Abstract
PURPOSE
Bevacizumab is associated with decreased vascular permeability that allows for more aggressive radiotherapy schedules. We conducted a phase II trial in newly-diagnosed glioblastoma utilizing a novel hypofractionated stereotactic radiotherapy (HFSRT) schedule combined with temozolomide and bevacizumab.
EXPERIMENTAL DESIGN
Patients with tumor volume ≤60cc were treated with HFSRT (6×6Gy to contrast-enhancement and 6×4Gy to FLAIR hyperintensity with dose painting) combined with concomitant/ adjuvant temozolomide and bevacizumab at standard doses. Primary endpoint was 1-year overall-survival (OS): Promising=70%; non-promising=50%; α=0.1; β=0.1.
RESULTS
Forty patients were enrolled (median age: 55y; methylated MGMT promoter: 23%; unmethylated: 70%). The 1-year OS was 93% (95%CI 84–100) and median OS was 19m. The median progression-free survival was 10m, with no pseudo-progression observed. The objective response rate (ORR) was 57%. Analysis of TCGA glioblastoma transcriptional subclasses (Nanostring assay) suggested patients with a proneural phenotype (26%) fared worse (ORR=14%, versus 77% for other subclasses; p=0.009). Dynamic susceptibility-contrast perfusion MRI showed marked decreases in rCBV over time (p<0.0001) but had no prognostic value, whereas higher baseline ADC ratios and persistent hypermetabolism at the 6-month FDG-PET predicted poor OS (p=0.05 and 0.0001, respectively). Quality-of-life (FACT-BR-4) and neuropsychological test scores were stable over time, although some domains displayed transient decreases following HFSRT.
CONCLUSIONS
This aggressive radiotherapy schedule was safe and more convenient for patients, achieving an OS that is comparable to historical controls. Analysis of advanced neuro-imaging parameters suggests ADC and FDG-PET as potentially useful biomarkers, whereas tissue correlatives uncovered the poor prognosis associated with the proneural signature in non-IDH-1 mutated glioblastoma.
Keywords: glioblastoma, hypofractionated radiotherapy, bevacizumab, temozolomide
Introduction
Glioblastoma, the most common and aggressive malignant glioma, is characterized not only by a short survival (1–3), but also by a particularly poor quality of life (QoL), as brain function is progressively compromised by both disease burden and effects of treatments (4,5); new therapeutic options are clearly needed.
The standard of care for newly-diagnosed glioblastoma was established in a phase III study testing focal radiotherapy (60 Gy in 2 Gy fractions over 6 weeks) with concomitant and adjuvant temozolomide versus radiotherapy alone (1). In that study, the chemoradiotherapy arm achieved a median overall survival (OS) of 15 months, compared to 12 months for radiotherapy alone. A post-hoc tissue analysis (6) suggested that tumors with promoter methylation of the DNA repair enzyme O6-methylguanine-DNA methyltransferase (MGMT) had a better prognosis; in the chemotherapy arm, the median OS was 22 months for methylated versus 13 months for unmethylated tumors.
In the present study, we sought to optimize this standard first line regimen through two innovative approaches: 1) utilization of an alternative radiotherapy schedule based on aggressive hypofractionation, made possible with the adoption of stereotactic technology and 2) addition of bevacizumab to prevent symptomatic radionecrosis and associated brain edema through decreased vascular permeability resulting from VEGF blockade (7). Exploratory correlative studies consisted of 1) advanced neuroimaging, including dynamic susceptibility contrast (DSC) MR perfusion, diffusion MRI and FDG-PET; 2) tissue correlates, including analysis of TCGA transcriptional subclasses (8,9) and expression of angiogenesis and hypoxia-related genes; and 3) evaluation of QoL and neuropsychological testing throughout treatment.
Patients and Methods
Patients
Main inclusion criteria (Supplementary Data 1, online) included histologically confirmed newly-diagnosed glioblastoma, tumor volume≤ 60 cc (approximately 5cm maximum diameter) and age≥18. Exclusion criteria included multicentric disease and contra-indication to the use of bevacizumab. Therapeutic anticoagulation (e.g. for venous thromboembolism) was allowed.
Treatment
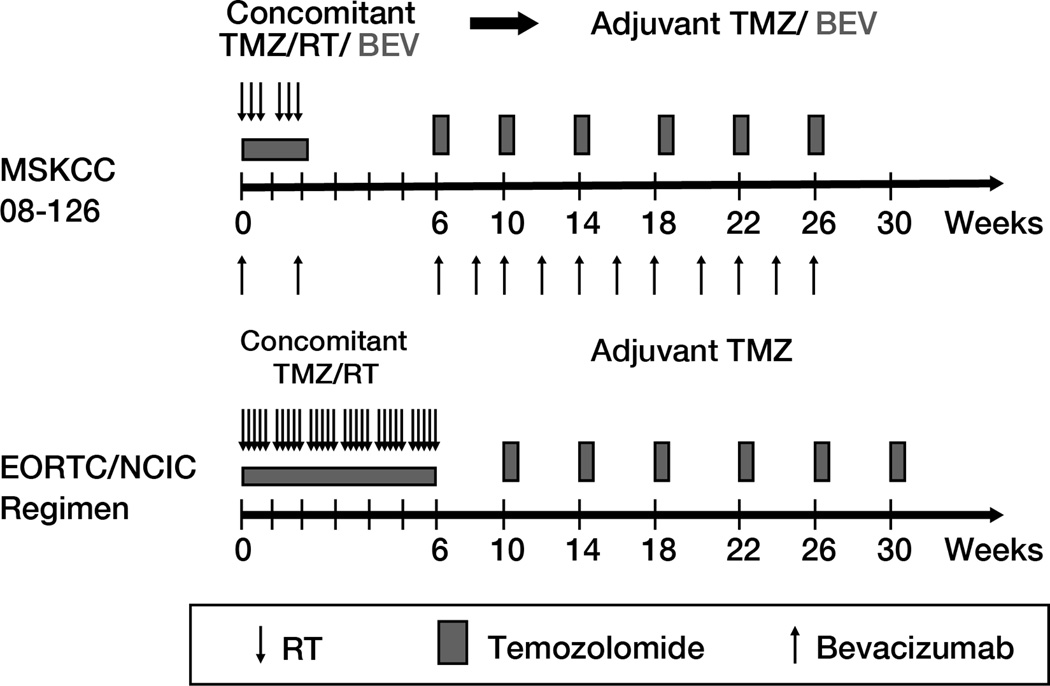
The study treatment schema is shown in Figure 1. Starting 4–6 weeks from surgery, the hypofractionated stereotactic radiotherapy (HFSRT) was delivered in 6 treatments over two weeks, typically on a Monday, Wednesday, Friday schedule, as detailed in Supplementary Data 2 (online). Areas with contrast enhancement received 6×6 Gy and areas with FLAIR hyperintensity received 6×4 Gy, with dose painting for homogeneous dose distribution.
Figure 1.
Study treatment schema and comparison with the EORTC/ NCIC standard glioblastoma regimen (1).
Chemotherapy concomitant with HFSRT consisted of bevacizumab 10mg/kg IV on days 1 and 15, and temozolomide 75mg/m2 given orally daily throughout HFSRT, for a total of 2 weeks. Supportive anti-emetic therapy (e.g. ondansentron) and pneumocystis prophylaxis were recommended. Corticosteroids were given at the discretion of the treating physician.
Adjuvant chemotherapy started approximately 3 weeks after HFSRT, consisting of 28-day cycles of temozolomide given at conventional doses (150–200 mg/m2) on days 1–5, combined with bevacizumab 10 mg/kg on days 1 and 15. A total of 6 adjuvant cycles were mandated per protocol; treatment continuation beyond 6 cycles was left to the treating physician’s discretion. Weekly CBCs, and monthly comprehensive metabolic panel and urine/ plasma creatinine (UPC) ratio were obtained.
