Abstract
Lymph node-positive breast tumors are more likely to express COX-2 than node-negative tumors. In preclinical studies, COX2 inhibition prevents breast tumor spread to lymph-nodes. Therefore, we examined the association between recent (1 year) pre-diagnostic use of aspirin (COX1/COX2 inhibitor), lymph node involvement at breast cancer diagnosis and breast cancer-specific mortality. Women with stage I-III breast cancer diagnosed from 2001-2006 (N=2,796) were identified from Ireland's National Cancer Registry. This data was linked to prescription-refill and mammographic-screening databases. Relative risks (RR) were estimated for associations between pre-diagnostic aspirin use and lymph node-positive status at diagnosis. Hazard ratios (HR) were estimated for associations between pre- and post-diagnostic aspirin use and 5-year mortality, stratified by lymph-node status. Women with pre-diagnostic aspirin use were statistically significantly less likely to present with a lymph node-positive tumor than non-users (RR=0.89, 95%CI 0.81-0.97), particularly those with larger (P-interaction=0.036), PR-negative (P-interaction<0.001) or ER-negative (P-interaction=0.056) tumors. The magnitude of this association increased with dose (P-trend<0.01) and dosing-intensity (P-trend<0.001) and was similar in women with or without screen-detected tumors (P-interaction=0.70). Pre-diagnostic aspirin use was associated with lower 5-year breast cancer-specific mortality among women with lymph node-negative tumors (HR=0.55 95%CI 0.33-0.92), but not node-positive tumors (HR=0.91 95%CI 0.37-1.22). Tests for effect-modification were, however, not statistically significant (P-interaction=0.087). Post-diagnostic aspirin use was not associated with breast cancer-specific mortality (HR=0.99 95%CI 0.68-1.45). Our findings indicate recent pre-diagnostic aspirin use is protective against lymph node-positive breast cancer. This is a plausible explanation for reductions in breast cancer mortality reported in observational studies of aspirin use.
Background
In a recent meta-analysis of randomized trials of aspirin for cardiovascular disease prevention, the use of aspirin, a cyclooxygenase (COX) −1/−2 inhibitor, prior to a cancer diagnosis, was associated with a 36% reduction in the risk of distant metastasis.(1) In further sub-analyses a statistically significant reduction in metastasis was observed among colorectal cancer patients taking aspirin OR 0.36, 95%CI 0.18–0.74. They made up the largest subgroup (N = 130). Aspirin use was also associated with a non-significant reduction in metastasis in women with breast cancer, OR=0.50, 95%CI 0.16-1.51 (N = 86). In the same meta-analysis, pre-diagnostic aspirin use was also associated with lower cancer-specific mortality. This mortality benefit was only observed among individuals with non-metastatic disease at diagnosis.(1) In other observational studies, aspirin use by women with breast cancer has been associated with statistically significant reductions in breast cancer recurrence and mortality.(2,3)
Preclinical data suggests that the cyclooxygenase/prostaglandin pathway is involved in the development of lymph node metastases through the regulation of vascular endothelial growth factor-C/-D (VEGF-C/-D) mediated lymphangiogenesis.(4,5) Inhibition of the cyclooxygenase/prostaglandin pathway has also been shown to suppress the development of lymphatic metastases in breast cancer animal models.(4,5) The COX-2 enzyme is expressed in up to 40% of breast cancers and is associated with larger tumor size, negative hormone receptor status, a high proliferation rate (identified by Ki-67) and the presence of HER-2 oncogene amplification.(6,7) Women with tumors that express COX-2 are also more likely to present with positive lymph nodes at diagnosis and die from breast cancer.(6,7)
In this study we aimed to investigate the following in women with breast cancer: (i) associations between recent pre-diagnostic aspirin use and the presence of lymph node metastasis at breast cancer diagnosis; (ii) associations between recent pre-diagnostic aspirin use and breast cancer mortality; and (iii) whether the presence of lymph node metastases at diagnosis modifies associations between recent pre-diagnostic aspirin use and breast cancer mortality.
Methods
Setting & Data Sources
We conducted this study using patient records from the National Cancer Registry Ireland (NCRI) linked to prescription dispensing data from Ireland's General Medical Services (GMS) pharmacy claims database(8) and information on mammographic screening from BreastCheck, a national breast cancer screening program.(9) The NCRI records detailed information on all incident cancers diagnosed in the population usually resident in Ireland. Information is collected by trained, hospital-based, tumor registration officers from multiple sources including pathology and radiology reports, medical records and death certificates. The use for research of anonymized data held by the NCRI is covered by the Health (Provision of Information) Act 1997.
Eligibility for the GMS prescription scheme is through means test or age (>70 years). The GMS database records details of all prescription drugs dispensed to GMS eligible patients since 2000. This includes all low-dose and most high-dose aspirin preparations which are prescription-only in Ireland, as in other European countries.(10) A small number of high-dose aspirin preparations are available over the counter, but only for specified short-term indications, in small pack sizes (≤24-50 doses) and at increased cost. Women with GMS eligibility can obtain high-dose aspirin preparations on-prescription without charge or restriction.
We used two independent sources of information to identify women with breast tumors detected by organized or opportunistic(11) screening-mammography. Firstly, individual screening histories from Ireland's population-based organized screening-mammography program, BreastCheck,(9) were linked to NCRI patient records, allowing the accurate identification of all organized screen-detected breast cancers.(12) Secondly, the NCRI provided information, collected by tumor registration officers, identifying breast tumors detected by any screening mammography. There was close to 100% agreement for organized screen-detected tumors between linked BreastCheck records and data collected by the NCRI.(12) This enabled us to identify women with tumors detected by opportunistic screening-mammography (i.e. screening-mammography use outside of BreastCheck).
Cohort & Exposure Definitions
The study cohort included all women with a diagnosis of stage I-III invasive breast cancer (ICD-10 C50)(13) between 1st January 2001 and 31st December 2006, aged 50 to 80 years at diagnosis and with GMS eligibility from at least one year prior to diagnosis. Women were excluded if they had a prior invasive cancer other than non-melanoma skin cancer, or if their breast cancer diagnosis was made at the time of death (Figure 1). The lower age limit was set at 50 years to restrict the study population to women with similar potential for aspirin exposure.(14) Of the 489 women less than the age of 50 excluded from the study analyses, 97.6% did not receive any aspirin (Figure 1). Women over the age of 80 were excluded from the analysis as they are less likely to receive a definitive lymph node evaluation.(15)
Figure 1.
Flow chart for study cohort inclusion and exclusion criteria.
All prescriptions for aspirin, dispensed to women in the study cohort, were identified from the GMS database using WHO-ATC drug classifications(16) (Appendix). The dose and number of days’ supply on each prescription were abstracted. This meant we could evaluate the full range of aspirin use starting at the level of one prescription per year. Pre-diagnostic aspirin use was defined as having received aspirin in the year prior to diagnosis. Patients initiating pre-diagnostic aspirin use between 0-1.5, 1.5-3 and ≥3 years prior to diagnosis were also identified. These exposure windows were selected based on prior preclinical(17) and clinical (1) data indicating that aspirin exposure in the years immediately prior to diagnosis can impact breast cancer progression. Aspirin dosing intensity, the proportion of days with a supply of aspirin available in the year prior to diagnosis, was calculated from the number of days’ supply on each prescription.(18) Post-diagnostic aspirin use was defined as having received aspirin between diagnosis and the end of follow-up.
Outcomes & Covariates
We used information from the NCRI database to identify lymph node status at diagnosis (positive, negative). Women were identified as lymph node-positive if they had a pathologic nodal status of pN1/2/3 or, if not available, a clinical nodal status of N1/2/3.(13) Death certificates were used to identify the date and cause of death (Appendix) for survival analyses.
