Abstract
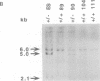
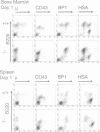
PU.1 is a member of the ets family of transcription factors and is expressed exclusively in cells of the hematopoietic lineage. Mice homozygous for a disruption in the PU.1 DNA binding domain are born alive but die of severe septicemia within 48 h. The analysis of these neonates revealed a lack of mature macrophages, neutrophils, B cells and T cells, although erythrocytes and megakaryocytes were present. The absence of lymphoid commitment and development in null mice was not absolute, since mice maintained on antibiotics began to develop normal appearing T cells 3-5 days after birth. In contrast, mature B cells remained undetectable in these older mice. Within the myeloid lineage, despite a lack of macrophages in the older antibiotic-treated animals, a few cells with the characteristics of neutrophils began to appear by day 3. While the PU.1 protein appears not to be essential for myeloid and lymphoid lineage commitment, it is absolutely required for the normal differentiation of B cells and macrophages.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson S., Miller R. G., Phillips R. A. The identification in adult bone marrow of pluripotent and restricted stem cells of the myeloid and lymphoid systems. J Exp Med. 1977 Jun 1;145(6):1567–1579. doi: 10.1084/jem.145.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austyn J. M., Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981 Oct;11(10):805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Bain G., Maandag E. C., Izon D. J., Amsen D., Kruisbeek A. M., Weintraub B. C., Krop I., Schlissel M. S., Feeney A. J., van Roon M. E2A proteins are required for proper B cell development and initiation of immunoglobulin gene rearrangements. Cell. 1994 Dec 2;79(5):885–892. doi: 10.1016/0092-8674(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Baribault H., Price J., Miyai K., Oshima R. G. Mid-gestational lethality in mice lacking keratin 8. Genes Dev. 1993 Jul;7(7A):1191–1202. doi: 10.1101/gad.7.7a.1191. [DOI] [PubMed] [Google Scholar]
- Chen H. M., Pahl H. L., Scheibe R. J., Zhang D. E., Tenen D. G. The Sp1 transcription factor binds the CD11b promoter specifically in myeloid cells in vivo and is essential for myeloid-specific promoter activity. J Biol Chem. 1993 Apr 15;268(11):8230–8239. [PubMed] [Google Scholar]
- Cline M. J., Moore M. A. Embryonic origin of the mouse macrophage. Blood. 1972 Jun;39(6):842–849. [PubMed] [Google Scholar]
- Crocker P. R., Gordon S. Mouse macrophage hemagglutinin (sheep erythrocyte receptor) with specificity for sialylated glycoconjugates characterized by a monoclonal antibody. J Exp Med. 1989 Apr 1;169(4):1333–1346. doi: 10.1084/jem.169.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick J. E., Magli M. C., Huszar D., Phillips R. A., Bernstein A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell. 1985 Aug;42(1):71–79. doi: 10.1016/s0092-8674(85)80102-1. [DOI] [PubMed] [Google Scholar]
- Doetschman T. C., Eistetter H., Katz M., Schmidt W., Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985 Jun;87:27–45. [PubMed] [Google Scholar]
- Faust E. A., Saffran D. C., Toksoz D., Williams D. A., Witte O. N. Distinctive growth requirements and gene expression patterns distinguish progenitor B cells from pre-B cells. J Exp Med. 1993 Apr 1;177(4):915–923. doi: 10.1084/jem.177.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I., Bernet E., Soriano E., del Rio T., Fonseca M. Naturally occurring cell death in the cerebral cortex of the rat and removal of dead cells by transitory phagocytes. Neuroscience. 1990;39(2):451–458. doi: 10.1016/0306-4522(90)90281-8. [DOI] [PubMed] [Google Scholar]
- Galson D. L., Hensold J. O., Bishop T. R., Schalling M., D'Andrea A. D., Jones C., Auron P. E., Housman D. E. Mouse beta-globin DNA-binding protein B1 is identical to a proto-oncogene, the transcription factor Spi-1/PU.1, and is restricted in expression to hematopoietic cells and the testis. Mol Cell Biol. 1993 May;13(5):2929–2941. doi: 10.1128/mcb.13.5.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galy A., Travis M., Cen D., Chen B. Human T, B, natural killer, and dendritic cells arise from a common bone marrow progenitor cell subset. Immunity. 1995 Oct;3(4):459–473. doi: 10.1016/1074-7613(95)90175-2. [DOI] [PubMed] [Google Scholar]
