Abstract
Background
High fructose diet (HFD) induces dyslipidemia and insulin resistance in experimental animals and humans with incomplete mechanistic understanding. By utilizing mice and hamsters as in vivo models, we investigated whether high fructose consumption affects serum PCSK9 and liver LDL receptor (LDLR) protein levels.
Results
Feeding mice with a HFD increased serum cholesterol and reduced serum PCSK9 levels as compared with the mice fed a normal chow diet (NCD). In contrast to the inverse relationship in mice, serum PCSK9 and cholesterol levels were co-elevated in HFD-fed hamsters. Liver tissue analysis revealed that PCSK9 mRNA and protein levels were both reduced in mice and hamsters by HFD feeding, however, liver LDLR protein levels were markedly reduced by HFD in hamsters but not in mice. We further showed that circulating PCSK9 clearance rates were significantly lower in hamsters fed a HFD as compared with the hamsters fed NCD, providing additional evidence for the reduced hepatic LDLR function by HFD consumption. The majority of PCSK9 in hamster serum was detected as a 53 kDa N-terminus cleaved protein. By conducting in vitro studies, we demonstrate that this 53 kDa truncated hamster PCSK9 is functionally active in promoting hepatic LDLR degradation.
Conclusion
Our studies for the first time demonstrate that high fructose consumption increases serum PCSK9 concentrations and reduces liver LDLR protein levels in hyperlipidemic hamsters. The positive correlation between circulating cholesterol and PCSK9 and the reduction of liver LDLR protein in HFD-fed hamsters suggest that hamster is a better animal model than mouse to study the modulation of PCSK9/LDLR pathway by atherogenic diets.
Keywords: high fructose diet, PCSK9, LDL receptor, hamsters, dyslipidemia
1. Background
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a liver-derived plasma protein that exerts a profound effect on the circulating LDL-cholesterol (LDL-C) levels through its ability to promote degradation of hepatic LDL receptor (LDLR) [1]. PCSK9 is synthesized as a ~75 kDa precursor in the endoplasmic reticulum that undergoes autocatalytic cleavage to release the N terminal propeptide of 14 kDa. The ~62 kDa mature form of PCSK9 and the 14 kDa propeptide are bound together noncovalently and are secreted into plasma as a heterodimer. In addition, the circulating 62 kDa form of human PCSK9 can be further cleaved at arginine 218 by other members of the PCSK family furin and/or PC5/6A to generate a ~53 kDa truncated form, which is designated as PCSK9-ΔN218 [2-4]. In plasma, PCSK9 binds to the EGF-A extracellular domain of LDLR and subsequently, the PCSK9-LDLR protein complex is endocytosed by the hepatocytes followed by its degradation in the lysosome compartment [5]. Thus, PCSK9 plasma levels by impacting LDLR protein directly influence the level of plasma LDL-C [6]. It has been shown that in the general population, plasma levels of PCSK9, despite large variations, positively correlated with plasma levels of LDL-C. A 100 ng/ml increase in plasma PCSK9 level was associated with a 4.5-mg/dl increase in plasma LDL-C concentration in women and a 3.2-mg/dl increase in LDL-C in men [7].
Given the critical function played by PCSK9 in the regulation of LDLR protein levels, PCSK9 has emerged as a key regulator of LDL-C homeostasis and a promising new therapeutic target in the clinical management of hypercholesterolemia [8]. Since the initial discovery of PCSK9 in 2003, many experimental and therapeutic approaches using anti-PCSK9 antibodies have been employed to interfere with the PCSK9-LDLR interactions [9]. In addition, strategies of antisense oligonucleotides [10] or small interference RNAs (siRNAs) [11] have been employed to reduce PCSK9 expression. In particular, large clinical trials involving anti-PCSK9 monoclonal antibodies have successfully established the efficacy and safety of PCSK9 inhibition in patients with hyperlipidemia [12].
Despite the wealth of knowledge currently available in understanding the impact of pharmacological interventions on plasma PCSK9, relatively limited studies have been carried out aimed at examining the influence of nutritional factors on the plasma PCSK9 levels and their relevance to hepatic LDLR protein abundance. Interestingly, a recent study to examine the effect of short term high-fat diet, high-fat/high-protein diet or high-fructose diet on plasma PCSK9 levels has revealed that while high-fat and high-fat/high-protein short-term diets did not significantly change PCSK9 concentrations, high-fructose diet increased plasma PCSK9 concentrations by 28% in healthy volunteers and by 34% in offspring of type 2 diabetic that are more prone to develop insulin resistance [13].
It is well recognized that feeding a fructose-enriched diet for more than one week can induce dyslipidemia, particularly hypertriglyceridemia and insulin resistance in both experimental animals and in human subjects [14]. The mechanisms underlying fructose-induced dyslipidemia have only been partially delineated, and some evidence suggests that induction of de novo lipogenesis through activation of SREBP1c in liver contributes to the elevated serum VLDL-cholesterol and triglycerides [15]. At present, however, it remains to be determined whether PCSK9 plays any role in the development of dyslipidemia in response to high dietary fructose consumption.
