Abstract
Hair follicles contain nestin-expressing pluripotent stem cells, the origin of which is above the bulge area, below the sebaceous gland. We have termed these cells hair follicle-associated pluripotent (HAP) stem cells. In the present study, we established efficient cryopreservation methods of the hair follicle that maintained the pluripotency of HAP stem cells. We cryopreserved the whole hair follicle from green fluorescent protein transgenic mice by slow-rate cooling in TC-Protector medium and storage in liquid nitrogen. After thawing, the upper part of the hair follicle was isolated and cultured in Dulbecco's Modified Eagle's Medium (DMEM) with fetal bovine serum (FBS). After 4 weeks of culture, cells from the upper part of the hair follicle grew out. The growing cells were transferred to DMEM/F12 without FBS. After 1 week of culture, the growing cells formed hair spheres, each containing ∼1×102 HAP stem cells. The hair spheres contained cells that differentiated to neurons, glial cells, and other cell types. The thawed and cultured upper part of the hair follicle produced almost as many pluripotent hair spheres as fresh follicles. The hair spheres derived from slow-cooling cryopreserved hair follicles were as pluripotent as hair spheres from fresh hair follicles. In contrast, rapid-cooling (vitrification) cryopreservation poorly preserved the pluripotency of the hair follicle stem cells. Stem cell marker genes (nestin, Sox2, and SSEA-1) were as highly expressed in slow-rate cooled cryopreserved follicles, after thawing, as in fresh follicles. However, in the vitrification cryopreserved follicles, the expression of the stem cell marker genes was greatly reduced. Direct cryopreservation of hair spheres by either the rapid-cooling, or slow-cooling method, resulted in loss of pluripotency. These results suggest that the slow-rate cooling cryopreservation of the whole hair follicle is effective to store HAP stem cells. Stored HAP stem cells would be very useful in personalized regenerative medicine, enabling any individual to maintain a bank of pluripotent stem cells for future clinical use.
Introduction
The hair follicle is dynamic, cycling between the growth (anagen), regression (catagen), and resting (telogen) phases throughout the life of a mammal.1–3 The neuronal stem cell marker, nestin, is expressed in hair follicles in cells located above the bulge area (BA), below the sebaceous gland. The nestin-expressing hair follicle cells were discovered in transgenic mice with nestin-driven green fluorescent protein (ND-GFP).4–7 Immunohistochemically, the nestin-positive hair follicle stem cells are keratin 15 (K15) negative. In vitro, the nestin-expressing cells can differentiate into neurons, glial cells, smooth muscle cells, keratinocytes, and other cell types. Therefore, we have termed these cells as hair follicle-associated pluripotent (HAP) stem cells.8
Upon transplantation to the severed sciatic nerve of the mouse, the HAP stem cells promoted axonal growth of preexisting neurons,9 which resulted in functional recovery. Transplanted HAP stem cells also promoted the functional recovery of the injured spinal cord, enabling mice to regain use of their hind legs.10
Nestin-expressing dermal papilla (DP) cells were also observed, but only in early and middle anagen. The BA had nestin expression throughout the hair cycle and to a greater extent than the DP. The cells from both regions had very long processes extending from them as shown by confocal microscopy. Nestin-expressing stem cells from both areas differentiated into neuronal cells at high frequency in vitro. Both nestin-expressing DP and BA cells differentiated into neuronal and glial cells after transplantation to the injured spinal cord and enhanced injury repair and locomotor recovery within 4 weeks.11
Using confocal imaging of whisker follicles from ND-GFP mice, we demonstrated that the BA is the source of the nestin-expressing HAP stem cells of the hair follicle. The nestin-expressing HAP stem cells migrated from the BA to the DP as well as into the surrounding skin tissues, including the epidermis, and during wound healing.12
The technique of long-term Gelfoam® histoculture of whiskers isolated from ND-GFP mice was established. Confocal imaging was used to monitor ND-GFP-expressing HAP stem cells trafficking in real time between the BA and DP to determine the fate of the stem cells. It was observed over a 2-week period that the stem cells trafficked from the BA toward the DP area and extensively grew out onto Gelfoam® forming nerve-like structures.13 The nerve-like structures contained β-III tubulin-positive fibers, consisting of ND-GFP-expressing cells, which extended up to 500 μm from the whisker nerve stump in Gelfoam® histoculture. The growing fibers had growth cones on their tips expressing F-actin. These findings indicate that β-III tubulin-positive fibers elongating from the whisker follicle sensory nerve stump were growing axons. The growing whisker sensory nerve was highly enriched in ND-GFP HAP stem cells, which appeared to play a major role in its elongation and interaction with other nerves in 3D culture, including the sciatic nerve, the trigeminal nerve, and the trigeminal nerve ganglion. These results suggest that a major function of the nestin-expressing HAP stem cells in the hair follicle is for growth of the follicle sensory nerve.14
In the present study, we evaluated cryopreservation methods of the whole hair follicle to preserve the differentiation potential of HAP stem cells.
