Abstract
Previous studies of mouse embryos concluded that after the optic vesicle evaginates from the ventral forebrain and contacts the surface ectoderm, signals from the ectoderm specify the distal region of the optic vesicle to become retina and signals from the optic vesicle induce the lens. Germline deletion of Bmp4 resulted in failure of lens formation. We performed conditional deletion of Bmp4 from the optic vesicle to test the function of Bmp4 in murine eye development. The optic vesicle evaginated normally and contacted the surface ectoderm. Lens induction did not occur. The optic cup failed to form and the expression of retina-specific genes decreased markedly in the distal optic vesicle. Instead, cells in the prospective retina expressed genes characteristic of the retinal pigmented epithelium. We conclude that Bmp4 is required for retina specification in mice. In the absence of Bmp4, formation of the retinal pigmented epithelium is the default differentiation pathway of the optic vesicle. Differences in the signaling pathways required for specification of the retina and retinal pigmented epithelium in chicken and mouse embryos suggest major changes in signaling during the evolution of the vertebrate eye.
Keywords: Bmp4, Retina, Optic cup, Retinal pigmented epithelium, Evolution
Introduction
Early in eye formation, the optic vesicles evaginate from the ventral diencephalon and contact the surface ectoderm on the sides of the head. The ectoderm in contact with the optic vesicle becomes the lens placode and the distal region of the optic vesicle in contact with the ectoderm begins the process of retina differentiation. The two adherent layers of cells then invaginate to form the lens vesicle and the bilayered optic cup, with the retina surrounded by an outer layer of retinal pigmented epithelium (RPE). The signals that specify the lens and retina have been of great interest to developmental biologists, because lens formation is the first example of embryonic induction (Spemann, 1901) and formation of the retina is essential for vision.
Evidence that Bmp4 is required for lens induction came from studies of Bmp4 germline knockout mice (Furuta and Hogan, 1998). The lens did not form in these mutants and beads delivering Bmp4 promoted the formation of the lens from the mutant ectoderm in the presence of a mutant optic vesicle. In mice, Bmp4 is expressed at the distal surface of the optic vesicle (prospective retina) (Furuta and Hogan, 1998) under control of the eye field transcription factor, Lhx2 (Yun et al., 2009). In Lhx2 knockout mice, the lens failed to form and pSmad1/5/8 staining decreased in the ectoderm, supporting the view that Bmp4 from the optic vesicle is responsible for lens induction. Studies from the Furuta lab showed that conditional deletion of two of the three type I BMP receptors in the optic vesicle decreased the expression of several genes characteristic of the early retina (Murali et al., 2005), suggesting that BMP signaling might also contribute to retina differentiation.
By contrast, studies in chicken and mouse embryos led to the conclusion that FGF signaling from the ectoderm specifies the location and differentiation of the retina (Hyer et al., 1998; Nguyen and Arnheiter, 2000; Pittack et al., 1997). Studies in chicken embryos indicated that BMPs from the surface ectoderm were required for the differentiation of the RPE, not the retina (Muller et al., 2007; Steinfeld et al., 2013). Exposing the optic vesicle to beads soaked in Bmp4 prevented retinal differentiation and optic cup formation (Hyer et al., 2003; Muller et al., 2007). These studies suggested that vertebrate retina specification and differentiation does not involve BMP signaling.
To directly address the function of Bmp4 in mammalian eye formation, we used Rx-Cre (Swindell et al., 2006) to conditionally delete floxed alleles of Bmp4 from the mouse optic vesicle. Alterations in gene expression were assessed by laser microdissection and microarray analysis of the prospective lens and retinal epithelia and confirmed using antibody staining and fluorescent in situ hybridization.
Results
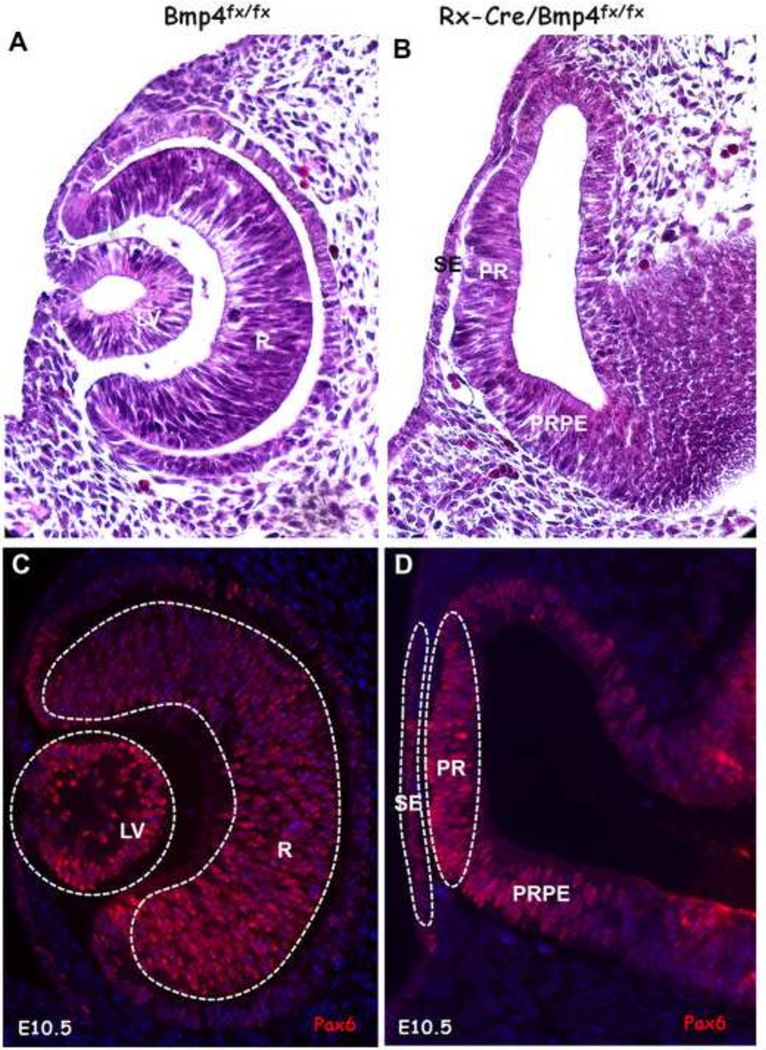
In embryos in which Bmp4 was conditionally deleted from the optic vesicles, the optic vesicles extended normally and contacted the surface ectoderm. As expected from previous results (Furuta and Hogan, 1998; Yun et al., 2009), deletion of Bmp4 from the optic vesicle prevented lens induction and optic cup formation (Fig. 1).