The NCRI database was also used to classify women by tumor size (T1, T2, T3, T4);(13) tumor stage (I, IIa, IIb, IIIa, IIIb-c);(13) tumor grade (low, intermediate, high, unspecified); tumor morphology (ductal, lobular, other; Appendix); tumor topography (outer, inner/central, unspecified; Appendix); ER, PR, HER2 status (positive, negative, unspecified; Appendix); age (years); smoking status (never, past, current, unspecified); and screen-detection (organized, opportunistic, not screen-detected). We used prescription data to identify the use of other medications that could be confounders (Appendix), including anti-diabetic medications which were taken to indicate a diagnosis of diabetes. A medication-based comorbidity score, based on a validated measure,(19) was calculated for each patient as the sum of distinct medication classes (defined by the first 5 ATC code characters) received in the year prior to breast cancer diagnosis.
Statistical Analyses
The distribution of clinical and socio-demographic covariates was compared between aspirin users and non-users. Univariate and multivariate log-binomial models(20,21) were used to estimate relative risks (RR) with 95% confidence intervals (CI) for associations between aspirin use prior to diagnosis and lymph node-positive breast cancer at diagnosis.(22,23) Covariates were identified for inclusion in the multivariate model based on prior knowledge of clinical, demographic and behavioral predictors of nodal status (tumor size; grade; morphology; topography; ER, PR, HER2 status; age; smoking status; screen-detection);(24–28) drugs associated with tumor invasiveness (beta-blockers, biguanides, bisphosphonates, statins, estrogen, estrogen/progesterone, NSAIDS);(29–34) specific comorbidities associated with lymphatic metastasis (diabetes);(35) and patient characteristics associated with extent of nodal evaluation (age, comorbidity score).(15) We selected the final multivariate model from these covariates using backwards elimination up to a 10% maximum cumulative change in the effect component of the fully adjusted RR.(36) Covariates consistently associated with nodal status in previous studies were fixed in the model a priori (tumor size, grade, age, screen-detection).
Subgroup analyses of nodal status were conducted by quartiles of pre-diagnostic aspirin dosing intensity; by low-dose (all prescriptions for <150mg) and high-dose aspirin use (at least one prescription for ≥150mg); and by duration of pre-diagnostic aspirin use (0-1.5, 1.5-3, ≥3 years).(37) Effect modification of associations between pre-diagnostic aspirin use and nodal status was assessed on an additive scale (risk difference, RD; interaction contrast, IC) and significance was tested using the Wald test.(38) The separate and joint effects of aspirin exposure and effect modifier are presented using a single reference category, in addition to the within strata effects and measures of interaction.(39,40) Breast tumor characteristics known to be associated with COX-2 expression, were identified a priori and considered as potential effect modifiers.(6,41,42) These were large tumor size, high grade, negative ER or PR status, positive HER2 status and tumor morphology.
In survival analyses multivariate Cox proportional hazards models were used to estimate hazard ratios (HR) with 95%CI for associations between pre-diagnostic aspirin use and (i) breast cancer-specific mortality, (ii) all-cause mortality. All women were followed from diagnosis to the first of either death, the 31st December 2008 or 5 years. Deaths from non-breast cancer causes were censored in analyses of breast cancer-specific mortality. Covariates were selected for inclusion in the multivariate model based on prior knowledge of clinical and demographic characteristics associated with breast cancer survival. These were age, comorbidity score, tumor stage (including nodal status), grade, ER, PR and HER2 status. Effect modification by nodal status at diagnosis was assessed on a multiplicative scale (ratio of hazard ratios, rHR) with 95%CI. We repeated survival analyses with the inclusion of post-diagnostic aspirin use (unexposed, exposed; time varying; lagged 2 years) and calculated hazard ratios (HR) with 95%CI for associations between post-diagnostic aspirin use and (i) all-cause mortality, (ii) breast cancer-specific mortality. Post-diagnostic aspirin use was lagged in survival analyses to reduce the possibility that worsening prognosis influenced prescribing patterns (time-dependent confounding).(43) This lag time was varied from one to three years in sensitivity analyses. Cumulative mortality was also estimated from directly adjusted survival curves.(44) All analyses were conducted using SAS® v9.2 (SAS Institute Inc, Cary, NC). Results were considered statistically significant at a two-sided α-level of 0.05.
Sensitivity Analyses
In addition to adjusting for screen-detection in nodal status analyses, the following sensitivity analyses were conducted to rule out early detection bias due to differential screening or intensity of medical surveillance among aspirin users as an explanation of our results: (i) associations between aspirin use and lymph node status were assessed in analyses stratified by screen-detection; (ii) a propensity-score matched analysis was conducted incorporating screening practices and comorbidities for aspirin users and non-users. We also conducted sensitivity analyses to rule out bias due to the potential misclassification of nodal status based on clinical evaluation alone. In addition, to minimize the effect of any differential bias due to unrecorded nodal status (N=165) we took a conservative approach in the main analysis and classified all women with unrecorded lymph node status as lymph node positive (aspirin user 4.9%; aspirin non-user 8.6%). Sensitivity analyses using complete cases were also undertaken.
To assess the presence of bias due to possible misclassification of breast cancer-specific cause of death, we repeated survival analyses with the inclusion of: (i) deaths where breast cancer was listed as a secondary cause of death on the death certificate; (ii) deaths from ill-defined or secondary cancers, cancers of unknown behavior and unspecified causes.
Results
Cohort Characteristics
The characteristics of aspirin users (n=740) and non-users (n=2,056), stratified by dosing intensity, are presented in Table 1. Aspirin users were older and had a higher comorbidity score than non-users. The proportion of organized and opportunistic screen-detected tumors was similar between aspirin users and non-users (user/non-user; organized 11.0%/12.5%; opportunistic 3.9%/4.6%; P=0.38). The reason for aspirin use was not recorded; however, 85.4% of women were taking low-dose (<150mg/day) aspirin exclusively; which is primarily indicated for cardiovascular disease prevention. The median proportion of days using aspirin in the year prior to diagnosis (dosing intensity) was 80.3%.
TABLE 1.