Materials and Methods
GFP-transgenic mice (green mice)
Transgenic C57/B6-GFP mice15 were obtained from the Research Institute for Microbial Diseases (Osaka University, Osaka, Japan). The C57/B6-GFP mice expressed the Aequorea victoria GFP under the control of the chicken β-actin promoter and cytomegalovirus enhancer. All of the tissues from this transgenic line, with the exception of erythrocytes and hair, were fluorescent green under excitation light. All animal experiments were conducted according to the Guidelines for Animal Experimentation at the Kitasato University.
Isolation of vibrissa hair follicles
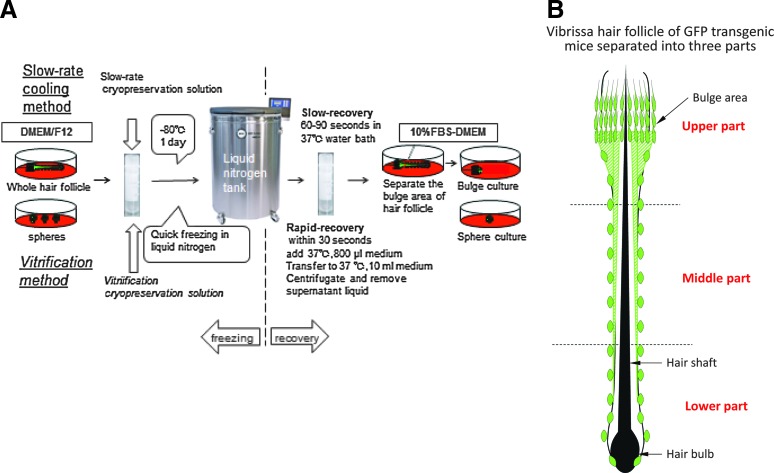
To isolate the vibrissa follicles from GFP-transgenic mice, the upper lip containing the vibrissa pad was cut under anesthesia and the inner surface was exposed. Entire vibrissa hair follicles were dissected under a binocular microscope and plucked from the pad by pulling them gently by the neck with fine forceps. The isolated vibrissa were washed in Dulbecco's Modified Eagle's Medium (DMEM)/F12 (Gibco-BRL) with 2% B-27 (Gibco-BRL) and 50 μg/mL gentamicin (Gibco-BRL). The follicles were divided into three parts using a surgical knife and fine forceps under a binocular microscope as previously described (Fig. 1B).16 All surgical procedures were done under a sterile environment.
FIG. 1.