Figure 1.
Sections of the optic cup and lens in E10.5 wild type eyes and the optic vesicles and surface ectoderm from Bmp4CKO embryos. Embryos were littermates. A. An H&E-stained wild type eye with lens vesicle and optic cup. B. An H&E-stained Bmp4CKO embryo in which the lens did not form and the optic cup did not invaginate. C. A wild type eye stained for the transcription factor Pax6. Dotted lines around the lens and retina indicate the tissues that were laser microdissected for microarray analysis. D. A Bmp4CKO embryo stained for Pax6. The dotted lines indicate the tissues that were laser microdissected for microarray analysis. For all embryos, dorsal is toward the top of the figure. LV, lens vesicle; R, retina; RPE, retinal pigmented epithelium; SE, surface ectoderm; PR, prospective retina; PRPE, prospective retinal pigmented epithelium.
Laser microdissection and microarray analysis of the prospective lens and retina of wild type and Bmp4 conditional knockout (Bmp4CKO) embryos at E10.5 provided insight about changes in gene expression resulting from loss of Bmp4 in the optic vesicle (Table 1). Transcripts characteristic of the lens and retina were significantly decreased in the surface ectoderm and distal optic vesicle, respectively. In the prospective retina of the conditional knockouts, numerous transcripts were present at significantly higher levels than in wild type eyes. Many of these genes with increased expression in the prospective retina are characteristic of the RPE (Table 1). Overall, a total of 2084 transcripts from the prospective retina were significantly increased or decreased in the knockout embryos (995 increased, 1089 decreased). In the prospective lens, 2044 transcripts were significantly increased or decreased in the knockouts (948 increased, 1096 decreased). Although lens transcripts were abundant among those significantly decreased in the ectoderm, several transcripts characteristic of the corneal epithelium were significantly increased in the surface ectoderm of the Bmp4 optic vesiclespecific knockouts (Lypd2, Trp63, Otx1, Tcfap2b, Trpm1; all p<0.001), suggesting that Bmp4 from the optic vesicle suppresses cornea differentiation while promoting lens formation.
Table 1.
Changes in gene expression in the prospective lens and retina at E10.5.
| Lens Transcripts | |||||
|---|---|---|---|---|---|
| Gene Name | Gene Symbol |
WT | CKO | Fold Change* |
Function in Lens Development |
| Prospero-related homeobox 1 | Prox1 | 976 | 28 | −35 | Required for lens fiber cell formation |
| βB3 crystallin | Crybb3 | 289 | 1 | −289 | Lens-preferred crystallin |
| γE-crystallin | Cryge | 1425 | 60 | −24 | Lens-preferred crystallin |
| γC-crystallin | Crygc | 186 | 1 | −186 | Lens-preferred crystallin |
| γD-crystallin | Crygd | 315 | 11 | −28 | Lens-preferred crystallin |
| Jagged 1 | Jag1 | 1007 | 82 | −12 | Promotes lens epithelial proliferation |
| Musculoaponeuroticfibrosarcoma oncogene | Maf | 1499 | 119 | −13 | Regulates crystallin gene expression |
| Paired-like homeobox-3 | Pitx3 | 153 | 4 | −40 | Lens formation and cell survival |
| N-cadherin | Cdh2 | 228 | 9 | −26 | Lens vesicle separation and survival |
| Forkhead box gene E3 | Foxe3 | 59 | 1 | −59 | Survival of the lens epithelium |
| SRY-box gene 2 | Sox2 | 1204 | 278 | −4 | Interacts with Pax6 for lens formation |
| Retina Transcripts | |||||
|---|---|---|---|---|---|
| Gene Name | Gene Symbol |
WT | CKO | Fold Change* |
Function in Retina Development |
| Aldehyde dehydrogenase family 1 | Aldh1a1 | 2649 | 100 | −26 | Production of retinoic acid |
| Growth and differentiation factor 6 | Gdf6 | 337 | 1 | −337 | Loss causes microphthalmia, coloboma |
| Bone morphogenetic protein 4 | Bmp4 | 311 | 6 | −56 | Retina formation, D-V polarity |
| T-box gene 3 | Tbx3 | 209 | 1 | −209 | Retina D-V patterning |
| Fibroblast growth factor 15 | Fgf15 | 869 | 77 | −11 | Function unknown, highly expressed |
| SRY-box gene 2 | Sox2 | 1528 | 298 | −5 | Maintains retinal progenitor cells |
| Visual system homeobox 2 | Vsx2 | 98 | 4 | −24 | Specifies retina, opposes RPE formation |
| Dickkopf homolog 3 | Dkk3 | 891 | 198 | −5 | Promotes retina gene expression |
| Frizzled 5 | Fzd5 | 109 | 7 | −16 | Wnt receptor, loss causes coloboma |
| Fibroblast growth factor 9 | Fgf9 | 91 | 1 | −91 | Establishes retina-RPE boundary |
| RPE Transcripts | |||||
|---|---|---|---|---|---|
| Gene Name | Gene Symbol |
WT | CKO | Fold Change* |
Function in RPE Development |
| Premelanosome protein | Pmel | 67 | 1460 | 22 | Scaffold for melanosome formation |
| Dopachrometautomerase (Tyrp2) | Dct | 1 | 138 | 138 | Melanin biosynthesis |
| Indolethylamine N-methyltransferase | Inmt | 192 | 1549 | 8 | Melanin biosynthesis |
| Tyrosinase-related protein 1 | Tyrp1 | 4 | 169 | 44 | Melanin biosynthesis |
| Microphthalmia transcription factor | Mitf | 33 | 278 | 8 | Regulation of RPE gene expression |
| Bone morphogenetic protein 7 | Bmp7 | 39 | 295 | 8 | Optic fissure formation and closure |
| RAS oncogene family-38 | Rab38 | 81 | 472 | 6 | Transport of Tyrp1 to the melanosome |
| Stimulated by retinoic acid gene 6 | Stra6 | 32 | 131 | 4 | Retinoic acid uptake |
| Orthodenticle homolog 2 | Otx2 | 472 | 1504 | 3 | Regulation of RPE gene expression |
All comparisons of gene expression in wild type and Bmp4CKO embryos at E10.5 were significantly different at p<0.01.
