. Mediat Inflamm 2013;2013:317503. [DOI] [PMC free article] [PubMed] [Google Scholar]