(A) Schema of slow-rate cooling and rapid-cooling (vitrification) methods of cryopreserving hair follicles and hair spheres. (B) Schema for dividing the hair follicle into upper, middle, and lower parts.16
Hair follicle and hair sphere culture
The upper part of the vibrissa hair follicle was isolated and cultured in DMEM with 10% fetal bovine serum (FBS). After 4 weeks of culture, cells growing out from the upper follicle were treated enzymatically with Accumax (Innovative Cell Technologies, Inc.) to detach them. The detached cells were then transferred to nonadhesive culture dishes with DMEM/F12 containing 2% B-27. After 1 week of culture, the growing cells formed hair spheres containing nestin-expressing HAP stem cells. After the change of medium to DMEM containing 10% FBS and 2 days of additional culture, the GFP-expressing HAP stem cells differentiated to β-III tubulin-positive neurons, glial fibrillary acidic protein (GFAP)-positive glial cells, K15-positive keratinocytes, and smooth muscle actin (SMA)-positive smooth muscle cells.16
Cryopreservation of the whole hair follicle
Slow-rate cooling method
Five whole vibrissa follicles were transferred to cryovials, and TC-Protector medium (DS Pharma Biomedical Co.) was added (500 μL). Eighteen cryovials containing the vibrissa follicles were stored in a −80°C freezer overnight and then transferred to a liquid nitrogen tank. Three mice were used for this method for three independent experiments involving one mouse each.
The cryopreserved vibrissa follicles were thawed at 37°C in a water bath for 60–90 s (slow recovery) with gentle shaking and separated in three parts (upper, middle, and lower). The upper part of hair follicle was isolated and cultured in DMEM with 10% FBS.
Vitrification rapid-cooling method
Five whole vibrissa hair follicles were transferred to cryovials, and StemCell Keep medium (Bio Verde, Inc.) was added (200 μL). Eighteen cryovials were immediately placed (within 15 s) in a liquid nitrogen tank for storage. Three mice were used for this method for three independent experiments involving one mouse each.
To thaw the vitrified follicles, DMEM with 10% FBS, prewarmed to 37°C, was added (800 μL) to the cryovials and the follicles were thawed quickly by pipetting. The cryopreserved follicles were thawed in less than 30 s (rapid recovery) so that they could be rapidly transferred out of the StemCell Keep medium, which may be toxic. The thawed follicles were transferred to 10 mL DMEM with 10% FBS and centrifuged (280×g for 5 min). After centrifugation, the top clear layer of the liquid was removed and the follicles were separated into three parts (upper, middle, and lower) (Fig. 1B). The upper part of the hair follicle was isolated and cultured in DMEM with 10% FBS.
A total of nine mice were used for these methods, including three mice for isolation of follicles that were not cryopreserved.
Cryopreservation of hair spheres
Slow-rate cooling method
The upper part of hair follicle was isolated and cultured in DMEM with 10% FBS. After 4 weeks of culture, cells that grew out from the follicles were transferred to DMEM/F12 with 2% B-27 as described above. After 1 week of culture, hair spheres formed. Fifty spheres were transferred to cryovials and TC-Protector medium was added (500 μL). Nine cryovials with spheres were stored in a −80°C freezer overnight and then transferred to a liquid nitrogen tank. The stored spheres were thawed at 37°C in a water bath with gentle shaking. The spheres were cultured in DMEM with 10% FBS.
Vitrification rapid-cooling method
The upper part of the vibrissa hair follicle was isolated and cultured in DMEM with 10% FBS. After 4 weeks of culture, cells that grew out were transferred to DMEM/F12 with 2% B-27 as described above. After 1 week of culture, the cells formed hair spheres. Fifty spheres were transferred to cryovials and StemCell Keep medium was added (200 μL). Nine cryovials were immediately (within 15 s) placed in a liquid nitrogen tank for storage.
To thaw the vitrified spheres, DMEM with 10% FBS, prewarmed to 37°C, was added (800 μL) in the vials and the spheres were thawed quickly by pipetting. The thawed spheres were transferred to 10 mL medium and centrifuged (280×g for 5 min). After centrifugation, the top clear layer of the liquid was removed. The spheres were plated on DMEM with 10% FBS and cultured (Fig. 1A).