CHARACTERISTICS OF WOMEN SELECTED FOR INCLUSION IN THE STUDY COHORT
| Aspirin use in the year prior diagnosis (dosing intensity by quartiles)A |
||||||
|---|---|---|---|---|---|---|
| Characteristic at diagnosis | Non-user N = 2,056 |
Dosing intensity (1%-37%) N = 186 |
Dosing intensity (38%-79%) N = 184 |
Dosing intensity (80%-97%) N = 185 |
Dosing intensity (98%-100%) N = 185 |
|
| Patient details | ||||||
| Age – Median (IQR) | Years | 67 (58, 73) | 72 (65, 77) | 71 (64, 77) | 73 (65, 76) | 72 (66, 77) |
| Comorbidity – Median (IQR) | Drug classes | 6 (3, 10) | 10 (7, 14) | 11 (8, 15) | 11 (7, 15) | 12 (9, 17) |
| Smoking status – (%) | Never | 1,022 (49.7) | 92 (49.5) | 95 (51.6) | 100 (54.1) | 88 (47.6) |
| Past | 245 (11.9) | 23 (12.4) | 20 (10.9) | 22 (11.9) | 23 (12.4) | |
| Current | 456 (22.2) | 33 (17.7) | 31 (16.8) | 30 (16.2) | 34 (18.4) | |
| Unspecified | 333 (16.2) | 38 (20.4) | 38 (20.7) | 33 (17.8) | 40 (21.6) | |
| Screen detected – (%) | Organized B | 257 (12.5) | 15 (8.1) | 24 (13.0) | 21 (11.4) | 21 (11.4) |
| Opportunistic | 94 (4.6) | 7 (3.8) | 5 (2.7) | 8 (4.3) | 9 (4.9) | |
| Concomitant drugs – (%) C | Estrogen | 107 (5.2) | 6 (3.2) | 8 (4.3) | 9 (4.9) | 8 (4.3) |
| Estrogen/Progesterone | 164 (8.0) | 9 (4.8) | 7 (3.8) | 6 (3.2) | 10 (5.4) | |
| Statins | 307 (14.9) | 68 (36.6) | 93 (50.5) | 92 (50.5) | 112 (60.5) | |
| NSAID | 876 (42.6) | 101 (54.3) | 89 (48.4) | 94 (50.8) | 101 (54.6) | |
| Beta blocker | 315 (15.3) | 62 (33.3) | 68 (37.0) | 69 (37.3) | 85 (45.9) | |
| Anti-diabetic | 79 (3.8) | 17 (9.1) | 28 (15.2) | 23 (12.4) | 32 (17.3) | |
| - Biguanide | 46 (2.2) | 14 (7.5) | 18 (9.8) | 16 (8.6) | 23 (12.4) | |
| Bisphosphonate | 109 (5.3) | 10 (5.4) | 16 (8.7) | 23 (12.4) | 18 (9.7) | |
| Tumor details | ||||||
| Nodal status – (%) D | Negative | 1,020 (49.6) | 86 (46.2) | 99 (53.8) | 112 (60.5) | 107 (57.8) |
| Positive | 1,036 (50.4) | 100 (53.8) | 85 (46.2) | 73 (39.5) | 78 (42.2) | |
| Tumor size – (%) D | T1 | 848 (41.2) | 71 (38.2) | 84 (45.7) | 78 (42.2) | 62 (33.5) |
| T2 | 916 (44.6) | 87 (46.8) | 81 (44.0) | 84 (45.4) | 98 (53.0) | |
| T3 | 130 (6.3) | 9 (4.8) | 12 (6.5) | 12 (6.5) | 13 (7.0) | |
| T4 | 162 (7.9) | 19 (10.2) | 7 (3.8) | 11 (5.9) | 12 (6.5) | |
| Tumor stage – (%) D | I | 624 (30.4) | 51 (27.4) | 66 (35.9) | 60 (32.4) | 48 (25.9) |
| IIa / IIb | 626/490 (30.4/23.8) | 60/46 (32.3/24.7) | 61/36 (33.2/19.6) | 68/35 (36.8/18.9) | 80/31 (43.2/16.8) | |
| IIIa / IIIb-c | 130/186 (9.0/6.3) | 9/20 (4.8/10.8) | 13/8 (7.1/4.3) | 8/14 (4.3/7.6) | 10/16 (5.4/8.6) | |
| Tumor grade – (%) | Low | 207 (10.1) | 18 (9.7) | 18 (9.8) | 27 (14.6) | 17 (9.2) |
| Intermediate | 921 (44.8) | 83 (44.6) | 105 (57.1) | 84 (45.4) | 81 (43.8) | |
| High | 692 (33.7) | 59 (31.7) | 50 (27.2) | 51 (27.6) | 64 (34.6) | |
| Unspecified | 236 (11.5) | 26 (14.0) | 11 (6.0) | 23 (12.4) | 23 (12.4) | |
| Tumor morphology – (%) | Ductal | 1,462 (71.1) | 135 (72.6) | 122 (66.3) | 128 (69.2) | 131 (70.8) |
| Lobular | 270 (13.1) | 29 (15.6) | 26 (14.1) | 24 (13.0) | 21 (11.4) | |
| Other | 324 (16.3) | 22 (11.8) | 36 (19.6) | 33 (17.8) | 33 (17.8) | |
| ER – (%) | +ve/-ve/Unspecified | 1,384/378/294 (67.3/18.4/14.3) | 125/45/16 (67.2/24.2/8.6) | 130/24/30 (70.7/13.0/16.3) | 127/28/30 (68.6/15.1/16.2) | 131/29/25 (70.8/15.7/13.5) |
| PR – (%) | +ve/-ve/Unspecified | 918/497/641 (44.6/24.2/31.2) | 85/57/44 (45.7/30.6/23.7) | 79/38/67 (42.9/20.7/36.4) | 82/41/62 (44.3/22.2/33.5) | 75/51/59 (40.5/27.6/31.9) |
| HER2 – (%) | +ve/-ve/Unspecified | 227/919/910 (11.0/44.7/44.3) | 27/86/73 (14.5/46.2/39.2) | 21/73/80 (12.1/42.0/46.0) | 15/91/79 (8.1/49.2/42.7) | 27/89/69 (14.6/48.1/37.3) |
| Aspirin exposure (year prior to diagnosis) | ||||||
| Number of Rx dispensed | - - | 522 - | 1,587 - | 2,166 - | 2,353 - | |
| Rx doses – (%) | 75mg/300mg/Other | - - | 379/107/36 (72.6/20.5/6.9) | 1,247/264/76 (78.6/16.6/4.8) | 1,914/186/66 (88.4/8.6/3.0) | 2,137/160/56 (90.8/6.8/2.4) |
| Dosing intensity-Median(IQR)A | % | - - | 16.4 (7.7, 26.6) | 57.0 (47.0, 71.6) | 90.4 (86.8, 94.2) | 100.0 (99.2, 100.0) |
| Aspirin exposure (diagnosis to end of follow-up) | ||||||
| Dosing intensity-Median(IQR) E | % | 0.0 (0.0, 0.0) F | 23.6 (0.0, 75.3) | 74.2 (44.5, 90.9) | 89.4 (65.0, 96.2) | 97.2 (88.5, 100.0) |
IQR: Inter-Quartile Range. ER: Estrogen Receptor. PR: Progesterone Receptor. HER2: Human Epidermal Growth Factor Receptor 2. Rx: Prescription. NSAID: Non-Steroidal Anti-Inflammatory Drug.
Dosing intensity calculated as the number of days with a supply of aspirin available in year prior to diagnosis, divided by 365.
Identified from linked BreastCheck national screening program records.
In the year prior to breast cancer diagnosis.
AJCC Cancer Staging Manual 6th Edition. Springer, 2002.
Post-diagnostic dosing intensity calculated as number of days with supply of aspirin available from diagnosis to end of follow-up, divided by the number of days from diagnosis to end of follow-up.
400 women initiated de-novo aspirin use between their breast cancer diagnosis and the end of follow-up.
Aspirin & Nodal Status
RRs for associations between aspirin use and lymph node-positive breast cancer are presented in Table 2. The proportion of women with node-positive breast cancer in the aspirin non-user and user groups was 50.4% and 45.4%, respectively. In analyses adjusted for tumor size, tumor grade, screen detection, age and comorbidity score, women taking aspirin were statistically significantly less likely to present with lymph node-positive breast cancer than women not taking aspirin (RR=0.89, 95%CI 0.81, 0.97). This translates to a 6% (95%CI 2%, 10%) lower absolute risk of having positive lymph nodes at breast cancer diagnosis in aspirin users compared to non-users.
TABLE 2.