Various mouse models have been used to study dyslipidemia and obesity induced by fructose-enriched diet as well as high cholesterol and high fat diets [16-21]. In addition to mice, the HFD-fed golden Syrian hamster model has been used as a metabolic model of insulin resistance and dyslipidemia [22-25]. Given the importance of plasma PCSK9 and its recent association with the dietary fructose consumption in humans, we initiated the current studies to use mice and hamsters as two in vivo models to examine the impact of chronic feeding of HFD on plasma PCSK9 levels and hepatic LDLR expression. Here we provide evidence that HFD differentially impacts circulating PCSK9 concentrations and hepatic LDLR levels in mice and hamsters. HFD-induced hyperlipidemia is inversely correlated with circulating PCSK9 in mice; however, in hamsters HFD feeding led to elevated circulating LDL-C concentrations and increased plasma PCSK9 levels, which were accompanied by a significant reduction of liver LDLR amount. Thus, similar to human situations, hamsters exhibit a positive correlation between circulating PCSK9 level and LDL-C concentration. Our study results suggest that while mice have been extensively used in the genetic study of PCSK9 function in inducing LDLR degradation, hamster is a better model than mouse to study the modulation of PCSK9/LDLR pathway by dyslipidemic and atherogenic diets.
2. Materials and methods
2.1. Animals and diets
Ten-week old male golden Syrian hamsters were purchased from Harlan. Eight-week old male C57BL/6J mice were purchased from Jackson Labs (Bar Harbor, Maine). All animals were housed (mice, 4 animals/cage; hamsters, 2 animals/cage) under controlled temperature (72°F) and lighting (12 h light/dark cycle). Animals had free access to autoclaved water and food. Mice were fed either a rodent normal chow diet (NCD, n=8) or a HFD (n=8) containing 60% fructose (Dyets Inc., Bethlehem, PA) for three weeks. For hamsters, in the first study, sixteen hamsters were fed a NCD (n=8) or a HFD (n=8) for three weeks. In the second study, twelve hamsters were fed NCD (n=6) or HFD (n=6) for four weeks. At the experimental termination, all animals were sacrificed. Mouse serum and liver samples were collected after a 4 h fasting (9 AM to 1 PM) and hamster serum and liver samples were collected after an overnight fasting (5 PM to 9 AM). All serum and liver samples were stored at –80°C until being analyzed. Animal use and all experimental procedures were approved by Institutional Animal Care and Use Committee of the VA Palo Alto Health Care System.
2.2 Serum isolation and cholesterol determination
Fasting blood samples (0.2 ml) were collected from the retro-orbital plexus using heparinized capillary tubes under anesthesia (2–3% isoflurane and 1–2 L/min oxygen) and serum was isolated at room temperature and stored at -80°C. Standard enzymatic methods were used to determine TC, TG, and HDL-C with commercially available kits purchased from Stanbio Laboratory (Texas, USA). Each sample was assayed in duplicate.
2.3 HPLC analysis of lipoprotein-cholesterol profiles
Fifty μl of hamster serum sample from two serum samples of the same diet group were pooled together and a total of 4 pooled samples from NCD group and 4 pooled samples from HFD group were analyzed for cholesterol and triglyceride levels of each of the major lipoprotein classes including chylomicron (CM, >80 mm), VLDL (30-80 nm), LDL (16-30 nm), and HDL (8-16 nm) with a dual detection HPLC system consisting of two tandem connected TSKgel Lipopropak XL columns (300 × 7.8-mm; Tosoh, Japan) at Skylight Biotech, Inc. (Tokyo, Japan) [26]. Likewise, pooled mouse serum samples of NCD and HFD groups were analyzed for cholesterol and triglyceride levels in different lipoprotein fractions after HPLC separation.
2.4 RNA isolation and quantitative real-time PCR
Total RNA isolation, generation of cDNA, and real-time PCR were conducted as previously reported [26]. Each cDNA sample was run in duplicate. The correct size of the PCR product and the specificity of each primer pair were validated by examination of PCR products on an agarose gel. Primer sequences of mouse and hamster genes used in real-time PCR are listed in Table 1. Target mRNA expression in each sample was normalized to the housekeeping gene GAPDH. The 2-ΔΔCt method was used to calculate relative mRNA expression levels.