Immunohistochemistry of differentiated hair follicles
After a 1-week culture of hair spheres in DMEM with 10% FBS, the hair spheres were stained in individual wells with the following antibodies: anti-III-β-tubulin mAb (1:500, Tuj1 clone; Covance Research Products); anti-GFAP chicken polyclonal Ab (1:200; Abcam); anti-SMA mAb (1:400; Lab Vision); and anti-K15 mAb (1:100; Lab Vision). Secondary antibodies used were as follows: Alexa Fluor 568-labeled goat anti-mouse IgG (1:400; Molecular Probes) was used for anti-III-β-tubulin, anti-SMA, and anti-K15 and Alexa Fluor 568-labeled goat anti-chicken IgG (1:1000; Molecular Probes) was used for anti-GFAP. For quantification of the percentage of cells producing a given marker protein, at least four fields were photographed in any given experiment. The number of positive cells was determined relative to the total number of cells with 4′,6-diamidino-2-phenylindole dihydrochloride (Molecular Probes) nuclear staining.
Expression of stem cell marker genes
The mRNA levels of stem cell marker genes (nestin, Sox2, SSEA-1) were examined using real-time polymerase chain reaction (RT-PCR) analysis. After 1 week of culture in DMEM/F12 containing 2% B-27, the growing cells formed hair spheres. We extracted the total RNA from 100 hair spheres using the RNeasy Plus Mini kit (Qiagen). c-DNA was synthesized by high-capacity RNA with a cDNA kit (ABI). Real-time PCR on CFX96 (Bio-Rad) with TaqMan Gene Expression Assays (ABI) and TaqMan Probes were used as follows: r18s:Hs99999901_s1; Nestin: Mm00450205_m1; Sox2: Mm03053810_s1; and SSEA-1: Mm00487448_s1. The mRNA levels were normalized by comparison with r18s.
Western blot analysis was also used to detect the expression of SSEA-1. After 1 week of culture in DMEM/F12 containing 2% B-27, the growing cells formed hair spheres. Total proteins (30 μg/well) from hair spheres were subjected to 4–20% SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) and then transferred to Immobilon-P membranes (Millipore Corporation). SSEA-1 was detected by the anti-SSEA-1 primary antibody (1:250; BioLegend), followed by a mixture of peroxidase-conjugated anti-mouse IgA, IgG and IgM (Chemicon) with enhanced chemiluminescence plus a Western blotting detection system (Amersham Biosciences). Results are expressed as mean±standard deviation (SD) for three samples each.
Statistical analysis
All experiments were conducted in three independent cultures. Results are expressed as mean±SD. P-values were calculated with the paired Student's t-test.
Result and Discussion
Effects of cryopreservation of the whisker hair follicle
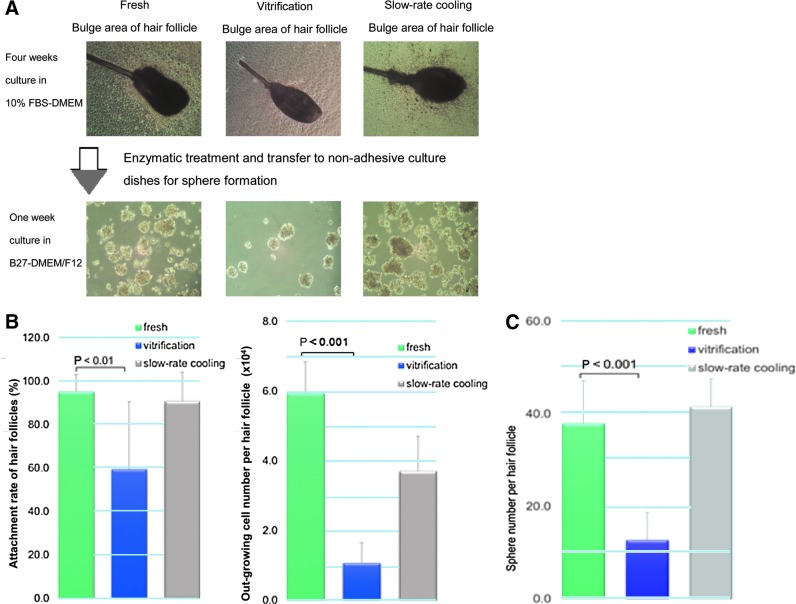
After thawing and 4 weeks culture in DMEM with 10% FBS, the upper parts of hair follicles were placed in dishes. The attachment rate of the hair follicles and number of cells growing out from the follicle were quantified as follows: 95.2%±7.7% of fresh hair follicles (nonfrozen control) attached and 5.9±0.9×104 cells per hair follicle grew out; 90.8%±13.3% of hair follicles cryopreserved by the slow-rate cooling method attached and 3.7±1.3×104 cells per hair follicle grew out; and 59.4%±31.1% of hair follicles cryopreserved by the vitrification method attached (p<0.01 compared to fresh hair follicles) and 1.1±0.6×104 cells per hair follicle grew out (p<0.001 compared to fresh hair follicles) (Fig. 2A, B).