UNIVARIATE AND MULTIVARIATE RELATIVE RISKS FOR ASPIRIN USE AND LYMPH NODE-POSITIVE BREAST CANCER AT DIAGNOSIS
| Risk-ratios for node-positive (N+ve) versus node-negative (N-ve) |
||||
|---|---|---|---|---|
| Aspirin Use | N+ve (%) | N-ve (%) | Univariate RR (95%CI) | Multivariate RR (95%CI) A |
| Non-user in year prior to diagnosis | 1,036 (50.4) | 1,020 (49.6) | Ref - | Ref - |
| Aspirin user in year prior to diagnosis | 336 (45.4) | 404 (54.6) | 0.90 (0.82, 0.99) | 0.89 (0.81, 0.97) |
| Aspirin dosing intensity | ||||
| Dosing intensity 1% - 37% B,C | 100 (53.8) | 86 (46.2) | 1.07 (0.93, 1.23) | 0.98 (0.87, 1.10) |
| Dosing intensity 38% - 79% | 85 (46.2) | 99 (53.8) | 0.92 (0.92, 0.78) | 0.96 (0.83, 1.11) |
| Dosing intensity 80% - 97% | 73 (39.5) | 112 (60.5) | 0.78 (0.78, 0.65) | 0.77 (0.65, 0.91) |
| Dosing intensity 98% - 100% | 78 (42.2) | 107 (57.8) | 0.84 (0.70, 0.99) | 0.81 (0.68, 0.96)** |
| Aspirin dose | ||||
| Low Dose < 150mg E | 288 (45.6) | 344 (54.4) | 0.90 (0.82, 0.99) | 0.90 (0.82, 0.98) |
| High Dose ≥ 150mg F | 48 (44.4) | 60 (55.6) | 0.88 (0.71, 1.09) | 0.82 (0.67, 1.00)* |
| Aspirin dosing intensity & dose | ||||
| Low dosing intensity 1% - 79% B,D | ||||
| Low dose < 150mg E | 152 (49.8) | 153 (50.2) | 0.99 (0.88, 1.12) | 0.99 (0.90, 1.10) |
| High dose ≥ 150mg F | 33 (50.8) | 32 (49.2) | 1.01 (0.79, 1.28) | 0.90 (0.72, 1.12) |
| High dosing intensity 80% - 100% | ||||
| Low dose < 150mg | 136 (41.6) | 191 (58.4) | 0.83 (0.72, 0.95) | 0.80 (0.71, 0.92) |
| High dose ≥ 150mg | 15 (34.9) | 28 (65.1) | 0.69 (0.46, 1.04) | 0.67 (0.45, 0.99)** |
| Aspirin duration G | ||||
| Non-user in 3 years prior to diagnosis | 543 (49.5) | 554 (50.5) | Ref - | Ref - |
| Aspirin user in 3 years prior to diagnosis | ||||
| Start aspirin <1.5 years prior to diagnosis | 61 (50.8) | 59 (49.2) | 1.03 (0.85, 1.24) | 1.01 (0.86, 1.18) |
| Start aspirin 1.5-3.0 years prior to diagnosis | 89 (47.1) | 100 (52.9) | 0.95 (0.81, 1.12) | 0.96 (0.83, 1.11) |
| Start aspirin ≥3.0 years prior to diagnosis | 100 (46.1) | 117 (53.9) | 0.93 (0.80, 1.09) | 0.89 (0.77, 1.03) |
| Aspirin dosing intensity & duration G | ||||
| Low dosing intensity 1%-82% D,H | ||||
| Start aspirin <1.5 years prior to diagnosis | 28 (47.6) | 31 (52.5) | 0.96 (0.73, 1.26) | 1.01 (0.80, 1.28) |
| Start aspirin 1.5-3.0 years prior to diagnosis | 60 (50.4) | 59 (48.2) | 1.02 (0.84, 1.23) | 1.08 (0.91, 1.29) |
| Start aspirin ≥3.0 years prior to diagnosis | 44 (51.8) | 41 (48.2) | 1.05 (0.84, 1.30) | 0.97 (0.80, 1.16) |
| High dosing intensity 83%-100% | ||||
| Start aspirin <1.5 years prior to diagnosis | 33 (54.1) | 28 (45.9) | 1.09 (0.86, 1.39) | 1.01 (0.83, 1.22) |
| Start aspirin 1.5-3.0 years prior to diagnosis | 29 (41.4) | 41 (58.6) | 0.84 (0.63, 1.11) | 0.82 (0.64, 1.06) |
| Start aspirin ≥3.0 years prior to diagnosis | 56 (42.4) | 76 (57.6) | 0.86 (0.70, 1.05) | 0.83 (0.68, 1.01) |
P-trend <0.01
P-trend <0.001; Ref: Referent Group. RR: Relative Risk. CI: Confidence Interval. N+ve: Node-Positive. N-ve: Node-Negative.
Adjusted for age (years, continuous), tumor size (T1, T2, T3, T4), tumor grade (low, intermediate, high, unspecified), comorbidity score (number of medication classes, continuous) and screen-detected tumor (organized screening, opportunistic screening, not screen detected).
Dosing intensity calculated as the number of days with supply of aspirin available in the year prior to diagnosis, divided by 365.
Dosing intensity by quartiles.
Dosing intensity by median.
All prescriptions in the year prior to diagnosis were for doses of < 150mg. The 150mg cutpoint represents twice the standard low-dose aspirin strength (75mg) used in Ireland.
At least one prescription in the year prior to diagnosis was for a dose of ≥ 150mg.
Women with at least three years of continuous GMS eligibility prior to diagnosis were included in this exposure response analysis.
Dosing intensity calculated as number of days with supply of aspirin available from the first aspirin exposure in the three years prior to diagnosis up to diagnosis, divided by the number of days from the first aspirin exposure in the three years prior to diagnosis up to diagnosis.
The risk of presenting with lymph node involvement at diagnosis decreased with increasing aspirin dosing intensity and dose (Table 2). A 19% relative reduction in node positive breast cancer was observed among women in the highest quartile of aspirin dosing intensity when compared to non-users (RR=0.81, 95%CI 0.68, 0.96). A greater reduction in node positive disease was also observed among women taking higher versus lower doses of aspirin (Table 2).
In sensitivity analyses, associations between aspirin use and lymph node metastasis were the same in women with (RD −0.09, 95%CI −0.21, 0.04) and without (RD −0.11, 95%CI −0.17, −0.05) screen-detected breast cancers (Appendix, P-interaction=0.70). Similar results were obtained in analyses matched by propensity-score (see Appendix). The results were also unchanged in sensitivity analyses classifying women with only clinical assessment of nodal status as node positive (data not shown) and analyses of complete cases (data not shown).
Aspirin & Nodal Status - Effect Modification
The associations between aspirin use and a lower risk of lymph node metastasis were statistically significantly stronger in women with larger tumors (Table 3; P-interaction=0.04); and PR-negative tumors (Table 3; P-interaction<0.001). Associations were also stronger in women with ER-negative tumors (P-interaction=0.056, data not shown); HER2-positive tumors (P-interaction=0.17, data not shown); and high grade tumors (P-interaction=0.24, data not shown), although these interactions did not reach statistical significance. There was no evidence of effect modification by tumor morphology (P-interaction=0.62, data not shown).
TABLE 3.
ASPIRIN USE & LYMPH NODE-POSITIVE BREAST CANCER - EFFECT MODIFICATION BY TUMOR CHARACTERISTICS AT DIAGNOSIS
| Aspirin use in the year prior to diagnosis |
||||||
|---|---|---|---|---|---|---|
| Tumor size | Non-user A |
Low dosing intensity (1% - 79%) A,B |
High dosing intensity (80% - 100%) A,B |
Low dosing intensity v non-user within strata C |
High dosing intensity v non-user within strata C |
|
| T1 | N+ve/N-ve | 266/582 | 55/100 | 40/100 | ||
| RD (95%CI) | Ref - | 0.02 (−0.06, 0.11) | −0.04 (−0.13, 0.04) | 0.02 (−0.06, 0.11) p = 0.57 | −0.04 (−0.13, 0.04) p = 0.29 | |
| T2-4 | N+ve/N-ve | 770/438 | 130/85 | 111/119 | ||
| RD (95%CI) | 0.24 (0.20, 0.29) | 0.21 (0.13, 0.28) | 0.09 (0.01, 0.16) | −0.04 (−0.11, 0.04) p = 0.29 | −0.16 (−0.23, −0.09) p < 0.001 | |
| Aspirin*Tumor size | Additive scale: IC (95%CI) | T2-4 v T1 | −0.06 (−0.17, 0.04) p = 0.26 | −0.11 (−0.22, −0.01) p = 0.036 | ||
| Adjusted for age, tumor size, tumor grade, comorbidity, screen detection | ||||||
| Aspirin use in the year prior to diagnosis |
||||||
|---|---|---|---|---|---|---|
| PR status | Non-user A |
Low dosing intensity (1% - 79%) A,B |
High dosing intensity (80% - 100%) A,B |
Low dosing intensity v non-user within strata C |
High dosing intensity v non-user within strata C |
|
| PR Positive | N+ve/N-ve | 456/462 | 80/84 | 73/84 | ||
| RD (95%CI) | Ref - | 0.00 (−0.08, 0.08) | −0.05 (−0.12, 0.03) | 0.00 (−0.08, 0.08) p = 0.93 | −0.05 (−0.12, 0.03) p = 0.24 | |
| PR Negative | N+ve/N-ve | 279/218 | 55/40 | 32/60 | ||
| RD (95%CI) | 0.06 (0.01, 0.11) | 0.04 (−0.05, 0.14) | −0.19 (−0.28, −0.10) | −0.01 (−0.12, 0.09) p = 0.78 | −0.25 (−0.35, −0.15) p < 0.001 | |
| Aspirin*PR status | Additive scale: IC (95%CI) | PR negative v positive | −0.01 (−0.14, 0.12) p = 0.86 | −0.21 (−0.33, −0.08) p < 0.001 | ||
| Adjusted for age, tumor size, tumor grade, comorbidity, screen detection | ||||||
N+ve: Node-Positive. N-ve: Node-Negative. RD: Risk Difference. IC: Interaction Contrast. CI: Confidence Interval
Separate and joint effects of aspirin exposure and the effect modifier using a single reference category.