Expression levels for transcripts below 1.0 were rounded up to 1 to calculate estimates of fold change.
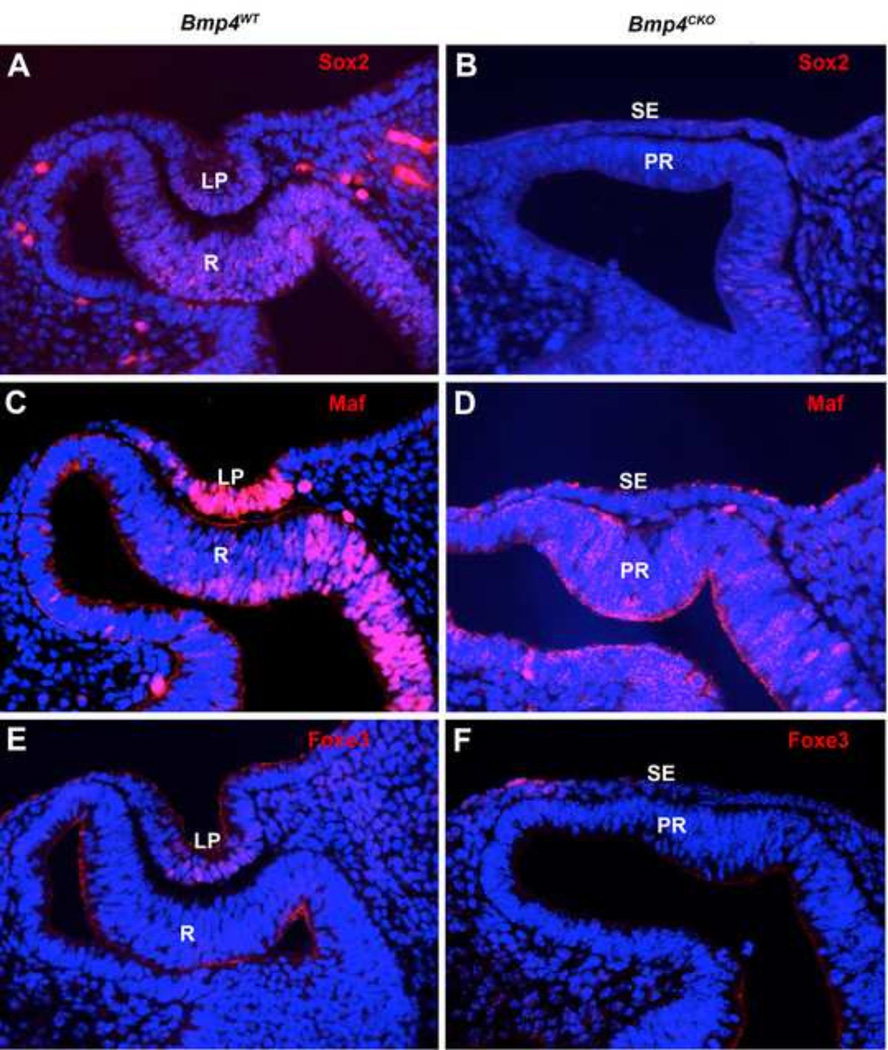
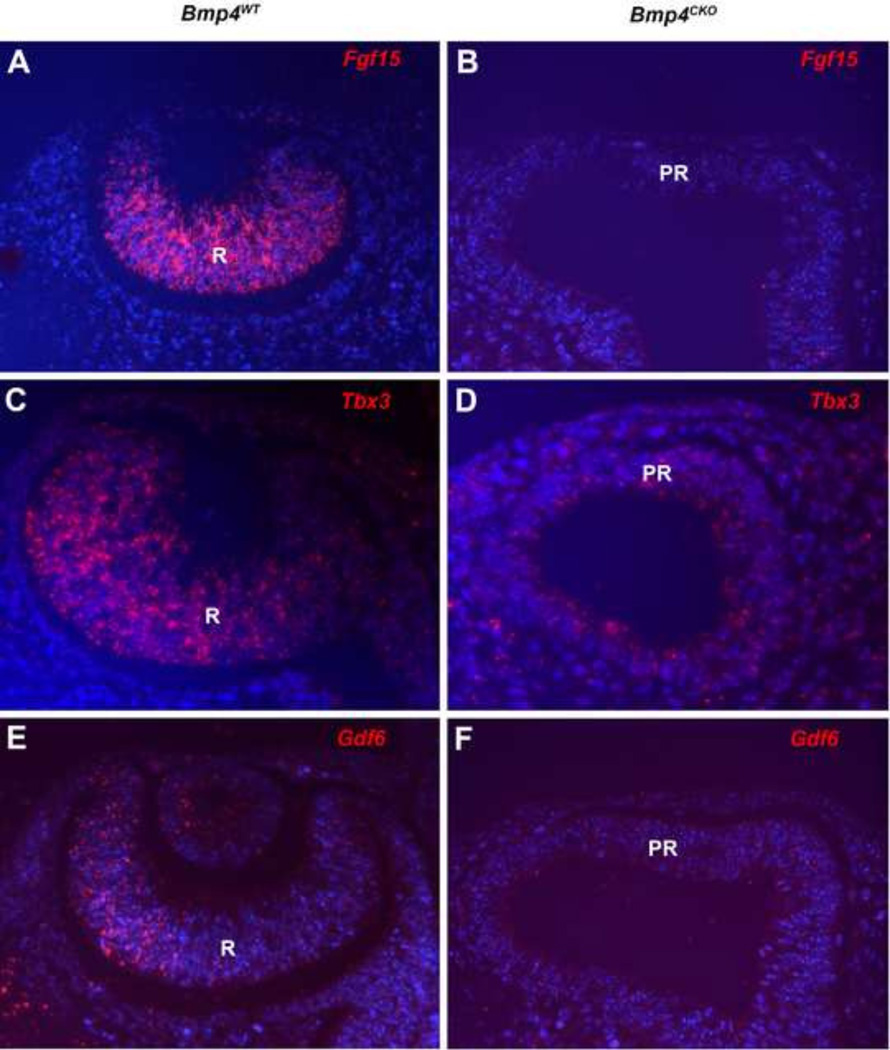
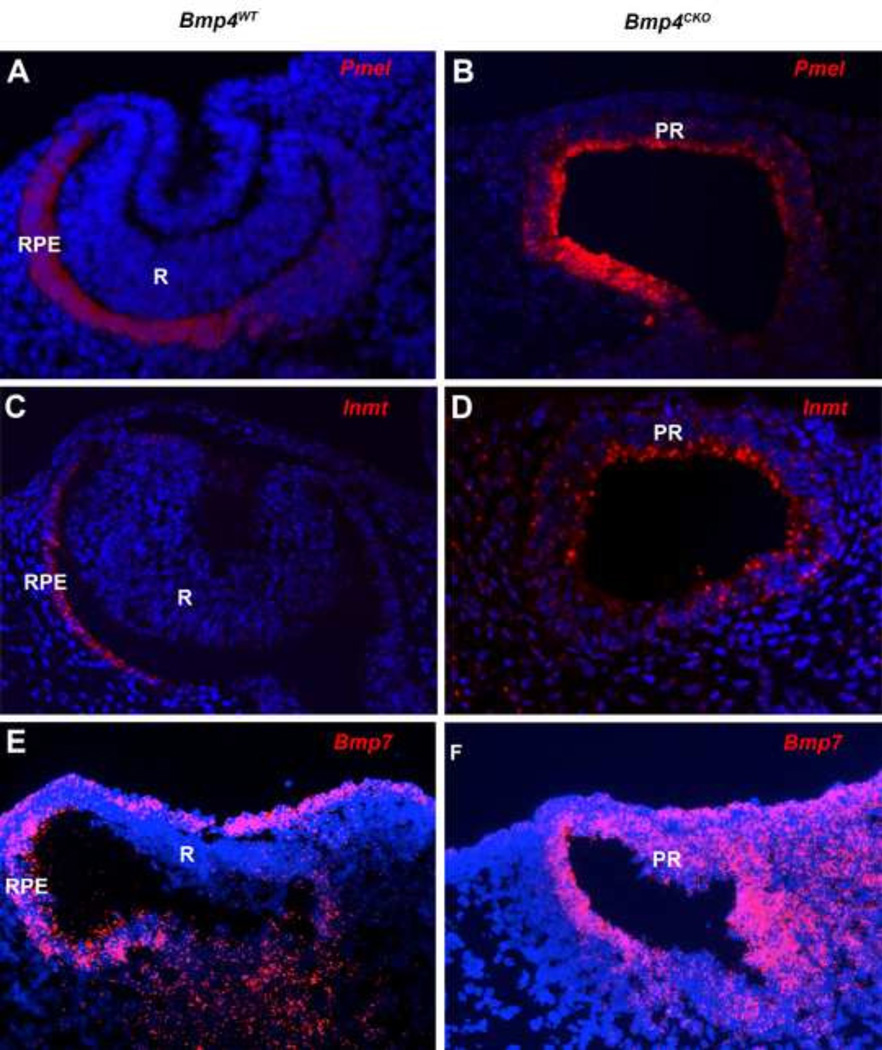
Changes in gene expression were confirmed by immunofluorescence and fluorescent in situ hybridization in wild type and Bmp4CKO embryos. Figure 2 shows that the lens transcription factors, Sox2, Maf and Foxe3 were greatly reduced or undetectable in the surface ectoderm of Bmp4CKO embryos. Sox2 was also greatly reduced in the prospective retina, as predicted from the microarray data (Table 1). Figure 3 illustrates the decreased expression of three retina transcripts, Fgf15, Tbx3 and Gdf6, in the prospective retina of Bmp4CKO embryos. Gdf6 expression was also present in the dorsal lens vesicle of wild type embryos and greatly decreased in the surface ectoderm of Bmp4CKO embryos. The RPE transcripts, Pmel, Inmt and Bmp7, were not detected in the wild type retina and were abundantly expressed in the distal optic vesicle (prospective retina) of Bmp4CKO embryos (Figure 4).
Figure 2.