Advanced Neuro-Imaging Evaluation
In addition to standard MRI, all patients underwent DSC perfusion MRI at baseline, as well as post-radiotherapy (pre-adjuvant chemotherapy), and every 2 months thereafter. Supplementary Data 3 (online) details imaging processing and acquisition parameters. Commercially available software (FuncTools 2.6.9, GE Healthcare) was used to determine perfusion parameters such as relative cerebral blood volume (rCBV), relative peak height (rPH) and peak signal recovery (PSR), as well as apparent diffusion coefficient (ADC). In addition, a dedicated brain FDG-PET scan was obtained after 6 adjuvant cycles for characterization of disease activity and correlation with outcome.
Tissue Correlates
As detailed in Supplementary Data 4, TCGA glioblastoma transcriptional subclasses (8) (proneural, mesenchymal, classical and neural) were assessed from mRNA extracted from formalin-fixed paraffin-embedded tissue obtained at initial surgery, using a gene expression profiling assay (Nanostring nCounter, Seattle, WA) based on 81 genes selected from the initial TCGA publication (8). Genes for which expression levels are known to directly reflect copy number alterations (Supplementary Data 4) were also added to the probe set, along with VEGF target genes (ANGPTL4, CA-9, EGLN-3, GLUT1 and PDK1).
In a separate analysis, tumor MGMT promoter methylation status was determined utilizing real-time methylation-specific PCR, as described previously (6). IDH1 R132H mutation status was determined by immunohistochemistry (Dianova, Hamburg, Germany; 1:30).
Neuropsychological evaluation
Prospective neuropsychological evaluations (10–12) were performed in consenting, progression-free, patients at baseline and four, eight and twelve months. Raw test scores were compared with published normative values according to age and education, and converted into z-scores; a z-score ≤ −1.5 represents impairment. Evaluated domains included Executive Function (Trail Making; Brief Test of Attention; Controlled Oral Word Association), Verbal Memory (Hopkins Verbal Learning Test-Revised) and Visual Memory (Brief Visuospatial Memory Test-Revised). QoL was evaluated with the Functional Assessment of Cancer Therapy-Brain Cancer v4 (FACT-BR-4). Fatigue was evaluated with the Functional Assessment of Chronic Illness Therapy-Fatigue Subscale 4 (FACIT-FS) and depression with the Beck Depression Inventory II (BDI).
Statistics
The primary endpoint was survival probability at one year (1y-OS). A single stage binomial design was utilized, with 1y-OS of 70% considered promising and 50% non-promising; α=0.1; β=0.1. Forty patients were to be enrolled; if ≥24 were alive at one year, the regimen would be considered worthy of further study. Secondary endpoints consisted of progression-free survival (PFS), response rate (Macdonald (13) and RANO criteria (14)) and toxicity profile (NCI-CTCAE v3). Exploratory endpoints included feasibility and preliminary evaluation of the prognostic value of advanced neuroimaging parameters and tissue-based biomarkers, as well as QoL and neuropsychological evaluation. Neuropsychological test scores were summarized using descriptive statistics, and longitudinal trajectories evaluated using linear mixed models (LMMs) controlling for age and fatigue. Exact follow-up assessment times from baseline were calculated, and both linear and quadratic terms were estimated by the LMMs.