Dosing intensity by median. Dosing intensity calculated as number of days with supply of aspirin available in year prior to diagnosis, divided by 365.
Association between aspirin exposure and nodal status within strata of the effect modifier.
Aspirin & Mortality
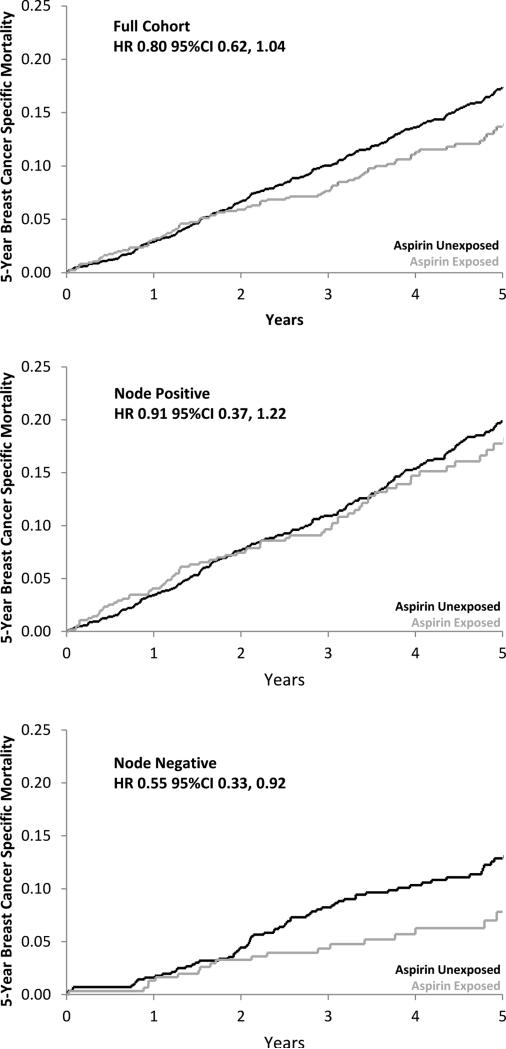
Overall, pre-diagnostic aspirin use was associated with a non-significant, 20% lower risk of breast cancer-specific mortality at 5 years (Table 4; Figure 2). In analyses of effect modification by nodal status, pre-diagnosis aspirin use was associated with a statistically significant 45% lower risk of 5- year breast cancer-specific mortality among women with node-negative tumors, and no reduction in mortality among women with node-positive tumors. The interaction between aspirin exposure and nodal status did not reach statistical significance P-interaction=0.087; Table 5; Figure 2). These results did not change after adjustment for post-diagnostic aspirin use, or in sensitivity analyses for misclassification of cause of death (data not shown). Post-diagnosis aspirin dosing intensity was similar for women with node-negative (84%) and node-positive tumors (78%). We observed no association between post-diagnostic aspirin use and breast cancer-specific mortality (Table 4). These results remained unchanged in sensitivity analyses varying the lag time for post-diagnostic aspirin use from one to three years (data not shown).
TABLE 4.
MULTIVARIATE HAZARD RATIOS FOR PRE- & POST-DIAGNOSTIC ASPIRIN USE & 5 YEAR ALL-CAUSE OR BREAST CANCER-SPECIFIC MORTALITY.
| 5-Year all-cause mortality |
5-Year breast cancer-specific mortality |
||||
|---|---|---|---|---|---|
| Aspirin use | Person years | Deaths | Multivariate HR (95%CI) | Deaths | Multivariate HR (95%CI) |
| Pre-diagnostic aspirin use A | |||||
| Non-user in year prior to diagnosis | 7,853 | 401 | Ref - | 274 | Ref - |
| Aspirin user in year prior to diagnosis | 2,720 | 148 | 0.81 (0.66, 0.99) | 87 | 0.80 (0.62, 1.04) |
| Post-diagnostic aspirin use B, C | |||||
| Non-user post diagnosis | 9096 | 459 | Ref - | 311 | Ref - |
| Aspirin user post diagnosis | 1477 | 90 | 1.11 (0.83, 1.50) | 50 | 0.99 (0.68, 1.45) |
Ref: Referent Group. HR: Hazard Ratio. CI: Confidence Interval.
Adjusted for age (years, continuous), tumor stage (I, IIa, IIb, IIIa, IIIb-c) tumor grade (low, intermediate, high, unspecified), estrogen receptor status (positive, negative unspecified), progesterone receptor status (positive, negative, unspecified), HER2 status (positive, negative, unspecified) and comorbidity score (number of medication classes, continuous)
Post diagnostic aspirin use defined as exposed, unexposed, time varying, lagged by 2 years.
Adjusted for pre-diagnostic aspirin use, age (years, continuous), tumor stage (I, IIa, IIb, IIIa, IIIb-c), tumor grade (low, intermediate, high, unspecified), estrogen receptor status (positive, negative unspecified), progesterone receptor status (positive, negative, unspecified), HER2 status (positive, negative, unspecified) and comorbidity score (number of medication classes, continuous)
Figure 2.
Adjusted cumulative probability of 5-year breast cancer–specific mortality for aspirin users and nonusers in the full cohort and by lymph node status at diagnosis (positive, negative), adjusted for age, tumor stage, tumor grade, ER, PR, HER2, and comorbidity.
TABLE 5.
PRE-DIAGNOSTIC ASPIRIN USE & 5-YEAR BREAST CANCER-SPECIFIC MORTALITY - EFFECT MODIFICATION BY LYMPH NODE STATUS AT DIAGNOSIS
| Aspirin use in the year prior to diagnosis |
||||
|---|---|---|---|---|
| Nodal status | Non-user A |
User A |
User v non-user within strata B |
|
| Positive | Person years | 3750 | 1141 | |
| Censored/Death | 827/209 | 268/68 | ||
| HR (95%CI) | Ref - | 0.91 (0.37, 1.22) | 0.91 (0.37, 1.22) p= 0.54 | |
| Negative | Person years | 4103 | 1579 | |
| Censored/Death | 955/65 | 385/19 | ||
| HR (95%CI) | 0.60 (0.42,0.86) | 0.33 (0.20,0.55) | 0.55 (0.33,0.92) p = 0.024 | |
| Aspirin*Nodal status | Multiplicative scale: rHR (95%CI) | Negative v Positive | 0.60 (0.34, 1.08) p = 0.087 | |
| Adjusted for age, comorbidity, tumor stage, tumor grade, ER, PR, HER2 | ||||
HR: Hazard Ratio. rHR: Ratio of Hazard Ratios. CI: Confidence Interval.
The separate and joint effects of aspirin exposure and nodal status in comparison to a single reference category.
The effects of aspirin exposure within strata of nodal status.