- Gruber D. F., Zucali J. R., Mirand E. A. Identification of erythropoietin producing cells in fetal mouse liver cultures. Exp Hematol. 1977 Sep;5(5):392–398. [PubMed] [Google Scholar]
- Hardy R. R., Carmack C. E., Shinton S. A., Kemp J. D., Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991 May 1;173(5):1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler M. E. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- Henkel G., Brown M. A. PU.1 and GATA: components of a mast cell-specific interleukin 4 intronic enhancer. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7737–7741. doi: 10.1073/pnas.91.16.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestdal K., Ruscetti F. W., Ihle J. N., Jacobsen S. E., Dubois C. M., Kopp W. C., Longo D. L., Keller J. R. Characterization and regulation of RB6-8C5 antigen expression on murine bone marrow cells. J Immunol. 1991 Jul 1;147(1):22–28. [PubMed] [Google Scholar]
- Hirsch E., Iglesias A., Potocnik A. J., Hartmann U., Fässler R. Impaired migration but not differentiation of haematopoietic stem cells in the absence of beta1 integrins. Nature. 1996 Mar 14;380(6570):171–175. doi: 10.1038/380171a0. [DOI] [PubMed] [Google Scholar]
- Hohaus S., Petrovick M. S., Voso M. T., Sun Z., Zhang D. E., Tenen D. G. PU.1 (Spi-1) and C/EBP alpha regulate expression of the granulocyte-macrophage colony-stimulating factor receptor alpha gene. Mol Cell Biol. 1995 Oct;15(10):5830–5845. doi: 10.1128/mcb.15.10.5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkinson-Woolley J., Hughes D., Gordon S., Martin P. Macrophage recruitment during limb development and wound healing in the embryonic and foetal mouse. J Cell Sci. 1994 May;107(Pt 5):1159–1167. doi: 10.1242/jcs.107.5.1159. [DOI] [PubMed] [Google Scholar]
- Hromas R., Orazi A., Neiman R. S., Maki R., Van Beveran C., Moore J., Klemsz M. Hematopoietic lineage- and stage-restricted expression of the ETS oncogene family member PU.1. Blood. 1993 Nov 15;82(10):2998–3004. [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Hume D. A., Perry V. H., Gordon S. Immunohistochemical localization of a macrophage-specific antigen in developing mouse retina: phagocytosis of dying neurons and differentiation of microglial cells to form a regular array in the plexiform layers. J Cell Biol. 1983 Jul;97(1):253–257. doi: 10.1083/jcb.97.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume D. A., Robinson A. P., MacPherson G. G., Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80. Relationship between macrophages, Langerhans cells, reticular cells, and dendritic cells in lymphoid and hematopoietic organs. J Exp Med. 1983 Nov 1;158(5):1522–1536. doi: 10.1084/jem.158.5.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Jameson S. C., Hogquist K. A., Bevan M. J. Positive selection of thymocytes. Annu Rev Immunol. 1995;13:93–126. doi: 10.1146/annurev.iy.13.040195.000521. [DOI] [PubMed] [Google Scholar]
- Janknecht R., Nordheim A. Gene regulation by Ets proteins. Biochim Biophys Acta. 1993 Dec 23;1155(3):346–356. doi: 10.1016/0304-419x(93)90014-4. [DOI] [PubMed] [Google Scholar]
- Karim F. D., Urness L. D., Thummel C. S., Klemsz M. J., McKercher S. R., Celada A., Van Beveren C., Maki R. A., Gunther C. V., Nye J. A. The ETS-domain: a new DNA-binding motif that recognizes a purine-rich core DNA sequence. Genes Dev. 1990 Sep;4(9):1451–1453. doi: 10.1101/gad.4.9.1451. [DOI] [PubMed] [Google Scholar]
- Kehrl J. H. Hematopoietic lineage commitment: role of transcription factors. Stem Cells. 1995 May;13(3):223–241. doi: 10.1002/stem.5530130304. [DOI] [PubMed] [Google Scholar]
- Keller G., Paige C., Gilboa E., Wagner E. F. Expression of a foreign gene in myeloid and lymphoid cells derived from multipotent haematopoietic precursors. Nature. 1985 Nov 14;318(6042):149–154. doi: 10.1038/318149a0. [DOI] [PubMed] [Google Scholar]
- Klemsz M. J., Maki R. A. Activation of transcription by PU.1 requires both acidic and glutamine domains. Mol Cell Biol. 1996 Jan;16(1):390–397. doi: 10.1128/mcb.16.1.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemsz M. J., McKercher S. R., Celada A., Van Beveren C., Maki R. A. The macrophage and B cell-specific transcription factor PU.1 is related to the ets oncogene. Cell. 1990 Apr 6;61(1):113–124. doi: 10.1016/0092-8674(90)90219-5. [DOI] [PubMed] [Google Scholar]