Immunofluorescent staining of E10.5 eyes from wild type and Bmp4CKO embryos for the transcription factors Sox2 (A, B), Maf (C, D) and Foxe3 (E, F). All three proteins were present in the nuclei of wild type lens pit cells, while nuclear staining was undetectable in the surface ectoderm (prospective lens) of Bmp4CKO embryos. Sox2 staining also disappeared from the nuclei of cells in the prospective retina of Bmp4CKO embryos. For all sections, dorsal is to the left. LP, lens pit; R, retina; SE, surface ectoderm; PR, prospective retina.
Figure 3.
Fluorescent in situ hybridization staining for Fgf15 (A, B), Tbx3 (C, D) and Gdf6 (E, F) in E10.5 eyes from wild type and Bmp4CKO embryos. In all cases staining was greatly decreased or undetectable in the prospective retina (distal optic vesicle) of Bmp4CKO embryos. Cells in the dorsal half of the wild type lens vesicle also expressed Gdf6, which was greatly decreased in the surface ectoderm of Bmp4CKO embryos. For all sections, dorsal is to the left. R, retina; PR, prospective retina.
Figure 4.
Fluorescent in situ hybridization staining for Pmel (A, B), Inmt (C, D) and Bmp7 (E, F) in eyes from wild type and Bmp4CKO embryos. All three transcripts were abundant in the RPE and undetectable in the retina of wild type embryos. All were expressed in the prospective retina (distal optic vesicle) of Bmp4CKO embryos. For all sections, dorsal is to the left. R, retina; RPE, retinal pigmented epithelium; PR, prospective retina.
In spite of these dramatic changes in gene expression, several genes known to be important in lens, retina and RPE differentiation were not significantly changed or only moderately changed in our microarray analysis. For the lens, these included Pax6 (decreased 50%; p<0.05), Six3 (not significantly changed) and Tcfap2a (not significantly changed). For the retina, Lhx2 was not significantly changed and Rx decreased approximately 60% (p<0.05 in two of four probe sets). The RPE gene Vax1 did not change in two of three probe sets (increased 60%; p<0.05 in the third) and Mlana was not significantly changed. These results suggest that, while BMP4 is required for lens and retina formation and to suppress RPE differentiation, it does not regulate the expression of all important genes in these tissues.
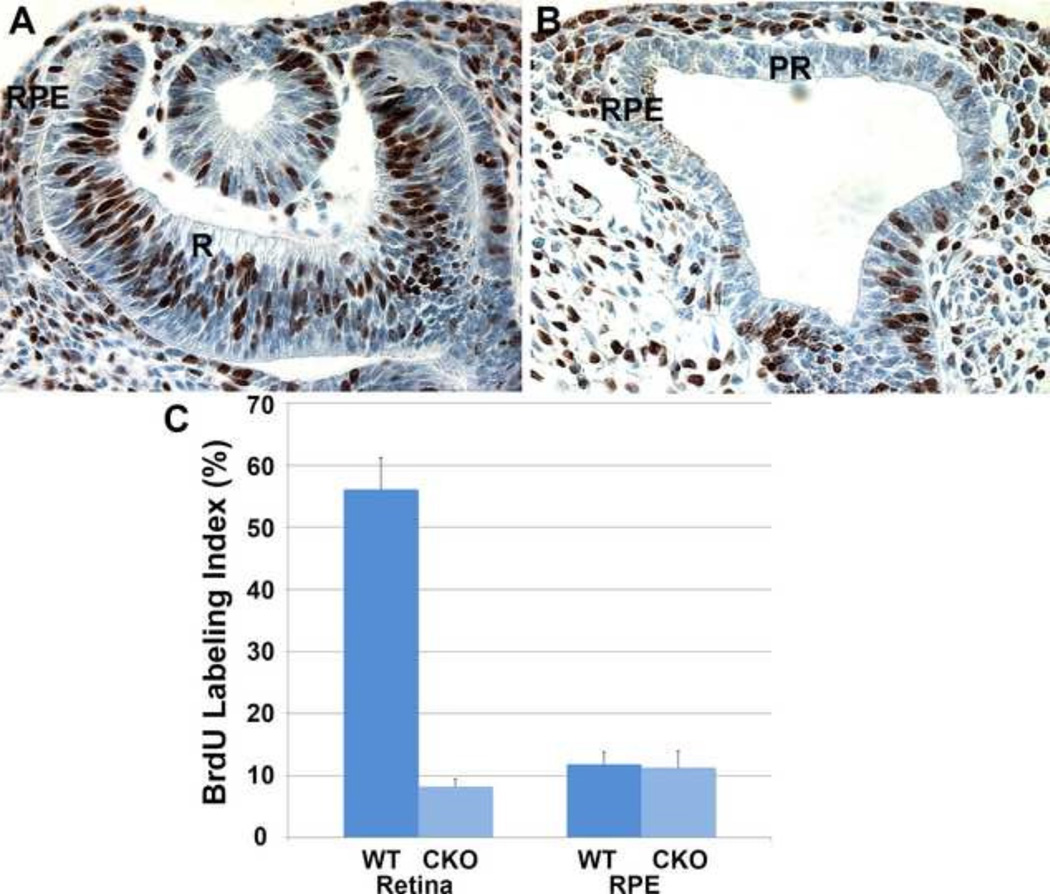
Cell proliferation in the prospective RPE is as high as in the prospective retina in the developing optic vesicle (Yamada et al., 2004), but decreases markedly soon after its differentiation begins. In the prospective retina of the Bmp4CKO embryos at E10.5, the BrdU labeling index, a measure of cells that are synthesizing DNA, was greatly reduced compared to the wild type retina and similar to that of the RPE (Fig. 5). The lower BrdU labeling index in the RPE remained similar in wild type and Bmp4CKO embryos. This observation is consistent with the expression of markers of the RPE in the prospective retinal tissue at E10.5.
Figure 5.