This investigation was performed after approval by the Institutional Review Board and in accordance with an assurance filed with and approved by the U.S. Department of Health and Human Services. Informed consent was obtained from each subject or subject's guardian.
Results
Patient Characteristics
Table 1 shows the characteristics of the 40 patients enrolled.
Table 1.
Patient characteristics (N=40).
| Age | Median: 55 (Range: 17–75) |
| KPS | Median: 90 (Range: 70–100) |
| Women | 14 (35%) |
| Men | 26 (65%) |
| Complete surgical resection | 10 (25%) |
| Partial resection or biopsy | 30 (75%) |
| MGMT promoter methylation | |
| Methylated | 9 (23%) |
| Unmethylated | 28 (70%) |
| Unknown | 3 (8%) |
Treatment and toxicity
Treatment was generally well tolerated and followed the established toxicity profile of each drug (Supplementary Data 5, online). One patient discontinued bevacizumab because of thrombotic microangiopathy with irreversible grade 4 renal failure. One patient developed grade 4 surgical wound infection without dehiscence but was able to resume treatment. Two patients had grade 4 pulmonary embolism and one experienced a late ischemic stroke. One patient with a history of difficult to control seizures died suddenly during sleep, while on treatment; autopsy found no tumor hemorrhage or thromboembolic event. CNS bleeding was reported in two patients, both asymptomatic: One grade 1 intra-tumoral hemorrhage and one grade 1 hemorrhage in a pre-existing cavernoma, in a patient receiving concomitant full-dose anticoagulation.
A total of 11 patients elected to continue treatment beyond the required 6 adjuvant cycles. Those patients received a median of 12 cycles in total (range: 7–23 cycles).
Efficacy
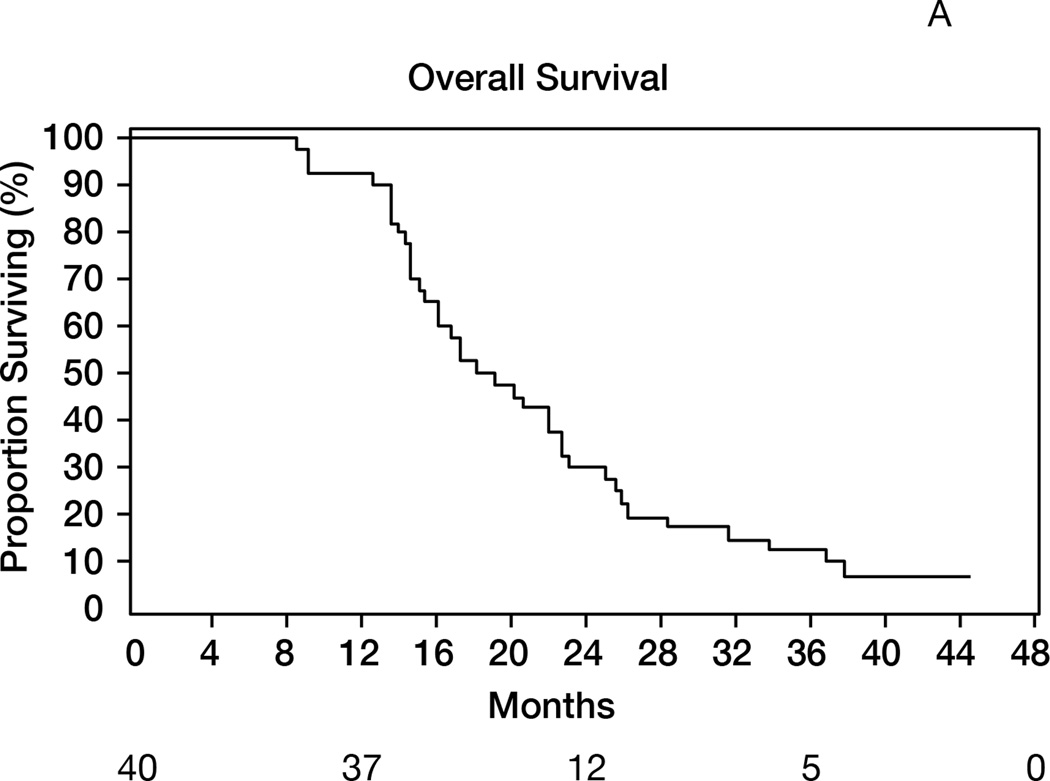
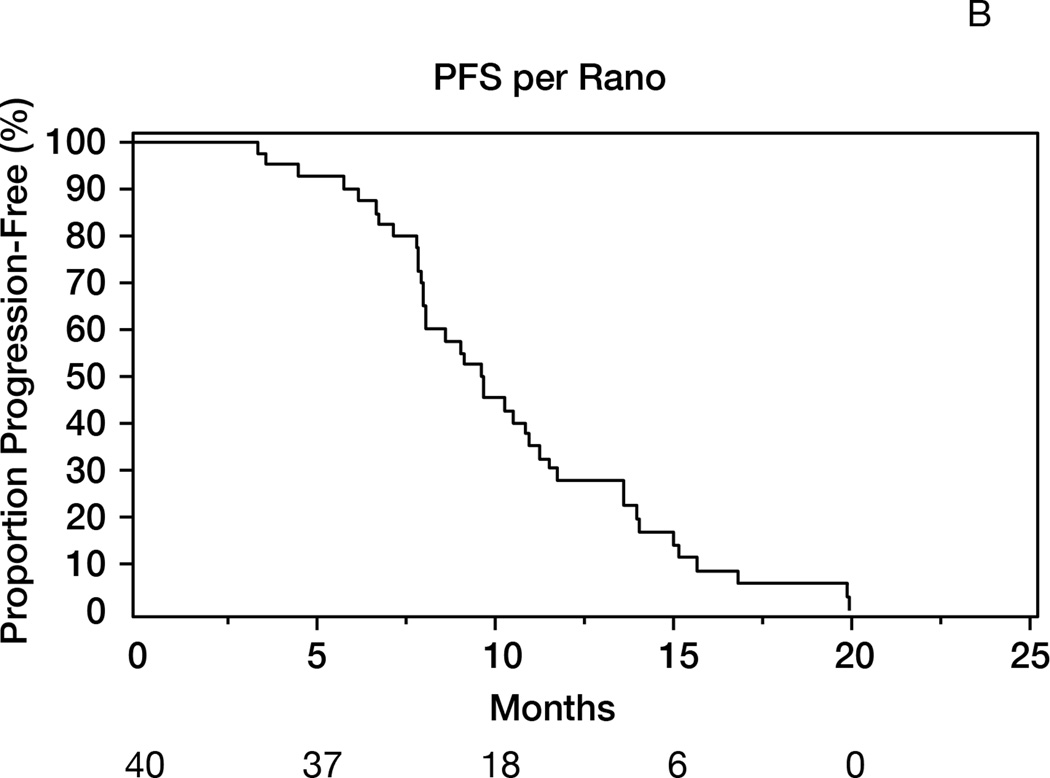
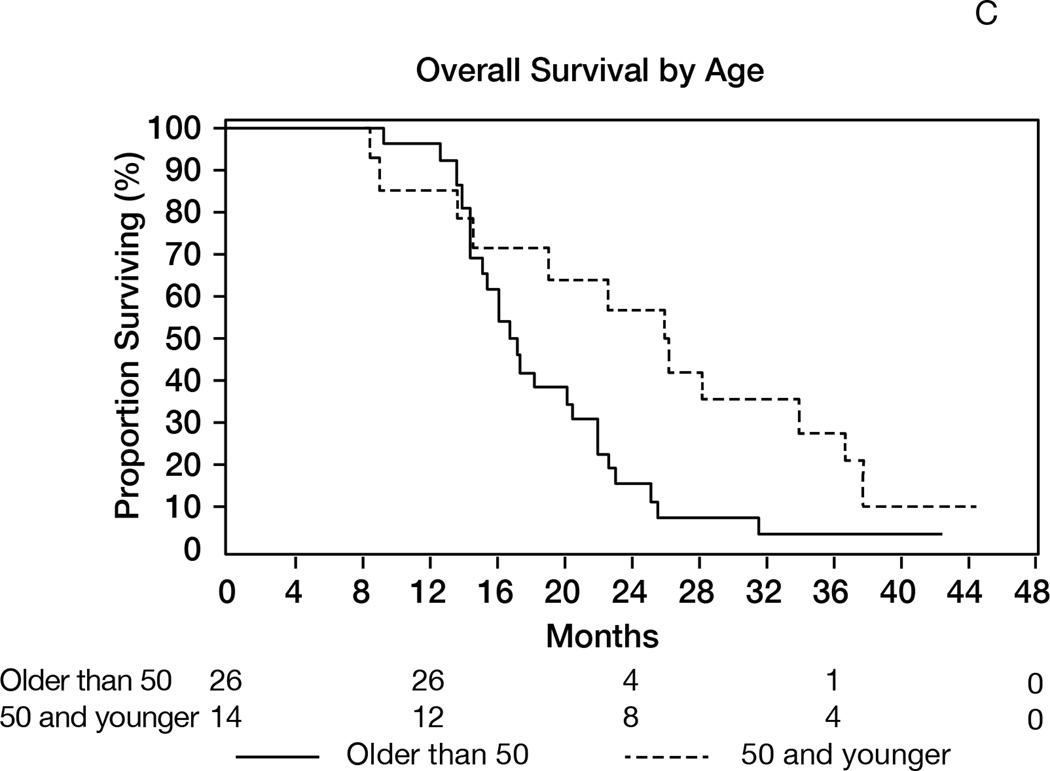
Thirty-seven patients were alive after one year (1-yr OS: 93% [95%CI 84–100]), meeting the study’s primary endpoint (Figure 2A). The median OS was 19 months (95%CI 15–23). The median follow-up of survivors was 42 months. The objective response rates (complete + partial responses) were 87% (Macdonald) and 57% (RANO) (Table 2). The median PFS was 10 months (95%CI 8–11) and 1-year PFS=28% (95%CI 14–41); the results were identical using RANO criteria, with median PFS of 10 months (95%CI: 8–11) and 1-year PFS of 28% (95%CI 14–41)(Figure 2B). Younger patients (age≤50) survived longer (median 26 months) than patients aged>50 (median 17 months, p=0.02, Figure 2C). Patients undergoing gross total resection achieved median OS of 26 months, as compared to 16 months in patients undergoing partial resection or biopsy (p=0.01).
Figure 2.