FIG. 2.
(A) Images demonstrating the recovery of sphere formation after cryopreservation by slow-rate cooling or rapid-cooling vitrification compared to fresh follicles. (B) Comparison of the attachment rate and growing cell number from fresh and cryopreserved hair follicles using slow-rate cooling or rapid-cooling vitrification after thawing and 4 weeks of culture in Dulbecco's Modified Eagle's Medium (DMEM) with 10% fetal bovine serum (FBS). Results are mean±standard deviation (SD) for three independent experiments (three independent mice for each method of cryopreservation and nonfrozen control). (C) Comparison of sphere number, produced from fresh follicles and follicles cryopreserved by slow-rate cooling or rapid-cooling vitrification, per upper part of the hair follicle. Results are mean±SD for three independent experiments (three samples each).
The upper part of the fresh vibrissa had 38.0±9.1 hair spheres growing, with each hair sphere containing ∼1×102 nestin-expressing HAP stem cells. Slow-rate cooling-cryopreserved hair follicles formed 41.6±5.9 hair spheres per upper part of hair follicle, with each hair sphere containing ∼1×102 nestin-expressing HAP stem cells. In contrast, with hair follicles cryopreserved by the vitrification method, only 12.7±3.7 hair spheres grew per upper part of hair follicle (p<0.001 compared to fresh hair follicles), with each hair sphere containing ∼1×102 nestin-expressing HAP stem cells (Fig. 2C).
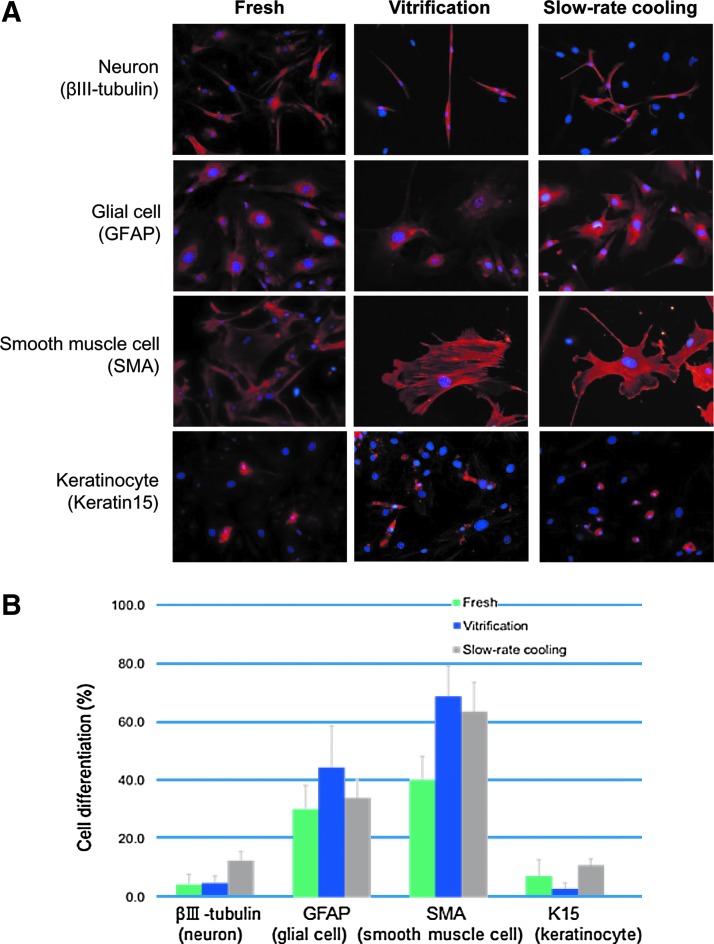
With hair spheres derived from fresh hair follicles, ∼4.3%±3.6% cells differentiated to anti-III-β-tubulin-positive neurons; 30.2%±8.1% of the cells differentiated to GFAP-positive glial cells; 40.2%±8.0% of the cells differentiated to SMA-positive smooth muscle cells; and 7.2%±5.7% of the cells differentiated to K15-positive keratinocytes. With hair spheres derived from slow-rate cooling-cryopreserved hair follicles, ∼12.6%±3.2% of the cells differentiated to