BrdU staining in wild type (A) and Bmp4CKO embryos at E10.5 (B). The BrdU labeling index decreased markedly in the prospective retina of Bmp4CKO embryos to levels at or below those seen in wild type RPE cells (C). For all sections, dorsal is to the left. R, Retina; RPE, retinal pigmented epithelium; PR, prospective retina.
Discussion
BMP signaling, retina and optic cup specification
Previous results from the Furuta laboratory are consistent with a function for Bmp4 in murine retina differentiation. Conditional deletion of Bmpr1a and Bmpr1b, two of the three type I BMP receptors, resulted in reduced retinal growth, failure of retinal neurogenesis and decreased expression of early retinal markers, like Fgf15, but did not prevent optic cup formation (Murali et al., 2005).
Given the greatly reduced proliferation and the absence of retinal transcripts in the distal optic vesicle of the E10.5 Bmp4CKO embryos, it is possible that Bmp4 specifies the retinal domain by promoting the selective proliferation of retinal progenitor cells. However, this is unlikely, because retina-specific proteins like Sox2 and Chx10 (Vsx2) are already expressed in the entire mouse distal optic vesicle as early as E9.0 or E9.5 [Figure 4A and 5A in (Miller et al., 2006)]. If Bmp4 promoted selective proliferation of retinal progenitors, the prospective retinal domain would be expected to be much smaller than the prospective RPE at this early stage. Therefore, the reduced proliferation and absence of retinal transcripts in the distal optic vesicle of the Bmp4CKO embryos at E10.5 is most likely due a fate switch in the distal optic vesicle from retina to RPE, since the RPE is characterized by decreased proliferation at this stage (Figure 5).
A major function of Bmp4 in chicken embryos appears to be the specification of the RPE, not the retina (Muller et al., 2007; Steinfeld et al., 2013). Consistent with this, exposure of the chicken optic vesicle to exogenous Bmp4 promoted RPE development and prevented retina and optic cup formation (Hyer et al., 2003; Muller et al., 2007). In mice, Bmp4 is expressed at the distal tip of the optic vesicle (Furuta and Hogan, 1998; Yun et al., 2009), but in chicken embryos Bmp4 is predominantly expressed in the surface ectoderm (Muller et al., 2007), which is consistent with the different functions of BMP signaling in these species.
FGF signaling and retina specification
FGF signaling within the retina is required for proper retinal cell differentiation in fish, chicken and mouse embryos (Cai et al., 2013; Martinez-Morales et al., 2005; Vogel-Hopker et al., 2000). Numerous studies have shown that exposure of the embryonic RPE to FGFs can transform it into retina (Guillemot and Cepko, 1992; Opas and Dziak, 1994; Park and Hollenberg, 1989; Pittack et al., 1991). As expected, exposure of the RPE to exogenous FGFs promoted retina formation in chicken and mouse embryos (Pittack et al., 1997; Hyer et al., 1998; Nguyen and Arnheiter, 2000). However, because exposure of the RPE to exogenous FGFs at this stage is known to cause the RPE to differentiate into retina, it is important to demonstrate that FGFs from the ectoderm provide the normal signal that promotes retina formation. Working with cultured chicken embryo optic vesicles, Pittack and colleagues showed that Fgf2 was present in the ectoderm and antibodies to Fgf2 blocked the differentiation of the retina, suggesting that Fgf2 from the ectoderm promotes retina formation in chicken embryos in vivo (Pittack et al., 1997). However, Nguyen and Arnheiter exposed mouse optic vesicles to exogenous FGF and performed no “loss of function” studies similar to those of Pittack et al. Conditional deletion of Fgfr1 and Fgfr2 in the mouse optic vesicle with Six3-Cre or Rx-Cre reduced cell proliferation, disrupted neurogenesis and caused coloboma formation, but did not prevent optic cup or retina formation (Cai et al., 2013; Chen et al., 2012). Given that loss of Bmp4 in the mouse optic vesicle results in absence of the retina, it seems unlikely that FGFs from the ectoderm are the normal signal for retina specification in mice.
It is possible that, in mice, Bmp4 from the optic vesicle induces the expression of FGFs in the surface ectoderm and that FGFs promote retina formation. However, FGFs were expressed at very low levels in the surface ectoderm in our microarray analysis and did not decrease significantly in Bmp4CKO embryos (Table S1), suggesting that FGFs from the ectoderm are not regulated by BMP signaling from the optic vesicle or involved in murine retina formation.
Implications for the study of cell-cell signaling in eye development
The marked switch in the functions of Bmp4 in birds and mammals from suppressing retina and promoting RPE formation to suppressing RPE and promoting retina formation suggests that major changes occurred in the signaling pathways that specify these ocular tissues during evolution from the common ancestor of birds and mammals. This perspective is supported by differences between the signaling pathways involved in lens induction in mice and other vertebrate classes. For example, FGF signaling contributes to lens induction in fish and birds (Kurose et al., 2005; Nakayama et al., 2008; Vogel-Hopker et al., 2000) and Notch signaling is required for lens induction in frogs (Ogino et al., 2008). However, FGF or Notch signaling are not required for lens induction in mice (Garcia et al., 2011; Le et al., 2012). Although Fgf2 was detected in the ocular surface ectoderm of chicken embryos and blocking Fgf2 prevented retina formation (Pittack et al., 1997), our previous PCR analysis did not detect Fgf2 transcripts in the mouse surface ectoderm (Garcia et al., 2011) (see also Table S1). These differences between lens and retina formation in different vertebrate classes suggest that caution should be applied when extrapolating the results of studies of cell-cell signaling in chicken embryo eye development to mammalian eye development.