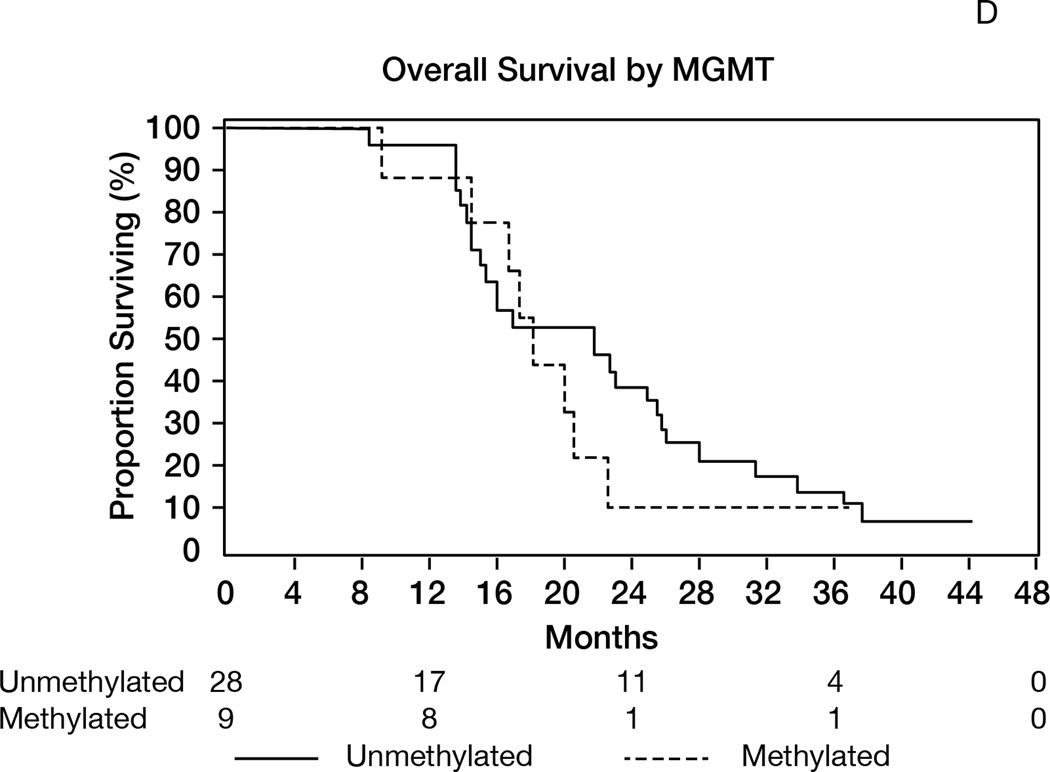
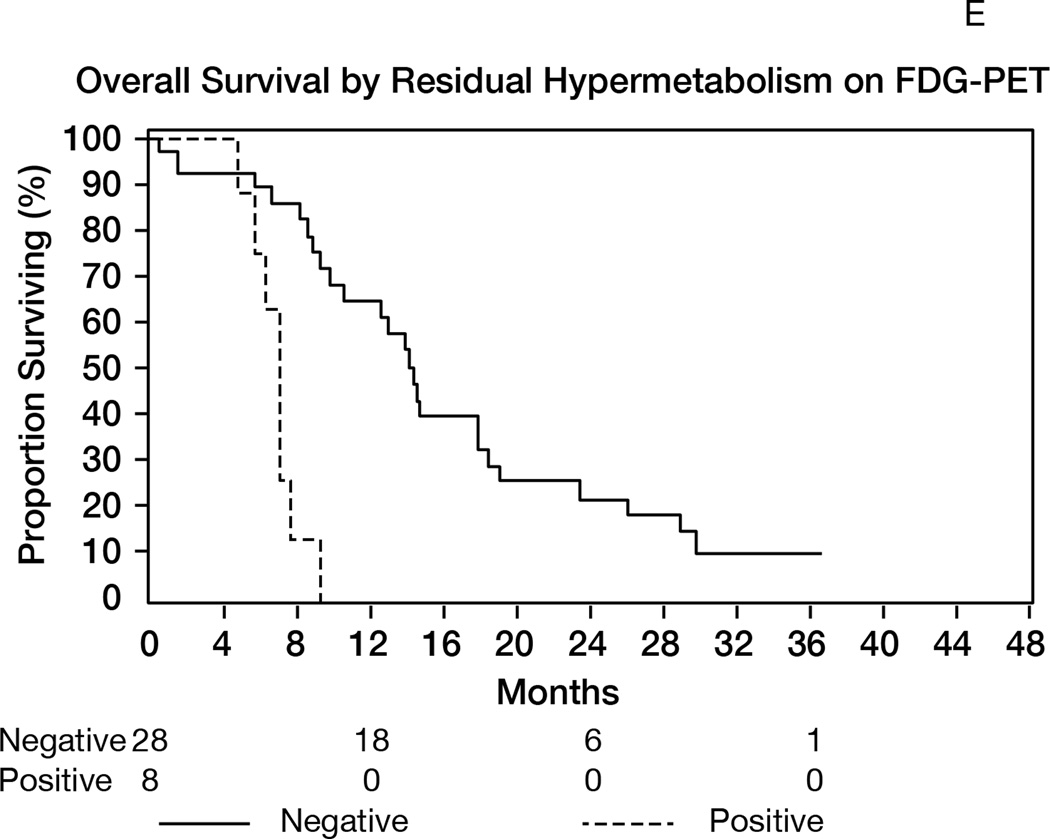
A: Overall survival (OS) for the entire population (N= 40). 1-y OS = 93% (95%CI 84–100); median OS= 19 months (95%CI 15–23). B: Progression-free survival (RANO criteria) for all patients (N=40). Median PFS= 10 months (95%CI: 8–11); 1-year PFS= 28% (95%CI 14–41). C: Overall survival in patients age>50 (median 17 months) versus age≤50 (median 26 months); p=0.02. D: Overall survival by MGMT promoter methylation status. Median OS in unmethylated tumors= 22 months; median OS in methylated tumors= 18 months; p=0.56. E: Overall survival according to presence or absence of hypermetabolism in the FDG-PET performed at the 6-month time point. Median OS from date of FDG-PET was 7 months in patients with hypermetabolism, versus 14 months in patients without; p<0.0001.
Table 2.
Response rates according to Macdonald and RANO criteria.
| N=30a | Macdonald | RANO | ||
|---|---|---|---|---|
| % | 95% CI | % | 95% CI | |
| CR | 27% | 12–46 | 13% | 4–31 |
| PR | 60% | 41–77 | 43% | 26–63 |
| SD | 7% | 1–22 | 37% | 20–56 |
| PD | 7% | 1–22 | 7% | 1–22 |
| ORR | 87% | 69–96 | 57% | 37–75 |
N=10 patients had no measurable disease post-operatively and accordingly were not evaluable for radiographic response.
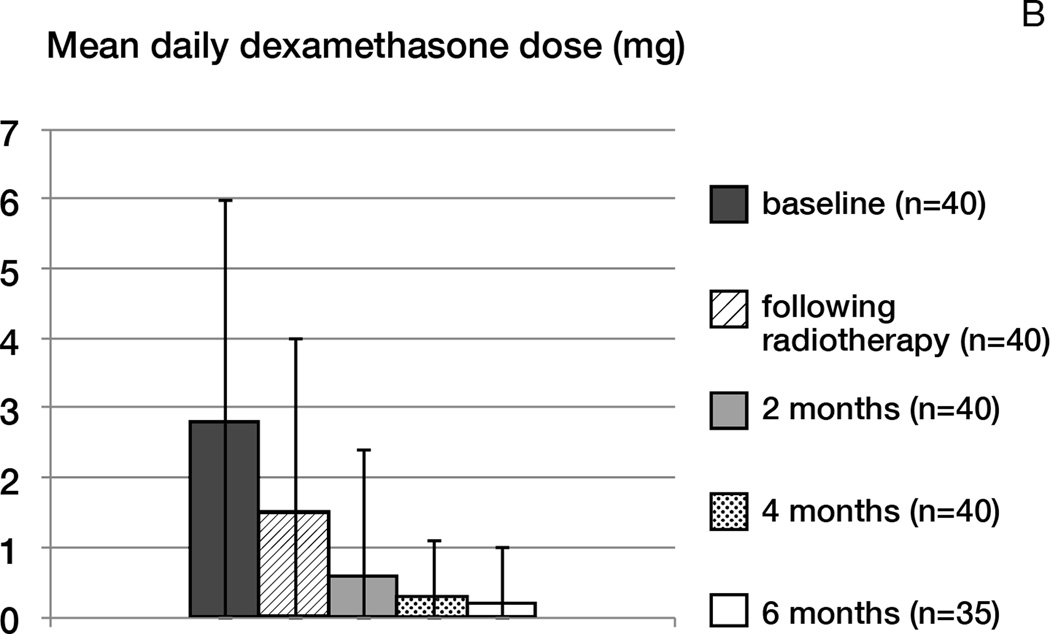
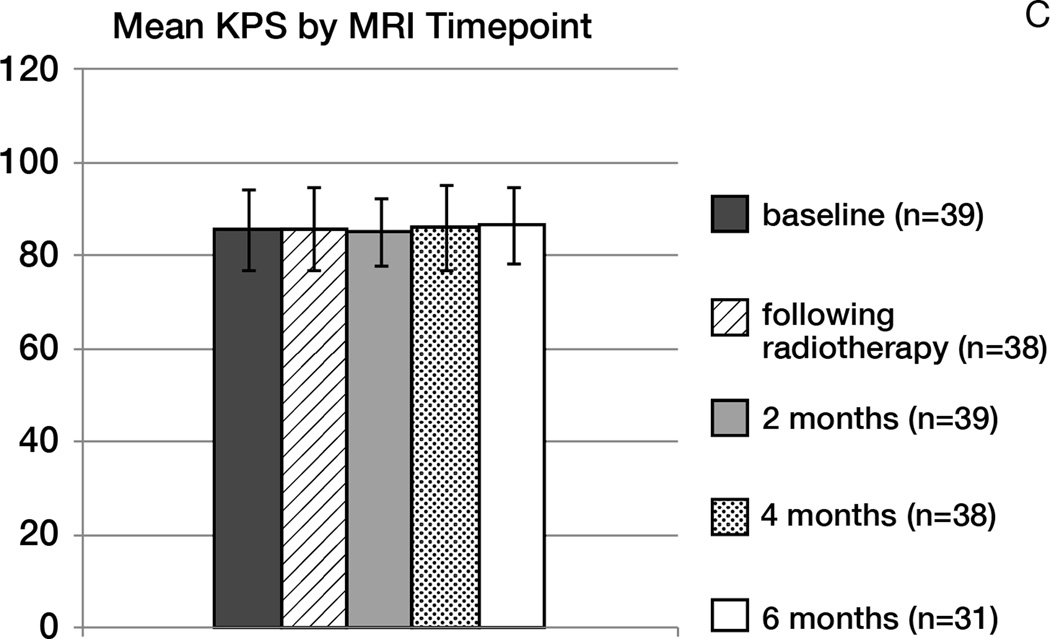
No patient developed worsening of symptoms or radiographic pseudoprogression within the first four months following radiotherapy. In patients free of progression, the KPS was maintained throughout treatment, and the use of corticosteroids significantly decreased over time (p< 0.0001; Figure 3B). The mean daily dexamethasone dose at baseline was 2.8 mg; following radiotherapy: 1.5 mg; at 6 months: 0.2 mg. Three patients thought to have tumor progression manifest by increased FLAIR hyperintensity without increased contrast enhancement underwent surgical resection; on pathology, tumor progression was confirmed in one patient, and two had mostly necrotic tissue.
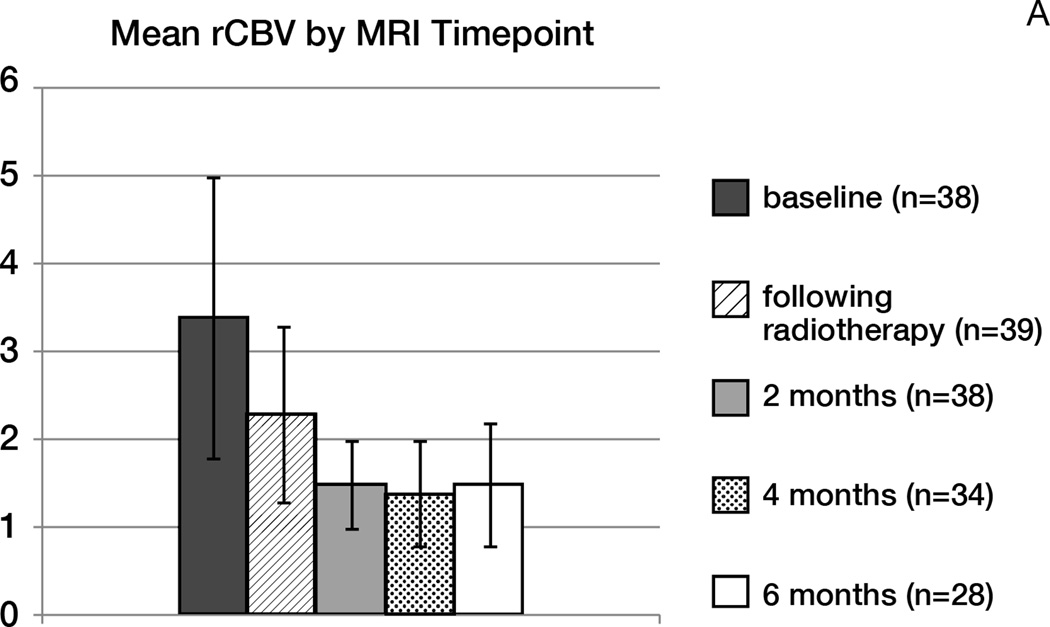
Figure 3.
Mean rCBV (A), corticosteroids use (B) and KPS (C) over time (error bars: standard deviation).
A- Mean rCBV
B- Mean corticosteroid dose (mg of dexamethasone)
C- Mean KPS
The pattern of radiographic progression (15) was determined in 29 patients during the trial. Among those, 25 (86%) had local progression, defined as focus of enhancing or nonenhancing tumor at, or within 3 cm of the primary site resection cavity. Four patients (14%) had distant progression, defined as new single focus of enhancement or FLAIR, developing more than 3 cm from the primary site. No patient displayed the so called diffuse pattern of progression (15) defined as recurrence centered or extending more than 3 cm from the primary site with poorly defined margins, or a multifocal pattern.