anti-III-β-tubulin-positive neurons; 34.0%±6.6% of the cells differentiated to GFAP-positive glial cells; 63.7%±10.0% of the cells differentiated to SMA-positive smooth muscle cells; and 11.0%±2.1% of the cells differentiated to K15-positive keratinocytes. With hair spheres derived from vitrified-cryopreserved hair follicles, ∼4.8%±2.5% of the cells differentiated to anti-III-β-tubulin-positive neurons; 44.4%±14.2% of the cells differentiated to GFAP-positive glial cells; 68.8%±10.4% of the cells differentiated to SMA-positive smooth muscle cells; and 2.9%±2.0% of the cells differentiated to K15-positive keratinocytes (Fig. 3A, B).
FIG. 3.
(A) Images demonstrating the recovery of differentiation potential after cryopreservation by slow-rate cooling or rapid-cooling vitrification compared to fresh follicles. Red color is for specific antibody staining and blue color is for nuclear staining. (B) Comparison of pluripotency of fresh hair follicles and hair follicles cryopreserved by slow-rate cooling or rapid-cooling vitrification after a one-week culture of hair spheres in DMEM with 10% FBS. Results are mean±SD for three independent experiments (three independent mice for each method of cryopreservation and nonfrozen control).
Nestin mRNA levels, relative to r18s, were 1.00±0.11 in fresh hair follicles; 1.01±0.02 in slow-rate cooled hair follicles; and 0.05±0.01 in vitrification rapid-cooled hair follicles. Sox2 mRNA levels, relative to r18s, were 1.00±0.02 in fresh hair follicles; 0.81±0.07 in slow-rate cooled hair follicles; and 0.00±0.00 in vitrification rapid-cooled hair follicles. SSEA-1 mRNA levels, relative to r18s, were 1.00±0.02 in fresh hair follicles; 0.90±0.03 in slow-rate cooled hair follicles; and 0.10±0.02 in vitrification rapid-cooled hair follicles (Fig. 4A). Thus, the mRNA levels of stem-cell marker genes were maintained in the slow-rate cooling method, but significantly reduced in the vitrification rapid-cooling method (Fig. 4A).
FIG. 4.