Discussion
In this study of 2,796 women with stage I-III breast cancer, women taking aspirin in the years immediately prior to their breast cancer diagnosis were statistically significantly less likely to present with a lymph node-positive breast cancer than non-users. The association was strongest among regular aspirin users and women taking higher aspirin doses. These results are not explained by differences in screening-mammography use or breast cancer surveillance between aspirin users and non-users for the following reasons: (i) there was no difference in the proportion of screen detected tumors between aspirin users and non-users; (ii) there was no difference in the distribution of tumor size at presentation between aspirin users and non-users; (iii) associations between aspirin use and nodal-status were unchanged in propensity-score matched analyses that incorporated co-medication use and screening; and (iv) we observed the same association between aspirin use and a reduced risk of nodal involvement in women with and without screen-detected breast cancers.
We identified two prior studies which have examined associations between pre-diagnostic aspirin use and breast cancer nodal status.(45,46) The first of these found no association between aspirin exposure and nodal status; although nodal status was missing in >20% of patients and analyses were not adjusted for relevant confounders such as tumor size or screening.(45) The second study examined associations between anti-coagulant use (aspirin, clopidogrel, dipyridamole) at the time of breast cancer diagnosis and the risk of lymph node metastasis.(46) Analyses were adjusted for some relevant confounders (tumor size) but the exposed group included patients using a variety of anticoagulants and it is unclear what proportion of these were taking aspirin. The authors reported a non-significant reduction in the risk of presenting with a lymph node positive tumor (RR=0.94, 95% CI: 0.87-1.03) and, similar to our study, evidence of effect modification by tumor size. Information on nodal status has also been reported in two prior observational studies of NSAID exposure and breast cancer mortality from which we were able to calculate a pooled univariate estimate of the association between regular NSAID exposure (≥3 tablets/week) and the risk of presenting with lymph node positive disease at diagnosis, RR=0.83 (95%CI 0.73-0.95). This is comparable to the results presented here for similar exposure intensity.
Our study did not examine associations between aspirin use and breast cancer incidence and therefore we cannot quantify the contribution that any reduction in breast cancer incidence due to aspirin exposure may have had on our results. However, studies that have evaluated aspirin use and breast cancer incidence have reported mixed results with the larger prospective cohorts and a single randomized trial finding no overall reduction in the risk of developing breast cancer.(47–50) A prior study of associations between NSAID exposure and breast cancer incidence, did stratify analyses by nodal status at diagnosis.(51) The authors reported no difference in the incidence of node-positive or node negative breast cancers. Three further studies have stratified their analyses by SEER summary stage classification.(52–54) Differences in the incidence of localized versus regional/distant disease were only observed in one of these studies (localized RR 0.8, 95%CI 0.63, 1.03; regional/distant RR 0.5, 95%CI 0.29, 0.88).(54) The timing of exposure assessment in these studies of breast cancer incidence did not capture exposure close to the time of diagnosis which is an important time window based on prior studies.(1,17) Also none of these studies have adjusted for potential confounders that may influence lymph node status such as tumor size and screen detection.
In analyses of effect modification we observed that recent pre-diagnostic aspirin use was associated with a greater reduction in the risk of presenting with node positive disease in women with breast tumor characteristics previously associated with COX-2 expression. This suggests that inhibition of lymphatic involvement by aspirin may be mediated at least in part through a COX-2 dependent pathway. Our findings are consistent with observations from in vivo breast cancer models which have shown that COX-2 inhibition suppresses the development of lymph node metastasis through the regulation of VEGF-C/-D mediated lymphatic dysregulation.(4,5) VEGF-C/-D overexpression has been shown to induce hyperplasia in peri-tumoral lymphatic vessels, increasing lymphatic flow and enhancing the rate of tumor cell delivery to lymph nodes, leading to increased lymph node metastasis.(5,17) Inhibition of lymphatic dysregulation represents one possible mechanism of action for aspirin in breast cancer, although a number of other mechanisms have been proposed, including the inhibition of platelet function and reductions in serum estrogen concentrations.(55,56) It is not clear whether regulation of lymphangiogenesis can restore dysregulated lymphatics in established tumors or inhibit the development of lymphatic metastases from tumor cells that have already seeded to the lymph nodes.(5,17) This may explain why associations with reduced lymph node metastasis were only observed in women with regular aspirin use for a sustained period prior to diagnosis.
Survival analyses were undertaken to examine the potential effect that the inhibition of lymphatic metastasis by pre-diagnostic aspirin use may have on associations between aspirin use and breast cancer mortality. We hypothesized that, in women using aspirin prior to their breast cancer diagnosis, the inhibition of lymph node involvement by aspirin would indicate women with aspirin responsive tumors and predict a subsequent survival benefit from aspirin use. Some evidence for this was suggested in analyses stratified by nodal status at diagnosis; where, pre-diagnostic aspirin use was associated with a statistically significant reduced risk of 5-year breast cancer-specific mortality among women with lymph node-negative disease at diagnosis, but not those with lymph node-positive disease, although tests for effect modification were not statistically significant. The length of mortality follow-up for women in our study was 5 years and longer follow-up will be needed to generalize these results beyond this time. While this study is the first to directly assess the modification of associations between pre-diagnostic aspirin use and breast cancer mortality by nodal status; the results from a previous study do provide some support for our observations.(3) Blair et al have reported a statistically significant association between NSAID exposure and mortality in women with localized breast cancer (100% node-negative; HR=0.37, 95%CI 0.16, 0.86) but not women with non-localized breast cancer (1.6% node-negative; HR=0.67, 95%CI 0.31, 1.43). Our results are also consistent with the findings from a recent meta-analyses of cardiovascular trials.(1) In this study, pre-diagnostic aspirin use was associated with a statistically significant reduced risk of presenting with distant metastatic disease in a range of cancers. Further, a reduction in mortality due to cancer was primarily observed among patients with local disease at diagnosis. In contrast with some prior studies,(2,3,57) we observed no association between post-diagnostic aspirin use and breast cancer specific mortality; however, the length of post diagnostic follow for women in our study was shorter than these prior studies. There are other possible reasons for this difference, unlike prior studies we adjusted our analyses for pre-diagnostic aspirin use, we also identified post-diagnostic aspirin exposure using objective prescription-refill data rather than patient self-report which can be less precise.(43)
The strengths of this study include its prospectively collected exposure and outcome data and the availability of high quality patient level information on mammographic screening. In addition, the prescription-only status of low-dose aspirin in Ireland allowed the objective assessment of detailed cumulative aspirin exposure histories for all women. The study also has some limitations. It is possible that the clinically relevant window of exposure for inhibiting lymphatic metastasis extends further than 3 years prior to diagnosis and that distant pre-diagnostic exposure – in patients who discontinued aspirin prior to diagnosis – is also of clinical relevance; future studies should examine longer durations of pre-diagnostic exposure. In addition, as aspirin use was based upon prescriptions dispensed, non-compliance with treatment will have resulted in exposure misclassification. The misclassification of aspirin exposures due to this would most likely be non-differential with respect to lymph node status and will usually, but not always, bias results towards the null.(58) Information on lifestyle factors that may affect nodal involvement and disease progression, such as obesity, alcohol use and Vitamin D was not available. The results from analyses of effect modification by PR and HER2 status should also be interpreted with caution due to the number of women with unspecified receptor status. In survival analyses, post-diagnostic exposures were lagged to reduce the possibility that changes in prognosis after diagnosis influenced post-diagnostic aspirin use. While this is an accepted approach in analyses of post-diagnostic exposures,(43) it may not fully eliminate time-dependent confounding.
Together, our findings provide insight into aspirin's potential mechanism of action in breast cancer progression. They indicate that recent pre-diagnostic aspirin exposure inhibits the development of lymph node metastases and, in women using aspirin prior to a breast cancer diagnosis, negative nodal status may predict a subsequent survival benefit from aspirin use at 5 years. studies with longer follow-up are required to confirm this. These results provide a plausible explanation for the reduction in breast cancer mortality seen in prior observational studies of aspirin use.
Supplementary Material
Acknowledgements
We would like to thank the National Cancer Registry Ireland and the Irish Health Services Executive Primary Care Reimbursements Services for providing access to the data upon which this study was based. In particular, we are grateful to the Data Team at the National Cancer Registry Ireland for linking the datasets and Dr Sandra Deady for preparing these for analysis. The interpretation and reporting of these data are the responsibility of the authors and should in no way be seen as the official policy or interpretation of the National Cancer Registry Ireland or the Irish Health Services Executive Primary Care Reimbursements Services.