Exploratory Tissue Correlates
MGMT promoter methylation status could be determined in 37 patients, and was methylated in 23% and unmethylated in 70%. There was no association between MGMT status and response (P=1.0), PFS (P=0.39) or OS (P=0.56, Figure 2D).
The transcriptional glioblastoma subclass was determined in 31 patients (Table 3): Proneural: 26%; mesenchymal: 42%; classical: 29%; and neural: 3%. Patients with proneural tumors had lower response rate compared to other phenotypes (P=0.009), and survived a median of 15m, as compared to 21m for other phenotypes (P=0.56). There were no statistically significant differences in age distribution according to glioblastoma subclass or MGMT promoter methylation.
Table 3.
Distribution of transcriptional glioblastoma subclasses and corresponding age, response rate (RANO) and overall survival
| N (%) | Median age |
Objective response rate by RANO (95% CI) |
Median overall survival |
|
|---|---|---|---|---|
| Proneural | 8 (26%) | 62 | 14%a (0–53%) | 15mb |
| Mesenchymal | 13 (42%) | 55 | 70% (35–93%) | 20m |
| Classical | 9 (29%) | 58 | 83% (36–100%) | 22m |
| Neural | 1 (3%) | N/A | N/A | N/A |
| All patients with evaluable tissue | 31 | 57 | 58% (37–78%) | 20m |
P= 0.009 (Fisher’s exact test, proneural vs others);
P= 0.56 (Logrank test, Proneural versus others);
N/A: Not applicable (only one patient presented with this phenotype)
Among the 25 patients with sufficient tissue for immunohistochemistry, none displayed the IDH-1 R132H mutation, including all of the evaluated proneural tumors.
The expression levels of angiogenesis and hypoxia-related genes had no prognostic value. Similarly, we found no correlations between inferred copy number alterations based on gene expression data and patient outcome.
Exploratory Imaging Correlates
Analysis of DSC MR perfusion and DWI parameters are shown in Figure 3A and Supplementary Data 6 (online). Initiation of treatment resulted in a progressive reduction in mean rCBV over time: Mean rCBV was 3.4 at baseline, versus 2.3 after radiotherapy (p<0.0001), 1.5 after 2 months, and 1.4 after 4 months. However, DSC MR perfusion parameters such as baseline rCBV, change in rCBV after radiotherapy, rPH and PSR did not predict PFS or OS. Conversely, lower baseline ADC was associated with prolonged OS (HR= 0.25; p=0.05), but not PFS (RANO p=0.13). The association between ADC ratios and OS was maintained even after patients with a complete resection were excluded (p=0.03). There was no association between ADC parameters and response.