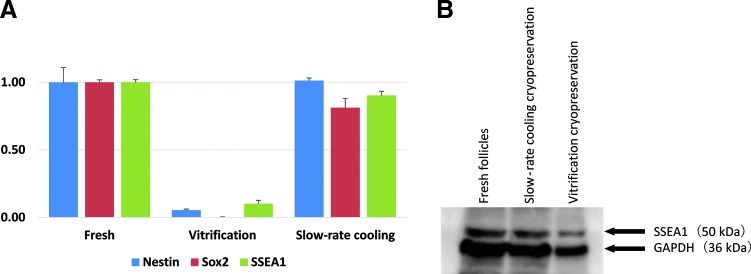
(A) Comparison of the mRNA levels of stem cell marker genes (nestin, Sox2, SSEA-1) were examined using real-time polymerase chain reaction (RT-PCR) analysis. Results are mean±SD for three samples each. The mRNA levels of stem cell marker genes were maintained in the slow-rate cooling method but significantly reduced in the rapid-cooling vitrification method. (B) Western-blot analysis was performed to detect the expression of SSEA-1. The protein level of SSEA-1 was maintained in the slow-rate cooling method but reduced in the rapid-cooling vitrification method.
Western blot analysis was performed to detect the expression of SSEA-1. The protein levels of SSEA-1 were maintained in the slow-rate cooling method and reduced in the vitrification rapid-cooling method (Fig. 4B).
Cryopreservation of hair spheres
Fresh hair spheres completely attached when placed in plastic dishes. In contrast, after slow-rate cooling cryopreservation, 5.4%±3.2% of the hair spheres survived. With vitrification rapid-cooled cryopreservation, ∼12.5%±10.8% of the hair spheres survived. However, after thawing, slow-rate cooled and vitrification rapid-cooled hair spheres did not differentiate.
Our results demonstrate that slow-rate cooling cryopreservation of hair follicles preserves the same attachment rate and production of pluripotent hair spheres as fresh hair follicles. Vitrification cooling-cryopreserved hair follicles were inferior to fresh hair follicles with respect to their attachment rate in culture and production of pluripotent hair spheres.
We have previously shown that human HAP stem cells from the scalp can also differentiate into neurons, glia, keratinocytes, smooth muscle cells, and melanocytes in vitro. Human HAP stem cells were transplanted in the severed sciatic nerve of the mouse, where they differentiated into GFAP-positive Schwann cells and promoted the recovery of preexisting axons, leading to nerve regeneration. The regenerated nerve recovered function.17
In previous experiments, the upper part of the mouse hair follicle containing HAP stem cells without culture was used directly for injection into the severed sciatic nerve in mice. The implanted HAP stem cells grew and promoted joining of the severed nerve. The transplanted HAP stem cells differentiated mostly to glial cells forming myelin sheaths, which promoted axonal growth and functional recovery of the severed nerve.18
HAP stem cells from human hair follicles offer high potential for clinical use in regenerative medicine. Autologous HAP stem cells would not be immunologically rejected and do not have the ethical or oncogenic problems as do human oocytes, embryonic stem cells, or iPS cells.
Our results suggest that the cryopreservation of the whole hair follicle with slow-rate cooling is useful to maintain the pluripotential of HAP stem cells of the hair follicle. The thawed follicles could be directly used for transplantation to severed nerves or spinal cords or cultured to produce hair spheres, which could be first differentiated or directly used for transplantation. The slow-rate cooling cryopreservation technique described in this report should be very useful for regenerative medicine, enabling each person to maintain a bank of accessible pluripotent stem cells for future clinical use. Slow-rate cooling cryopreservation of the hair follicle also preserved the expression of stem cell marker genes nestin, Sox2, and SSEA-1. Future experiments will study how differentiation is regulated in HAP stem cells and how cryopreservation may facilitate specific differentiation of HAP stem cells.
Acknowledgments
This study was supported, in part, by the National Institute of Neurological Disorders and Stroke grant NS086217 and the Parents' Association Grant of Kitasato University, School of Medicine.
Dedication
This article is dedicated to the memory of A.R. Moossa, MD.
Disclosure Statement
No competing financial interests exist.