Materials and Methods
Mice
Bmp4 flox mice (Chang et al., 2008) were mated to Rx-Cre mice (Swindell et al., 2006) to delete Bmp4 in the optic vesicle. Mice were genotyped with the universal PCR genotyping assay (Stratman et al., 2003) using the following primers for Bmp4: 5’-agactctttagtgagcattttcaac-3’; 5’–agcccaatttccacaacttc-3’ (WT 180 bp, flox 220 bp).
Immunostaining
Embryo heads were collected at E10.5, fixed in 4% paraformaldehyde, washed in PBS, embedded in agarose for orientation and then in paraffin and sectioned for histological analysis. Sections were reacted with a rabbit antibody to Pax6 (1:100; Novus Biologicals, #H00005080-P01, Littleton, CO), stained with an Alexafluor488-labeled anti-rabbit secondary antibody (1:1,000; Life Technologies, Grand Island, NY) and examined by fluorescence microscopy. For the lens transcription factors, antibodies to Sox2 (1:500; Cell Signaling Technology, Boston, MA), Maf (1:100; #sc-7866, Santa Cruz Biotechnology, Santa Cruz, CA) and Foxe3 (1:1,000; a gift from Peter Carlsson, Goteborg University, Goteborg, Sweden) were detected using the Tyramide Signal Amplification Kit (PerkinElmer, Waltham, MA). For BrdU labeling, pregnant mice were injected intraperitoneally with 100ul of 25mg/ml BrdU and 2.5mg/ml FdU (Fluorodeoxyuridine) and sacrificed one hour later. Sections were reacted with a mouse monoclonal antibody to BrdU (1:200; #M0744, Dako, Carpinteria, CA) and stained using a Vectastain Elite Mouse IgG ABC kit (Vector Laboratories, Burlingame, CA). Stained and total nuclei were counted to calculate the BrdU labeling index.
Fluorescent in situ hybridization
In situ hybridization was performed using QuantiGene View probes generated by Affymetrix (Santa Clara, CA), stained with an Affymetrix QuantiGene ViewRNA ISH Tissue 1-plex Assay Kit and a QuantiGene ViewRNA Chromogenic Signal Amplification Kit and imaged by fluorescence microscopy.
Laser microdissection and microarray analysis
Frozen sections of three wild type and three conditional knockout E10.5 embryo eyes were laser microdissected using a Leica Microsystems LMD6000 instrument (Buffalo Grove, IL). The surface ectoderm or lens pit and distal optic vesicle or retina were separately collected, RNA was purified and amplified using a NuGEN Ovation Pico WTA system V2 kit (San Carlos, CA), as described previously (Huang et al., 2011). Triplicate samples were used to probe an Illumina Mouse6 V2 bead microarray and results were analyzed using Genome Studio software (Illumina, San Diego, CA). Results of the microarray analysis are available in the GEO repository at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE62536
Supplementary Material
Highlights.
Conditional deletion of Bmp4 from the optic vesicle prevented retina and lens formation in mice
In the absence of Bmp4 the entire optic vesicle became retinal pigmented epithelium (RPE)
Bmp4 is required for RPE formation in chicken embryos, the opposite of its role in mice
Caution should be exercised in extrapolating results from chicken eye development to eye development in mammals
Acknowledgements
The authors are grateful to Dr. Brigid Hogan for providing the Bmp4 flox mice and to Dr. Milan Jamrich for the Rx-Cre mice. We also thank Dr. Peter Carlsson for providing the antibody to Foxe3. Ms. Helen Li assisted with the antibody staining and in situ hybridization. Research was supported by NIH grants EY04853 (DCB), NIH Core Grant EY02687 and an unrestricted grant to the DOVS from Research to Prevent Blindness. Partial support to the Genome Technologies Access Center for microarray analysis was provided by a NIH Clinical and Translational Science Award to Washington University [UL1TR000448].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Jie Huang developed the approach, performed experiments and data analysis. Ying Liu performed experiments and data analysis. Alina Oltean performed experiments. David Beebe developed the approach, performed data analysis and prepared the manuscript.
Literature Cited
- Cai Z, Tao C, Li H, Ladher R, Gotoh N, Feng G-S, Wang F, Zhang X. Deficient FGF signaling causes optic nerve dysgenesis and ocular coloboma. Development. 2013;140:2711–2723. doi: 10.1242/dev.089987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W, Lin Z, Kulessa H, Hebert J, Hogan BLM, Wu DK. Bmp4 Is Essential for the Formation of the Vestibular Apparatus that Detects Angular Head Movements. PLoS Genet. 2008;4:e1000050. doi: 10.1371/journal.pgen.1000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SJ, Li H, Zueckert-Gaudenz K, Paulson A, Guo F, Trimble R, Peak A, Seidel C, Deng C, Furuta Y, Xie T. Defective FGF signaling causes coloboma formation and disrupts retinal neurogenesis. Cell Res. 2012 doi: 10.1038/cr.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Hogan BLM. BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 1998;12:3764–3775. doi: 10.1101/gad.12.23.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia CM, Huang J, Madakashira BP, Liu Y, Rajagopal R, Dattilo L, Robinson ML, Beebe DC. The function of FGF signaling in the lens placode. Dev Biol. 2011;351:176–185. doi: 10.1016/j.ydbio.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot F, Cepko CL. Retinal fate and ganglion cell differentiation are potentiated by acidic FGF in an in vitro assay of early retinal development. Development. 1992;114:743–754. doi: 10.1242/dev.114.3.743. [DOI] [PubMed] [Google Scholar]