The presence of hypermetabolism on FDG-PET performed at the 6-month time point was associated with poor overall survival (p<0.0001, Figure 2E). Patients with tumors displaying hypermetabolism on the 6-month FDG-PET survived a median of 7 months from the date of the exam, as compared to 14 months in patients without hypermetabolism.
Exploratory Neurocognitive Findings
A total of N=37 (93%) patients agreed to undergo neuropsychological evaluations (Supplementary Data 7, online). At baseline, cognitive test z-scores were in the low-average to average range on most tests. The LMMs showed significant linear improvement in verbal fluency over time (COWA; p=0.001). There were no changes in grapho-motor speed (TMTA), attention (BTA) or visuospatial delayed recall (BVMT-R-DEL). After decreasing at the 4-month assessment, verbal learning (HVLT-R-TL) and verbal recognition memory (HVLT-R-DI) scores improved on subsequent evaluations to baseline levels or higher (quadratic time p=0.01 and 0.08, respectively). Cognitive flexibility scores (TMTB) decreased at the 4-month assessment (linear time p=0.023) before somewhat stabilizing (quadratic time p=0.07) below baseline levels at the 8 and 12-month assessments. Visuospatial learning (BVMT-R-TL) was stable at 4-months, with a possible indication of decline at subsequent follow-ups (quadratic time p=0.08). There were no significant changes in mood, self-reported QoL or fatigue across the study period.
Discussion
In this phase II trial, patients with newly diagnosed glioblastoma were treated with a hypofractionated radiotherapy schedule in combination with bevacizumab and temozolomide, and achieved a 1y-OS of 93%, median OS of 19 months and median PFS of 10 months. As compared to standard chemoradiotherapy, this regimen constituted a more convenient treatment option for patients, given the decreased use of corticosteroids and a notably shorter radiotherapy schedule, consisting of 6 treatments within two weeks, instead of the standard 30 treatments delivered daily over 6–7 weeks.
The value of adding bevacizumab to standard chemoradiotherapy for newly diagnosed glioblastoma is currently a matter of debate. Prospective phase II studies testing this combination reported a median PFS of 10–14 months and median OS of 19–21 months (16,17). More recently, preliminary results of two phase III trials examining standard chemoradiotherapy with or without bevacizumab, AVAGLIO (18) and RTOG 0825 (19), suggested that adding bevacizumab resulted in improvements in PFS (AVAGLIO: 11 vs 6 months; RTOG 0825: 11 vs 7 months), but not in OS (AVAGLIO: 17 months in both arms; RTOG 0825: 16 months in both arms).
While meaningful anti-tumor effects and survival benefit with anti-VEGF therapies remain to be demonstrated in glioblastoma, the effects on vascular permeability are unquestionable, as demonstrated by rapid reductions in contrast enhancement and peritumoral edema following treatment initiation (20). This “corticosteroid-like” effect made possible the hypofractionated schedule used in this trial, designed to produce radiobiological effects comparable to the standard regimen of 60 Gy/1.8-2Gy fractions. Advances in radiotherapy techniques, particularly IMRT and stereotactic technology, have allowed for remarkable improvements in spatial targeting, but the development of hypofractionated schedules has been limited (21). In addition to convenience, the biological rationale for hypofractionation is based on potentially enhanced direct cell kill and reduction in the accelerated tumor cell repopulation (22), although late-responding neural tissue injury and toxicity may increase. To date, the best studied use of hypofractionated or abbreviated radiotherapy schedules has been in poor prognosis populations, such as the elderly or poor performance status patients, who have been treated with biological doses that are equivalent to, or lower than standard schedules. Such studies have found median OS of 5–12 months (21,23–27), which in small randomized trials (23,25) did not appear inferior to standard schedules, although non-inferiority trial designs have not been used. Also under investigation is the use of hypofractionation and IMRT in escalation studies to deliver higher tumor biological effective doses, although an increased corticosteroid requirement and radionecrosis are major deterrents to the wide application of such schedules (21,28–32). In our study, the addition of bevacizumab appears to have offset the development of symptoms and radiographic signs of radionecrosis typically associated with hypofractionation, decreasing the need for corticosteroids. Interestingly, pathology in some of our re-operated patients showed signs of significant tissue damage; it is possible that other patients thought to have non-enhancing tumor progression in fact had non-enhancing treatment effects. It must also be noted that results of our exploratory neuropsychological testing showed some transient radiotherapy effects on neurocognitive functions, which seemed less marked in comparison to standard fractionation schedules (33), although definitive conclusions are limited by our small patient number, lack of a control arm and confounding effects of bevacizumab. Analysis of RTOG 0825 (19) suggested that the addition of bevacizumab resulted in more frequent cognitive decline as compared to standard chemoradiotherapy, although interpretation of such findings is limited by the fact that progressing patients were not evaluated, and patients remained longer on study in the bevacizumab arm, which implies that the cognitive decline could represent unrecognized tumor progression, rather than direct bevacizumab effects.
The survival curve in our trial followed the typical pattern observed in bevacizumab glioblastoma studies, with a decrease in early death rates, but no improvement in later survival endpoints in comparison to contemporary phase II historical controls, which have reported median OS of 18–19 months (34). Therefore, identifying imaging and tissue-based biomarkers and subgroups of patients who benefit from, or who are harmed by, this type of treatment remains crucial (35). We examined bevacizumab effects on tumor blood perfusion as measured by DSC-MRI, and found a nearly universal decrease in blood perfusion over time, demonstrating that the intended targeting of angiogenesis was accomplished. However, for prognostic purposes, diffusion-based parameters were more helpful. Tumors with the lowest ADCs appeared to benefit most perhaps representing highly hypoxic, or densely cell-packed phenotypes, therefore constituting optimal candidates for anti-angiogenic treatments (36). Interestingly, we found the FDG-PET at the 6-month time point to be a reliable marker of treatment failure (Figure 2E), even when other imaging modalities suggested stability. Taken together, our findings support the use of imaging techniques based on cell proliferation and metabolism, rather than perfusion and vasculature imaging, to guide future development of anti-angiogenic therapies.
Finally, we evaluated a range of potential tissue-based biomarkers implicated in glioblastoma and angiogenesis. A previous study in recurrent malignant gliomas suggested that high VEGF expression as determined by immunohistochemistry was associated with increased response to bevacizumab but did not predict survival, whereas expression of CA9 was associated with poor survival outcome (35). In our study, we focused on applying the TCGA-based transcriptional classification, under the hypothesis that the marked differences in oncogenetic and angiogenic drivers across distinct expression signatures might translate into differential responses to anti-VEGF therapy. Unexpectedly, results suggested that proneural glioblastomas were associated with worse outcomes as compared to other subclasses. Proneural glioblastomas had previously been associated with dysregulated PDGF signaling (8,37,38) and a relatively favorable prognosis (37), in sharp contrast to our findings. Subsequent work, however, suggested that the majority, if not the entirety, of this prognostic advantage derived from the enrichment of the proneural subclass with IDH-mutant tumors, a well-known predictor of prolonged survival (8,9). None of the proneural tumors in our study expressed mutant IDH-1, which may explain their poor outcome in this group. Based on our results, a post hoc analysis of tissue collected in the AVAGLIO study has been initiated, utilizing our Nanostring-based methodology. Preliminary results showed that proneural glioblastomas without IDH-1 mutation were associated with a poor prognosis in the placebo arm, confirming our finding that this molecular signature is associated with a poor prognosis, and suggesting this is not associated with a detrimental effect of bevacizumab in these patients. In fact, additional analysis suggested that adding bevacizumab may actually improve survival in these poor prognosis patients, a finding that will require further validation in larger, prospective studies. Interestingly, in our trial, patients with mesenchymal tumors benefitted from treatment and did not fare worse than other subclasses, which is consistent with their high VEGF expression. This finding contrasts with a preliminary analysis of the RTOG 0825 study, which found that some mesenchymal-associated genes conferred a worse prognosis in patients exposed to bevacizumab (39). Further studies are needed to determine whether this discrepancy is explained by our small number of patients, differing tissue study techniques, or to an effect unique to the hypofractionated radiotherapy schedule utilized in this study.