References
- 1.Hoffman R.M. The hair follicle as a gene therapy target. Nat Biothechnol 18, 20, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Oshima H., Rochat A., Kedzia C., Kobayashi K., and Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell 104, 233, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Cotsarelis G., Sun T.T., and Lavker R.M. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 61, 1329, 1990 [DOI] [PubMed] [Google Scholar]
- 4.Li L., Mignone J., Yang M., Matic M., Penman S., Enikolopov G., et al. Nestin expression in hair follicle sheath progenitor cells. Proc Natl Acad Sci U S A 100, 9958, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amoh Y., Li L., Yang M., Moossa A.R., Katsuoka K., Penman S., et al. Nascent blood vessels in the skin arise from nestin-expressing hair-follicle cells. Proc Natl Acad Sci U S A 101, 13291, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amoh Y., Li L., Katsuoka K., Penman S., and Hoffman R.M. Multipotent nestin-positive, keratin-negative hair-follicle bulge stem cells can form neurons. Proc Natl Acad Sci U S A 102, 5530, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amoh Y., Li L., Katsuoka K., and Hoffman R.M. Chemotherapy targets the hair-follicle vascular network but not the stem cells. J Invest Dermatol 127, 11, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Hoffman R.M. Nestin-expressing hair follicle-accessible-pluripotent (HAP) stem cells for nerve and spinal cord repair. Cells Tissues Organs, 2015. [Epub ahead of print]; DOI: 10.1159/000366098 [DOI] [PubMed] [Google Scholar]
- 9.Amoh Y., Li L., Campillo R., Kawahara K., Katsuoka K., Penman S., et al. Implanted hair follicle stem cells form Schwann cells that support repair of severed peripheral nerves. Proc Natl Acad Sci U S A 102, 17734, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amoh Y., Li L., Katsuoka K., and Hoffman R.M. Multipotent hair follicle stem cells promote repair of spinal cord injury and recovery of walking function. Cell Cycle 7, 1865, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Liu F., Uchugonova A., Kimura H., Zhang C., Zhao M., Zhang L., et al. The bulge area is the major hair follicle source of nestin-expressing pluripotent stem cells which can repair the spinal cord compared to the dermal papilla. Cell Cycle 10, 830, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Uchugonova A., Duong J., Zhang N., König K., and Hoffman R.M. The bulge area is the origin of nestin-expressing pluripotent stem cells of the hair follicle. J Cell Biochem 112, 2046, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Duong J., Mii S., Uchugonova A., Liu F., Moossa A.R., and Hoffman R.M. Real-time confocal imaging of trafficking of nestin-expressing multipotent stem cells in mouse whiskers in long-term 3-D histoculture. In Vitro Cell Dev Biol Anim 48, 301, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Mii S., Duong J., Tome Y., Uchugonova A., Liu F., Amoh Y., et al. The role of hair follicle nestin-expressing stem cells during whisker sensory-nerve growth in long-term 3D culture. J Cell Biochem 114, 1674, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Okabe M., Ikawa M., Kominami K., Nakanishi T., and Nishimune Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett 407, 313, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Amoh Y., Mii S., Aki R., Hamada Y., Kawahara K., Hoffman R.M., et al. Multipotent nestin-expressing stem cells capable of forming neurons are located in the upper, middle, and lower part of the vibrissa hair follicle. Cell Cycle 11, 3513, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amoh Y., Kanoh M., Niiyama S., Hamada Y., Kawahara K., Sato Y., et al. Human hair follicle pluripotent stem (hfPS) cells promote regeneration of peripheral-nerve injury: an advantageous alternative to ES and iPS cells. J Cell Biochem 107, 1016, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Amoh Y., Hamada Y., Aki R., Kawahara K., Hoffman R.M., and Katsuoka K. Direct transplantation of uncultured hair-follicle pluripotent stem (hfPS) cells promotes the recovery of peripheral nerve injury. J Cell Biochem 110, 272, 2010 [DOI] [PubMed] [Google Scholar]