- Huang J, Rajagopal R, Liu Y, Dattilo LK, Shaham O, Ashery-Padan R, Beebe DC. The mechanism of lens placode formation: A case of matrix-mediated morphogenesis. Developmental Biology. 2011;355:32–42. doi: 10.1016/j.ydbio.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyer J, Kuhlman J, Afif E, Mikawa T. Optic cup morphogenesis requires pre-lens ectoderm but not lens differentiation. Developmental Biology. 2003;259:351–363. doi: 10.1016/s0012-1606(03)00205-7. [DOI] [PubMed] [Google Scholar]
- Hyer J, Mima T, Mikawa T. FGF1 patterns the optic vesicle by directing the placement of the neural retina domain. Development. 1998;125:869–877. doi: 10.1242/dev.125.5.869. [DOI] [PubMed] [Google Scholar]
- Kurose H, Okamoto M, Shimizu M, Bito T, Marcelle C, Noji S, Ohuchi H. FGF19-FGFR4 signaling elaborates lens induction with the FGF8-L-Maf cascade in the chick embryo. Dev Growth Differ. 2005;47:213–223. doi: 10.1111/j.1440-169X.2005.00795.x. [DOI] [PubMed] [Google Scholar]
- Le TT, Conley KW, Mead TJ, Rowan S, Yutzey KE, Brown NL. Requirements for Jag1-Rbpj mediated Notch signaling during early mouse lens development. Developmental Dynamics. 2012;241:493–504. doi: 10.1002/dvdy.23739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Morales J-R, Del Bene F, Nica G, Hammerschmidt M, Bovolenta P, Wittbrodt J. Differentiation of the Vertebrate Retina Is Coordinated by an FGF Signaling Center. Developmental Cell. 2005;8:565–574. doi: 10.1016/j.devcel.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Muller F, Rohrer H, Vogel-Hopker A. Bone morphogenetic proteins specify the retinal pigment epithelium in the chick embryo. Development, dev.02884. 2007 doi: 10.1242/dev.02884. [DOI] [PubMed] [Google Scholar]
- Murali D, Yoshikawa S, Corrigan RR, Plas DJ, Crair MC, Oliver G, Lyons KM, Mishina Y, Furuta Y. Distinct developmental programs require different levels of Bmp signaling during mouse retinal development. Development. 2005;132:913–923. doi: 10.1242/dev.01673. [DOI] [PubMed] [Google Scholar]
- Nakayama Y, Miyake A, Nakagawa Y, Mido T, Yoshikawa M, Konishi M, Itoh N. Fgf19 is required for zebrafish lens and retina development. Developmental Biology. 2008;313:752–766. doi: 10.1016/j.ydbio.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Nguyen M, Arnheiter H. Signaling and transcriptional regulation in early mammalian eye development: a link between FGF and MITF. Development. 2000;127:3581–3591. doi: 10.1242/dev.127.16.3581. [DOI] [PubMed] [Google Scholar]
- Ogino H, Fisher M, Grainger RM. Convergence of a head-field selector Otx2 and Notch signaling: a mechanism for lens specification. Development. 2008;135:249–258. doi: 10.1242/dev.009548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opas M, Dziak E. bFGF-Induced Transdifferentiation of RPE to Neuronal Progenitors Is Regulated by the Mechanical Properties of the Substratum. Developmental Biology. 1994;161:440–454. doi: 10.1006/dbio.1994.1043. [DOI] [PubMed] [Google Scholar]
- Park C, Hollenberg M. Basic fibroblast growth factor induces retinal regeneration in vivo. Dev Biol. 1989;134:201–205. doi: 10.1016/0012-1606(89)90089-4. [DOI] [PubMed] [Google Scholar]
- Pittack C, Grunwald GB, Reh TA. Fibroblast growth factors are necessary for neural retina but not pigmented epithelium differentiation in chick embryos. Development. 1997;124:805–816. doi: 10.1242/dev.124.4.805. [DOI] [PubMed] [Google Scholar]
- Pittack C, Jones M, Reh T. Basic fibroblast growth factor induces retinal pigment epithelium to generate neural retina in vitro. Development. 1991;113:577–588. doi: 10.1242/dev.113.2.577. [DOI] [PubMed] [Google Scholar]
- Spemann H. Uber Korrelationen in der Entwicklung des Auges. Verh. Anat. Ges. 1901;15:61–79. [Google Scholar]
- Steinfeld J, Steinfeld I, Coronato N, Hampel M-L, Layer PG, Araki M, Vogel-Höpker A. RPE specification in the chick is mediated by surface ectoderm-derived BMP and Wnt signalling. Development. 2013;140:4959–4969. doi: 10.1242/dev.096990. [DOI] [PubMed] [Google Scholar]
- Stratman JL, Barnes WM, Simon TC. Universal PCR genotyping assay that achieves single copy sensitivity with any primer pair. Transgenic research. 2003;12:521–522. doi: 10.1023/a:1024225408961. [DOI] [PubMed] [Google Scholar]
- Swindell EC, Bailey TJ, Loosli F, Liu C, Amaya-Manzanares F, Mahon KA, Wittbrodt J, Jamrich M. Rx-Cre, a tool for inactivation of gene expression in the developing retina. Genesis. 2006;44:361–363. doi: 10.1002/dvg.20225. [DOI] [PubMed] [Google Scholar]
- Vogel-Hopker A, Momose T, Rohrer H, Yasuda K, Ishihara L, Rapaport DH. Multiple functions of fibroblast growth factor-8 (FGF-8) in chick eye development. Mech Dev. 2000;94:25–36. doi: 10.1016/s0925-4773(00)00320-8. [DOI] [PubMed] [Google Scholar]
- Yamada R, Mizutani-Koseki Y, Koseki H, Takahashi N. Requirement for Mab21l2 during development of murine retina and ventral body wall. Developmental Biology. 2004;274:295–307. doi: 10.1016/j.ydbio.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Yun S, Saijoh Y, Hirokawa KE, Kopinke D, Murtaugh LC, Monuki ES, Levine EM. Lhx2 links the intrinsic and extrinsic factors that control optic cup formation. Development. 2009;136:3895–3906. doi: 10.1242/dev.041202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.