FUNDING
TIB is supported by the Health Research Board Ireland (HRA-2009-221, ICE-2011-9) and the Irish Cancer Society (CCRC13GAL). EMF is supported by the Irish Cancer Society (CRS10FLA). KV is supported in part by the Breast Cancer Research Foundation. This study is based upon works supported by the Irish Cancer Society Collaborative Cancer Research Centre BREAST-PREDICT (CCRC13GAL). The Health Research Board Ireland, the Irish Cancer Society and the Breast Cancer Research Foundation had no role in the study design; collection, analysis, and interpretation of data; writing of the report; or the decision to submit for publication.
Footnotes
Conflict of Interest
The authors have no conflicts of interest to declare
REFERENCES
- 1.Rothwell PM, Wilson M, Price JF, Belch JFF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012;379:1591–601. doi: 10.1016/S0140-6736(12)60209-8. [DOI] [PubMed] [Google Scholar]
- 2.Holmes MD, Chen WY, Li L, Hertzmark E, Spiegelman D, Hankinson SE. Aspirin intake and survival after breast cancer. J Clin Oncol. 2010;28:1467–72. doi: 10.1200/JCO.2009.22.7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blair CK, Sweeney C, Anderson KE, Folsom AR. NSAID use and survival after breast cancer diagnosis in post-menopausal women. Breast Cancer Res Treat. 2007;101:191–7. doi: 10.1007/s10549-006-9277-x. [DOI] [PubMed] [Google Scholar]
- 4.Xin X, Majumder M, Girish GV, et al. Targeting COX-2 and EP4 to control tumor growth, angiogenesis, lymphangiogenesis and metastasis to the lungs and lymph nodes in a breast cancer model. Lab Invest, Advance Online Publication. 2012 doi: 10.1038/labinvest.2012.90. doi: 10.1038/labinvest.2012.90. [DOI] [PubMed] [Google Scholar]
- 5.Karnezis T, Shayan R, Caesar C, Roufail S, Harris NC, Ardipradja K, et al. VEGF-D promotes tumor metastasis by regulating prostaglandins produced by the collecting lymphatic endothelium. Cancer Cell. 2012;21:181–95. doi: 10.1016/j.ccr.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 6.Ristimäki A, Sivula A, Lundin J, Lundin M, Salminen T, Haglund C, et al. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002;62:632–5. [PubMed] [Google Scholar]
- 7.Holmes MD, Chen WY, Schnitt SJ, Collins L, Colditz GA, Hankinson SE, et al. COX-2 expression predicts worse breast cancer prognosis and does not modify the association with aspirin. Breast Cancer Res Treat. 2011;130:657–62. doi: 10.1007/s10549-011-1651-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population-based study. J Clin Oncol. 2011;29:2635–44. doi: 10.1200/JCO.2010.33.5422. [DOI] [PubMed] [Google Scholar]
- 9. [2012 Oct 17];BreastCheck The National Breast Screening Programme History [Internet] Available from: http://www.breastcheck.ie/content/history.
- 10.Bastiaannet E, Sampieri K, Dekkers OM, de Craen AJM, van Herk-Sukel MPP, Lemmens V, et al. Use of aspirin postdiagnosis improves survival for colon cancer patients. Br J Cancer. 2012;106:1564–70. doi: 10.1038/bjc.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bulliard J-L, Ducros C, Jemelin C, Arzel B, Fioretta G, Levi F. Effectiveness of organised versus opportunistic mammography screening. Ann Oncol. 2009;20:1199–202. doi: 10.1093/annonc/mdn770. [DOI] [PubMed] [Google Scholar]
- 12.NCRI [2013 Nov 23];Data Quality and Completeness at the Irish National Cancer Registry [Internet] 2012 Available from: http://www.ncri.ie/pubs/pubfiles/CompletenessQuality.pdf.
- 13.Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, et al. AJCC Cancer Staging Manual. 6th ed. Springer; 2002. [Google Scholar]
- 14.Schneeweiss S, Patrick AR, Stürmer T, Brookhart MA, Avorn J, Maclure M, et al. Increasing levels of restriction in pharmacoepidemiologic database studies of elderly and comparison with randomized trial results. Med Care. 2007;45:S131–142. doi: 10.1097/MLR.0b013e318070c08e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hillner BE, Penberthy L, Desch CE, McDonald MK, Smith TJ, Retchin SM. Variation in staging and treatment of local and regional breast cancer in the elderly. Breast Cancer Res Treat. 1996;40:75–86. doi: 10.1007/BF01806004. [DOI] [PubMed] [Google Scholar]
- 16.WHO . ATC classification index with DDDs, 2011. WHO Collaborating Centre for Drug Statistics Methodology; Obslo: 2010. [Google Scholar]
- 17.Hoshida T, Isaka N, Hagendoorn J, di Tomaso E, Chen Y-L, Pytowski B, et al. Imaging steps of lymphatic metastasis reveals that vascular endothelial growth factor-C increases metastasis by increasing delivery of cancer cells to lymph nodes: therapeutic implications. Cancer Res. 2006;66:8065–75. doi: 10.1158/0008-5472.CAN-06-1392. [DOI] [PubMed] [Google Scholar]
- 18.Peterson AM, Nau DP, Cramer JA, Benner J, Gwadry-Sridhar F, Nichol M. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007;10:3–12. doi: 10.1111/j.1524-4733.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 19.Schneeweiss S, Seeger JD, Maclure M, Wang PS, Avorn J, Glynn RJ. Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol. 2001;154:854–64. doi: 10.1093/aje/154.9.854. [DOI] [PubMed] [Google Scholar]
- 20.Skov T, Deddens J, Petersen MR, Endahl L. Prevalence proportion ratios: estimation and hypothesis testing. Int J Epidemiol. 1998;27:91–5. doi: 10.1093/ije/27.1.91. [DOI] [PubMed] [Google Scholar]
- 21.Wacholder S. Binomial regression in GLIM: estimating risk ratios and risk differences. Am J Epidemiol. 1986;123:174–84. doi: 10.1093/oxfordjournals.aje.a114212. [DOI] [PubMed] [Google Scholar]
- 22.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 23.Greenland S. Interpretation and choice of effect measures in epidemiologic analyses. Am J Epidemiol. 1987;125:761–8. doi: 10.1093/oxfordjournals.aje.a114593. [DOI] [PubMed] [Google Scholar]
- 24.Bevilacqua J, Cody H, 3rd, MacDonald KA, Tan LK, Borgen PI, Van Zee KJ. A prospective validated model for predicting axillary node metastases based on 2,000 sentinel node procedures: the role of tumour location [corrected]. Eur J Surg Oncol. 2002;28:490–500. doi: 10.1053/ejso.2002.1268. [DOI] [PubMed] [Google Scholar]
- 25.Gann PH, Colilla SA, Gapstur SM, Winchester DJ, Winchester DP. Factors associated with axillary lymph node metastasis from breast carcinoma: descriptive and predictive analyses. Cancer. 1999;86:1511–9. doi: 10.1002/(sici)1097-0142(19991015)86:8<1511::aid-cncr18>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 26.Reyal F, Rouzier R, Depont-Hazelzet B, Bollet MA, Pierga J-Y, Alran S, et al. The molecular subtype classification is a determinant of sentinel node positivity in early breast carcinoma. PLoS ONE. 2011;6:e20297. doi: 10.1371/journal.pone.0020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daniell HW. Increased lymph node metastases at mastectomy for breast cancer associated with host obesity, cigarette smoking, age, and large tumor size. Cancer. 1988;62:429–35. doi: 10.1002/1097-0142(19880715)62:2<429::aid-cncr2820620230>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 28.Bucchi L, Barchielli A, Ravaioli A, Federico M, De Lisi V, Ferretti S, et al. Screen-detected vs clinical breast cancer: the advantage in the relative risk of lymph node metastases decreases with increasing tumour size. Br J Cancer. 2005;92:156–61. doi: 10.1038/sj.bjc.6602289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V, et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70:7042–52. doi: 10.1158/0008-5472.CAN-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hwang YP, Jeong HG. Metformin blocks migration and invasion of tumour cells by inhibition of matrix metalloproteinase-9 activation through a calcium and protein kinase Calpha-dependent pathway: phorbol-12-myristate-13-acetate-induced/extracellular signal-regulated kinase/activator protein-1. Br J Pharmacol. 2010;160:1195–211. doi: 10.1111/j.1476-5381.2010.00762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boissier S, Ferreras M, Peyruchaud O, Magnetto S, Ebetino FH, Colombel M, et al. Bisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastases. Cancer Res. 2000;60:2949–54. [PubMed] [Google Scholar]