Our study has a number of limitations. Results are applicable to unifocal tumors, with a volume of 60 cc or less. However, for the most part, our population of patients had prognostic factors comparable to historical controls (34). Many trials used as historical controls have also excluded multifocal tumors (2,19) and larger tumors are also often excluded because of poor KPS. Another limitation is that a baseline FDG-PET was not obtained, and therefore it is not possible to determine whether the hypermetabolism observed at the sixth month was already present at the time of treatment start, or if developed throughout treatment. Finally, our correlative studies and neuropsychological testing are limited by small patient numbers, and few long-term survivors. Our analyses should be regarded as exploratory and hypothesis-generating; further investigations in larger, randomized studies will be necessary to validate our findings and determine whether potential biomarkers are of a predictive or prognostic value.
In summary, we describe a new use for bevacizumab in newly diagnosed glioblastoma, capitalizing on the anti-permeability effects to develop a convenient hypofractionated radiotherapy schedule. In our hands this regimen was found to be safe, associated with minimal detrimental effects on QoL, and with survival results comparable to other regimens. Our prospective correlative studies provide new insights into the biology of bevacizumab and hypofractionated radiotherapy in glioblastoma, and uncover candidate imaging and tissue-based biomarkers with either prognostic or predictive value that warrant further investigation in adequately powered randomized studies.
Supplementary Material
Translational relevance.
We describe a new hypofractionated radiotherapy schedule for newly-diagnosed glioblastoma, made possible through anti-permeability and corticosteroid-like effects of bevacizumab. The resulting treatment schedule (6 treatments in two weeks) is more convenient than the standard 30 treatments in 6–7 weeks, and was associated with comparable survival. Comprehensive exploratory correlative studies were performed to optimize patient selection, and provided new insights into the biology of bevacizumab and hypofractionated radiotherapy in glioblastoma. Analysis of TCGA glioblastoma transcriptional subclasses (Nanostring assay) showed patients with a proneural phenotype achieved unexpectedly poor outcomes, suggesting that in the absence of IDH-1 mutation, this phenotype is a marker of poor, and not better prognosis as previously thought. Dynamic susceptibility-contrast perfusion MRI showed marked decreases in rCBV over time, suggesting a role as a biomarker of anti-angiogenic effects. However, meaningful anti-tumor effects were better captured by imaging techniques based on cell proliferation and metabolism, such as ADC and FDG-PET, which were of prognostic value in this trial.
Acknowledgments
Research funding: K.S.P. and A.S.R. (NIH P30 CA008748); A.B.L. (Abbott, Abbvie, Aeterna Zentaris, Agenus, Amgen, Angiochem, Bayer/Onyx, BMS, Celldex, Millennium, Northwest Biotherapeutics, Novartis, Novocure, NPM Pharma, DCVax, Pfizer, Plexxicon, Roche, Theorum, Merck Sharp & Dohme/ Schering Plough, Genentech, Keryx, Sigma Tau, Astra Zeneca, Medimmune, Boehringer Ingelheim, Novartis).
Footnotes
Disclosures:
Genentech provided drug and partial financial support.
The authors report the following disclosures:
Employment: L.E.A. is currently an employee of Roche.
Consulting: A.O. (Roche, Novocure, CarThera); L.M.D. (Genentech, CarThera); P.G. (Genentech); A.B.L. (Merck Sharp & Dohme/ Schering Plough, BMS, Campus Bio, Cephalon, Eisai, Genentech, Roche, GSK, Imclone, Sigma Tau, Abbot, Novartis, Amgen, Kyowa Hakko Kirin Pharma, RadMD, Stemline, Agenus).
Honoraria: A.B.L. (Merck Sharp & Dohme/ Schering Plough, Omniprex)
Partial results presented at the American Society of Clinical Oncology (ASCO) Annual Meeting, 2010 (poster discussion) and Society for Neuro-Oncology Annual Meeting, 2011 (platform presentation).
Clinical trials.gov identifier: NCT01392209 (available at http://clinicaltrials.gov/ct2/show/NCT01392209?term=omuro&rank=2)
References
- 1.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert MR, Wang M, Aldape KD, Stupp R, Hegi ME, Jaeckle KA, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31:4085–4091. doi: 10.1200/JCO.2013.49.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong TS, Wefel JS, Wang M, Gilbert MR, Won M, Bottomley A, et al. Net clinical benefit analysis of radiation therapy oncology group 0525: a phase III trial comparing conventional adjuvant temozolomide with dose-intensive temozolomide in patients with newly diagnosed glioblastoma. J Clin Oncol. 2013;31:4076–4084. doi: 10.1200/JCO.2013.49.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310:1842–1850. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- 6.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 7.Gutin PH, Iwamoto FM, Beal K, Mohile NA, Karimi S, Hou BL, et al. Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2009;75:156–163. doi: 10.1016/j.ijrobp.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Correa DD, DeAngelis LM, Shi W, Thaler H, Glass A, Abrey LE. Cognitive functions in survivors of primary central nervous system lymphoma. Neurology. 2004;62:548–555. doi: 10.1212/01.wnl.0000109673.75316.d8. [DOI] [PubMed] [Google Scholar]
- 11.Correa DD, Maron L, Harder H, Klein M, Armstrong CL, Calabrese P, et al. Cognitive functions in primary central nervous system lymphoma: literature review and assessment guidelines. Ann Oncol. 2007;18:1145–1151. doi: 10.1093/annonc/mdl464. [DOI] [PubMed] [Google Scholar]
- 12.Correa DD, Rocco-Donovan M, DeAngelis LM, Dolgoff-Kaspar R, Iwamoto F, Yahalom J, et al. Prospective cognitive follow-up in primary CNS lymphoma patients treated with chemotherapy and reduced-dose radiotherapy. J Neurooncol. 2009;91:315–321. doi: 10.1007/s11060-008-9716-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 14.Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 15.Pope WB, Xia Q, Paton VE, Das A, Hambleton J, Kim HJ, et al. Patterns of progression in patients with recurrent glioblastoma treated with bevacizumab. Neurology. 2011;76:432–437. doi: 10.1212/WNL.0b013e31820a0a8a. [DOI] [PubMed] [Google Scholar]
- 16.Vredenburgh JJ, Desjardins A, Reardon DA, Peters KB, Herndon JE, 2nd, Marcello J, et al. The addition of bevacizumab to standard radiation therapy and temozolomide followed by bevacizumab, temozolomide, and irinotecan for newly diagnosed glioblastoma. Clin Cancer Res. 2011;17:4119–4124. doi: 10.1158/1078-0432.CCR-11-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai A, Tran A, Nghiemphu PL, Pope WB, Solis OE, Selch M, et al. Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2011;29:142–148. doi: 10.1200/JCO.2010.30.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370:709–722. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 19.Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370:699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hingorani M, Colley WP, Dixit S, Beavis AM. Hypofractionated radiotherapy for glioblastoma: strategy for poor-risk patients or hope for the future? Br J Radiol. 2012;85:e770–e81. doi: 10.1259/bjr/83827377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaaijk P, Troost D, Sminia P, Hulshof MC, van der Kracht AH, Leenstra S, et al. Hypofractionated radiation induces a decrease in cell proliferation but no histological damage to organotypic multicellular spheroids of human glioblastomas. Eur J Cancer. 1997;33:645–651. doi: 10.1016/s0959-8049(96)00503-5. [DOI] [PubMed] [Google Scholar]