- Kodandapani R., Pio F., Ni C. Z., Piccialli G., Klemsz M., McKercher S., Maki R. A., Ely K. R. A new pattern for helix-turn-helix recognition revealed by the PU.1 ETS-domain-DNA complex. Nature. 1996 Apr 4;380(6573):456–460. doi: 10.1038/380456a0. [DOI] [PubMed] [Google Scholar]
- Lang R. A., Bishop J. M. Macrophages are required for cell death and tissue remodeling in the developing mouse eye. Cell. 1993 Aug 13;74(3):453–462. doi: 10.1016/0092-8674(93)80047-i. [DOI] [PubMed] [Google Scholar]
- Lang R., Lustig M., Francois F., Sellinger M., Plesken H. Apoptosis during macrophage-dependent ocular tissue remodelling. Development. 1994 Dec;120(12):3395–3403. doi: 10.1242/dev.120.12.3395. [DOI] [PubMed] [Google Scholar]
- Lemischka I. R., Raulet D. H., Mulligan R. C. Developmental potential and dynamic behavior of hematopoietic stem cells. Cell. 1986 Jun 20;45(6):917–927. doi: 10.1016/0092-8674(86)90566-0. [DOI] [PubMed] [Google Scholar]
- Lund-Johansen F., Terstappen L. W. Differential surface expression of cell adhesion molecules during granulocyte maturation. J Leukoc Biol. 1993 Jul;54(1):47–55. doi: 10.1002/jlb.54.1.47. [DOI] [PubMed] [Google Scholar]
- Löffert D., Schaal S., Ehlich A., Hardy R. R., Zou Y. R., Müller W., Rajewsky K. Early B-cell development in the mouse: insights from mutations introduced by gene targeting. Immunol Rev. 1994 Feb;137:135–153. doi: 10.1111/j.1600-065x.1994.tb00662.x. [DOI] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Marshall A. J., Wu G. E., Paige G. J. Frequency of VH81x usage during B cell development: initial decline in usage is independent of Ig heavy chain cell surface expression. J Immunol. 1996 Mar 15;156(6):2077–2084. [PubMed] [Google Scholar]
- Mombaerts P., Iacomini J., Johnson R. S., Herrup K., Tonegawa S., Papaioannou V. E. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992 Mar 6;68(5):869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- Moore T. A., Zlotnik A. T-cell lineage commitment and cytokine responses of thymic progenitors. Blood. 1995 Sep 1;86(5):1850–1860. [PubMed] [Google Scholar]
- Moreau-Gachelin F. Spi-1/PU.1: an oncogene of the Ets family. Biochim Biophys Acta. 1994 Dec 30;1198(2-3):149–163. doi: 10.1016/0304-419x(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Morris L., Graham C. F., Gordon S. Macrophages in haemopoietic and other tissues of the developing mouse detected by the monoclonal antibody F4/80. Development. 1991 Jun;112(2):517–526. doi: 10.1242/dev.112.2.517. [DOI] [PubMed] [Google Scholar]
- Olson E. N., Arnold H. H., Rigby P. W., Wold B. J. Know your neighbors: three phenotypes in null mutants of the myogenic bHLH gene MRF4. Cell. 1996 Apr 5;85(1):1–4. doi: 10.1016/s0092-8674(00)81073-9. [DOI] [PubMed] [Google Scholar]
- Orkin S. H. Transcription factors and hematopoietic development. J Biol Chem. 1995 Mar 10;270(10):4955–4958. doi: 10.1074/jbc.270.10.4955. [DOI] [PubMed] [Google Scholar]
- Orlic D., Bodine D. M. What defines a pluripotent hematopoietic stem cell (PHSC): will the real PHSC please stand up! Blood. 1994 Dec 15;84(12):3991–3994. [PubMed] [Google Scholar]
- Pahl H. L., Scheibe R. J., Zhang D. E., Chen H. M., Galson D. L., Maki R. A., Tenen D. G. The proto-oncogene PU.1 regulates expression of the myeloid-specific CD11b promoter. J Biol Chem. 1993 Mar 5;268(7):5014–5020. [PubMed] [Google Scholar]
- Penit C., Vasseur F. Cell proliferation and differentiation in the fetal and early postnatal mouse thymus. J Immunol. 1989 May 15;142(10):3369–3377. [PubMed] [Google Scholar]
- Pfeffer K., Mak T. W. Lymphocyte ontogeny and activation in gene targeted mutant mice. Annu Rev Immunol. 1994;12:367–411. doi: 10.1146/annurev.iy.12.040194.002055. [DOI] [PubMed] [Google Scholar]
- Phillips R. A. Hematopoietic stem cells: concepts, assays, and controversies. Semin Immunol. 1991 Nov;3(6):337–347. [PubMed] [Google Scholar]
- Pongubala J. M., Van Beveren C., Nagulapalli S., Klemsz M. J., McKercher S. R., Maki R. A., Atchison M. L. Effect of PU.1 phosphorylation on interaction with NF-EM5 and transcriptional activation. Science. 1993 Mar 12;259(5101):1622–1625. doi: 10.1126/science.8456286. [DOI] [PubMed] [Google Scholar]