- 32.Denoyelle C, Vasse M, Körner M, Mishal Z, Ganné F, Vannier JP, et al. Cerivastatin, an inhibitor of HMG-CoA reductase, inhibits the signaling pathways involved in the invasiveness and metastatic properties of highly invasive breast cancer cell lines: an in vitro study. Carcinogenesis. 2001;22:1139–48. doi: 10.1093/carcin/22.8.1139. [DOI] [PubMed] [Google Scholar]
- 33.Connolly EM, Harmey JH, O'Grady T, Foley D, Roche-Nagle G, Kay E, et al. Cyclo-oxygenase inhibition reduces tumour growth and metastasis in an orthotopic model of breast cancer. Br J Cancer. 2002;87:231–7. doi: 10.1038/sj.bjc.6600462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antoine C, Liebens F, Carly B, Pastijn A, Rozenberg S. Influence of HRT on prognostic factors for breast cancer: a systematic review after the Women's Health Initiative trial. Hum Reprod. 2004;19:741–56. doi: 10.1093/humrep/deh112. [DOI] [PubMed] [Google Scholar]
- 35.Wolf I, Sadetzki S, Gluck I, Oberman B, Ben-David M, Papa MZ, et al. Association between diabetes mellitus and adverse characteristics of breast cancer at presentation. Eur J Cancer. 2006;42:1077–82. doi: 10.1016/j.ejca.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 36.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138:923–36. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 37.Rothwell PM, Price JF, Fowkes FGR, Zanchetti A, Roncaglioni MC, Tognoni G, et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379:1602–12. doi: 10.1016/S0140-6736(11)61720-0. [DOI] [PubMed] [Google Scholar]
- 38.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. Third. Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 39.Botto LD, Khoury MJ. Commentary: facing the challenge of gene-environment interaction: the two-by-four table and beyond. Am J Epidemiol. 2001;153:1016–20. doi: 10.1093/aje/153.10.1016. [DOI] [PubMed] [Google Scholar]
- 40.Knol MJ, VanderWeele TJ. Recommendations for presenting analyses of effect modification and interaction. Int J Epidemiol. 2012;41:514–20. doi: 10.1093/ije/dyr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Denkert C, Winzer K-J, Müller B-M, Weichert W, Pest S, Köbel M, et al. Elevated expression of cyclooxygenase-2 is a negative prognostic factor for disease free survival and overall survival in patients with breast carcinoma. Cancer. 2003;97:2978–87. doi: 10.1002/cncr.11437. [DOI] [PubMed] [Google Scholar]
- 42.Wülfing P, Diallo R, Müller C, Wülfing C, Poremba C, Heinecke A, et al. Analysis of cyclooxygenase-2 expression in human breast cancer: high throughput tissue microarray analysis. J Cancer Res Clin Oncol. 2003;129:375–82. doi: 10.1007/s00432-003-0459-1. [DOI] [PubMed] [Google Scholar]
- 43.Chubak J, Boudreau DM, Wirtz HS, McKnight B, Weiss NS. Threats to Validity of Nonrandomized Studies of Postdiagnosis Exposures on Cancer Recurrence and Survival. J Natl Cancer Inst. 2013 doi: 10.1093/jnci/djt211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X, Loberiza FR, Klein JP, Zhang M-J. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007;88:95–101. doi: 10.1016/j.cmpb.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 45.Jonsson F, Yin L, Lundholm C, Smedby KE, Czene K, Pawitan Y. Low-dose aspirin use and cancer characteristics: a population-based cohort study. Br J Cancer. 2013 doi: 10.1038/bjc.2013.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ljung R, Sennerstam R, Mattsson F, Auer G, Lagergren J. Anticoagulant medication at time of needle biopsy for breast cancer in relation to risk of lymph node metastasis. Int J Cancer. 2013 doi: 10.1002/ijc.28671. [DOI] [PubMed] [Google Scholar]
- 47.Zhang X, Smith-Warner SA, Collins LC, Rosner B, Willett WC, Hankinson SE. Use of aspirin, other nonsteroidal anti-inflammatory drugs, and acetaminophen and postmenopausal breast cancer incidence. J Clin Oncol. 2012;30:3468–77. doi: 10.1200/JCO.2012.42.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cook NR, Lee I-M, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Low-dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294:47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 49.Cook NR, Lee I-M, Zhang SM, Moorthy MV, Buring JE. Alternate-Day, Low-Dose Aspirin and Cancer Risk: Long-Term Observational Follow-up of a Randomized Trial. Ann Intern Med. 2013;159:77–85. doi: 10.7326/0003-4819-159-2-201307160-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jacobs EJ, Thun MJ, Connell CJ, Rodriguez C, Henley SJ, Feigelson HS, et al. Aspirin and other nonsteroidal anti-inflammatory drugs and breast cancer incidence in a large U.S. cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:261–4. [PubMed] [Google Scholar]
- 51.Egan KM, Stampfer MJ, Giovannucci E, Rosner BA, Colditz GA. Prospective study of regular aspirin use and the risk of breast cancer. J Natl Cancer Inst. 1996;88:988–93. doi: 10.1093/jnci/88.14.988. [DOI] [PubMed] [Google Scholar]
- 52.Marshall SF, Bernstein L, Anton-Culver H, Deapen D, Horn-Ross PL, Mohrenweiser H, et al. Nonsteroidal anti-inflammatory drug use and breast cancer risk by stage and hormone receptor status. J Natl Cancer Inst. 2005;97:805–12. doi: 10.1093/jnci/dji140. [DOI] [PubMed] [Google Scholar]
- 53.Ready A, Velicer CM, McTiernan A, White E. NSAID use and breast cancer risk in the VITAL cohort. Breast Cancer Res Treat. 2008;109:533–43. doi: 10.1007/s10549-007-9665-x. [DOI] [PubMed] [Google Scholar]
- 54.Johnson TW, Anderson KE, Lazovich D, Folsom AR. Association of aspirin and nonsteroidal anti-inflammatory drug use with breast cancer. Cancer Epidemiol Biomarkers Prev. 2002;11:1586–91. [PubMed] [Google Scholar]
- 55.Thun MJ, Jacobs EJ, Patrono C. The role of aspirin in cancer prevention. Nat Rev Clin Oncol. 2012;9:259–67. doi: 10.1038/nrclinonc.2011.199. [DOI] [PubMed] [Google Scholar]
- 56.Gates MA, Tworoger SS, Eliassen AH, Missmer SA, Hankinson SE. Analgesic use and sex steroid hormone concentrations in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2010;19:1033–41. doi: 10.1158/1055-9965.EPI-09-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wernli KJ, Hampton JM, Trentham-Dietz A, Newcomb PA. Use of antidepressants and NSAIDs in relation to mortality in long-term breast cancer survivors. Pharmacoepidemiol Drug Saf. 2011;20:131–7. doi: 10.1002/pds.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jurek AM, Greenland S, Maldonado G, Church TR. Proper interpretation of non-differential misclassification effects: expectations vs observations. Int J Epidemiol. 2005;34:680–7. doi: 10.1093/ije/dyi060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.