- 23.Roa W, Brasher PM, Bauman G, Anthes M, Bruera E, Chan A, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol. 2004;22:1583–1588. doi: 10.1200/JCO.2004.06.082. [DOI] [PubMed] [Google Scholar]
- 24.Hulshof MC, Schimmel EC, Andries Bosch D, Gonzalez Gonzalez D. Hypofractionation in glioblastoma multiforme. Radiother Oncol. 2000;54:143–148. doi: 10.1016/s0167-8140(99)00183-8. [DOI] [PubMed] [Google Scholar]
- 25.Malmstrom A, Gronberg BH, Marosi C, Stupp R, Frappaz D, Schultz H, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13:916–926. doi: 10.1016/S1470-2045(12)70265-6. [DOI] [PubMed] [Google Scholar]
- 26.Chang EL, Yi W, Allen PK, Levin VA, Sawaya RE, Maor MH. Hypofractionated radiotherapy for elderly or younger low-performance status glioblastoma patients: outcome and prognostic factors. Int J Radiat Oncol Biol Phys. 2003;56:519–528. doi: 10.1016/s0360-3016(02)04522-4. [DOI] [PubMed] [Google Scholar]
- 27.Cao JQ, Fisher BJ, Bauman GS, Megyesi JF, Watling CJ, Macdonald DR. Hypofractionated radiotherapy with or without concurrent temozolomide in elderly patients with glioblastoma multiforme: a review of ten-year single institutional experience. J Neurooncol. 2012;107:395–405. doi: 10.1007/s11060-011-0766-3. [DOI] [PubMed] [Google Scholar]
- 28.Floyd SR, Kasper EM, Uhlmann EJ, Fonkem E, Wong ET, Mahadevan A. Hypofractionated Radiotherapy and Stereotactic Boost with Concurrent and Adjuvant Temozolamide for Glioblastoma in Good Performance Status Elderly Patients - Early Results of a Phase II Trial. Front Oncol. 2012;2:122. doi: 10.3389/fonc.2012.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sultanem K, Patrocinio H, Lambert C, Corns R, Leblanc R, Parker W, et al. The use of hypofractionated intensity-modulated irradiation in the treatment of glioblastoma multiforme: preliminary results of a prospective trial. Int J Radiat Oncol Biol Phys. 2004;58:247–252. doi: 10.1016/s0360-3016(03)00819-8. [DOI] [PubMed] [Google Scholar]
- 30.Reddy K, Damek D, Gaspar LE, Ney D, Waziri A, Lillehei K, et al. Phase II trial of hypofractionated IMRT with temozolomide for patients with newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2012;84:655–660. doi: 10.1016/j.ijrobp.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 31.Panet-Raymond V, Souhami L, Roberge D, Kavan P, Shakibnia L, Muanza T, et al. Accelerated hypofractionated intensity-modulated radiotherapy with concurrent and adjuvant temozolomide for patients with glioblastoma multiforme: a safety and efficacy analysis. Int J Radiat Oncol Biol Phys. 2009;73:473–478. doi: 10.1016/j.ijrobp.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 32.Tsien CI, Brown D, Normolle D, Schipper M, Piert M, Junck L, et al. Concurrent temozolomide and dose-escalated intensity-modulated radiation therapy in newly diagnosed glioblastoma. Clin Cancer Res. 2012;18:273–279. doi: 10.1158/1078-0432.CCR-11-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wefel JS, Pugh SL, Armstrong TS, Gilbert MR, Won M, Wendland MM, et al. Neurocognitive function (NCF) outcomes in patients with glioblastoma (GBM) enrolled in RTOG 0825. ASCO Meeting Abstracts. 2013;31:2004. [Google Scholar]
- 34.Grossman SA, Ye X, Piantadosi S, Desideri S, Nabors LB, Rosenfeld M, et al. Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res. 2010;16:2443–2449. doi: 10.1158/1078-0432.CCR-09-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sathornsumetee S, Cao Y, Marcello JE, Herndon JE, 2nd, McLendon RE, Desjardins A, et al. Tumor angiogenic and hypoxic profiles predict radiographic response and survival in malignant astrocytoma patients treated with bevacizumab and irinotecan. J Clin Oncol. 2008;26:271–278. doi: 10.1200/JCO.2007.13.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pope WB, Lai A, Mehta R, Kim HJ, Qiao J, Young JR, et al. Apparent diffusion coefficient histogram analysis stratifies progression-free survival in newly diagnosed bevacizumab-treated glioblastoma. AJNR Am J Neuroradiol. 2011;32:882–889. doi: 10.3174/ajnr.A2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 38.Brennan C, Momota H, Hambardzumyan D, Ozawa T, Tandon A, Pedraza A, et al. Glioblastoma subclasses can be defined by activity among signal transduction pathways and associated genomic alterations. PLoS One. 2009;4:e7752. doi: 10.1371/journal.pone.0007752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sulman EP, Won M, Blumenthal DT, Vogelbaum MA, Colman H, Jenkins RB, et al. Molecular predictors of outcome and response to bevacizumab (BEV) based on analysis of RTOG 0825, a phase III trial comparing chemoradiation (CRT) with and without BEV in patients with newly diagnosed glioblastoma (GBM) ASCO Meeting Abstracts. 2013;31:LBA2010. [Google Scholar]
- 40.Wetzel SG, Cha S, Johnson G, Lee P, Law M, Kasow DL, et al. Relative cerebral blood volume measurements in intracranial mass lesions: interobserver and intraobserver reproducibility study. Radiology. 2002;224:797–803. doi: 10.1148/radiol.2243011014. [DOI] [PubMed] [Google Scholar]
- 41.Young R, Babb J, Law M, Pollack E, Johnson G. Comparison of region-of-interest analysis with three different histogram analysis methods in the determination of perfusion metrics in patients with brain gliomas. J Magn Reson Imaging. 2007;26:1053–1063. doi: 10.1002/jmri.21064. [DOI] [PubMed] [Google Scholar]
- 42.Cha S, Knopp EA, Johnson G, Wetzel SG, Litt AW, Zagzag D. Intracranial mass lesions: dynamic contrast-enhanced susceptibility-weighted echo-planar perfusion MR imaging. Radiology. 2002;223:11–29. doi: 10.1148/radiol.2231010594. [DOI] [PubMed] [Google Scholar]
- 43.Barajas RF, Chang JS, Sneed PK, Segal MR, McDermott MW, Cha S. Distinguishing Recurrent Intra-Axial Metastatic Tumor from Radiation Necrosis Following Gamma Knife Radiosurgery Using Dynamic Susceptibility-Weighted Contrast-Enhanced Perfusion MR Imaging. AJNR Am J Neuroradiol. 2009;30:367–372. doi: 10.3174/ajnr.A1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mangla R, Kolar B, Zhu T, Zhong J, Almast J, Ekholm S. Percentage signal recovery derived from MR dynamic susceptibility contrast imaging is useful to differentiate common enhancing malignant lesions of the brain. AJNR Am J Neuroradiol. 2011;32:1004–1010. doi: 10.3174/ajnr.A2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Law M, Young R, Babb J, Pollack E, Johnson G. Histogram analysis versus region of interest analysis of dynamic susceptibility contrast perfusion MR imaging data in the grading of cerebral gliomas. AJNR Am J Neuroradiol. 2007;28:761–766. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.