- Rodewald H. R., Kretzschmar K., Swat W., Takeda S. Intrathymically expressed c-kit ligand (stem cell factor) is a major factor driving expansion of very immature thymocytes in vivo. Immunity. 1995 Sep;3(3):313–319. doi: 10.1016/1074-7613(95)90116-7. [DOI] [PubMed] [Google Scholar]
- Rosmarin A. G., Caprio D., Levy R., Simkevich C. CD18 (beta 2 leukocyte integrin) promoter requires PU.1 transcription factor for myeloid activity. Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):801–805. doi: 10.1073/pnas.92.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J. W., Jr Death in embryonic systems. Science. 1966 Nov 4;154(3749):604–612. doi: 10.1126/science.154.3749.604. [DOI] [PubMed] [Google Scholar]
- Scott E. W., Simon M. C., Anastasi J., Singh H. Requirement of transcription factor PU.1 in the development of multiple hematopoietic lineages. Science. 1994 Sep 9;265(5178):1573–1577. doi: 10.1126/science.8079170. [DOI] [PubMed] [Google Scholar]
- Shinkai Y., Rathbun G., Lam K. P., Oltz E. M., Stewart V., Mendelsohn M., Charron J., Datta M., Young F., Stall A. M. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992 Mar 6;68(5):855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Shivdasani R. A., Orkin S. H. The transcriptional control of hematopoiesis. Blood. 1996 May 15;87(10):4025–4039. [PubMed] [Google Scholar]
- Sibilia M., Wagner E. F. Strain-dependent epithelial defects in mice lacking the EGF receptor. Science. 1995 Jul 14;269(5221):234–238. doi: 10.1126/science.7618085. [DOI] [PubMed] [Google Scholar]
- Singh H. Gene targeting reveals a hierarchy of transcription factors regulating specification of lymphoid cell fates. Curr Opin Immunol. 1996 Apr;8(2):160–165. doi: 10.1016/s0952-7915(96)80053-7. [DOI] [PubMed] [Google Scholar]
- Smith L. T., Hohaus S., Gonzalez D. A., Dziennis S. E., Tenen D. G. PU.1 (Spi-1) and C/EBP alpha regulate the granulocyte colony-stimulating factor receptor promoter in myeloid cells. Blood. 1996 Aug 15;88(4):1234–1247. [PubMed] [Google Scholar]
- Sollbach A. E., Wu G. E. Inversions produced during V(D)J rearrangement at IgH, the immunoglobulin heavy-chain locus. Mol Cell Biol. 1995 Feb;15(2):671–681. doi: 10.1128/mcb.15.2.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A., Dustin M. L., Kishimoto T. K., Marlin S. D. The lymphocyte function-associated LFA-1, CD2, and LFA-3 molecules: cell adhesion receptors of the immune system. Annu Rev Immunol. 1987;5:223–252. doi: 10.1146/annurev.iy.05.040187.001255. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994 Jan 28;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Surh C. D., Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994 Nov 3;372(6501):100–103. doi: 10.1038/372100a0. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Threadgill D. W., Dlugosz A. A., Hansen L. A., Tennenbaum T., Lichti U., Yee D., LaMantia C., Mourton T., Herrup K., Harris R. C. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science. 1995 Jul 14;269(5221):230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- Till J. E., McCulloch E. A. Hemopoietic stem cell differentiation. Biochim Biophys Acta. 1980 Nov 26;605(4):431–459. doi: 10.1016/0304-419x(80)90009-8. [DOI] [PubMed] [Google Scholar]
- Voso M. T., Burn T. C., Wulf G., Lim B., Leone G., Tenen D. G. Inhibition of hematopoiesis by competitive binding of transcription factor PU.1. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):7932–7936. doi: 10.1073/pnas.91.17.7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER R. ULTRASTRUCTURAL CHANGES IN REGRESSING TAIL MUSCLES OF XENOPUS LARVAE AT METAMORPHOSIS. J Cell Biol. 1964 Aug;22:481–487. doi: 10.1083/jcb.22.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A. M., Till J. E., Siminovitch L., McCulloch E. A. Cytological evidence for a relationship between normal hemotopoietic colony-forming cells and cells of the lymphoid system. J Exp Med. 1968 Mar 1;127(3):455–464. doi: 10.1084/jem.127.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- Young R. W. Cell death during differentiation of the retina in the mouse. J Comp Neurol. 1984 Nov 1;229(3):362–373. doi: 10.1002/cne.902290307. [DOI] [PubMed] [Google Scholar]
- Zhang D. E., Hetherington C. J., Chen H. M., Tenen D. G. The macrophage transcription factor PU.1 directs tissue-specific expression of the macrophage colony-stimulating factor receptor. Mol Cell Biol. 1994 Jan;14(1):373–381. doi: 10.1128/mcb.14.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]