Table 1.
real-time PCR primer sequences.
| Forward | Reverse | |
|---|---|---|
| Mouse | ||
| GAPDH | AACTTTGGCATTGTGGAAGG | GGATGCAGGGATGATGTTCT |
| FASN | GGCATCATTGGGCACTCCTT | GCTGCAAGCACAGCCTCTCT |
| HMGCR | CTTTCAGAAACGAACTGTAGCTCAC | CTAGTGGAAGATGAATGGACATGAT |
| HNFlα | GCACCAGAGACCCACGTGCC | GGCTTCCCCTCAGCTCCCGA |
| IDOL | AGGAGATCAACTCCACCTTCTG | ATCTGCAGACCGGACAGG |
| LDLR | ACCTGCCGACCTGATGAATTC | GCAGTCATGTTCACGGTCACA |
| PCSK9 | TTGCAGCAGCTGGGAACTT | CCGACTGTGATGACCTCTGGA |
| SORT1 | CCCGGACTTCATCGCCAAG | AGGACGAGAATAACCCCAGTG |
| SREBP1 | CAAGGCCATCGACTACATCCG | CACCACTTCGGGTTTCATGC |
| SREBP2 | CCAAAGAAGGAGAGAGGCGG | CGCCAGACTTGTGCATCTTG |
| Hamster | ||
| GAPDH | AACTTTGGCATTGTGGAAGG | GGATGCAGGGATGATGTTCT |
| HMGCR | GACGGTGACACTTACCATCTGT | GATGCACCGTGTTATGGTGA |
| HNFlα | GAGGTGGCTCAGCAATTCAC | CACTCCTCCACCAAGGTCTC |
| IDOL | CACCCACACCAGTCTTCTCA | ACCTGGCATGTCCAGTAAGC |
| LDLR | TTGGGTTGATTCCAAACTCC | GATTGGCACTGAAAATGGCT |
| PCSK9 | TGCTCCAGAGGTCATCACAG | GTCCCACTCTGTGACATGAAG |
| SORT1 | CCCGGACTTCATCGCCAAG | AGGACGAGAATAACCCCAGTG |
| SREBP1 | GCACTTTTTGACACGTTTCTTC | CTGTACAGGCTCTCCTGTGG |
| SREBP2 | GAGAGCTGTGAATTTTCCAGTG | CTACAGATGATATCCGGACCAA |
2.5 Western blot analysis
Approximately 50 mg of frozen liver tissue was homogenized in RIPA buffer containing 1 mM PMSF and protease inhibitor cocktail (Roche). After protein quantitation using BCA protein assay reagent (Pierce), 50 μg of homogenate proteins from individual liver samples were resolved by SDS-PAGE and transferred to nitrocellulose membranes. Anti-LDLR antibody was purchase from BioVision (Mountain View, CA). Anti-hamster PCSK9 antibody that recognizes the C-terminal end of hamster PCSK9 (CRNRPSAKASWVHQ) was developed in our laboratory and previously reported [27]. Anti-mouse PCSK9 antibody was obtained from R&D System. Anti-actin and anti-V5 antibodies were purchased from Sigma-Aldrich. Anti-ApoB antibody was a gift from Dr. Khosrow Adeli (Hospital for Sick Children, University of Toronto, Canada). Anti-ApoA1 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Immunoreactive bands of predicted molecular mass were visualized using SuperSignal West Substrate (Thermo Scientific) and quantified with the Alpha View Software with normalization by signals of β-actin.
2.6 Detection of mouse PCSK9 in serum
Secreted PCSK9 in sera of C57BL/6J mice were measured using mouse PCSK9 ELISA kit obtained from R&D System according to the instruction.
2.7 Detection of hamster PCSK9 in serum
Detection of PCSK9 in hamster serum samples was conducted by immuoprecipitation (IP), followed by Western blotting using anti-hamster PCSK9 antibody as we previously described [27].
2.8 Human PCSK9 cloning, purification, and injection into hamsters
Human PCSK9 coding sequence was cloned into the pcDNA3.1/V5-His TOPO vector (Invitrogen) and transfected into HEK293 cells. His-tagged PCSK9 was purified from the medium of transfected HEK293 cells using the ProBond protein purification system (Life technology). Protein purity was examined by gel electrophoresis with Coomassie blue staining. The purified PCSK9 concentration was determined using a human PCSK9 ELISA kit purchased from R&D System. Hamsters were fed a NCD or a HFD for three weeks prior to the injection of purified human PCSK9 (5μg per hamster) through the retro-orbital plexus under anesthesia. Blood was collected before injection and at the indicated time points after injection. Hamsters were euthanized at the end of the experiment and liver samples were collected. The level of human PCSK9 in hamster serum was determined using human PCSK9 ELISA kit (R & D system).
2.9 Plasmids and transient transfections
pIR-hFurin and pIR-mPC5A were previously described [2]. Hamster PCSK9 was cloned into pcDNA™3.1/V5-His TOPO [27]. HEK293 cells transfected with His-hamPCSK9 without or with pIR-hFurin or with pIR-mPC5A. The HEK293 medium was collected 48 h after transfection. The conditioned HEK293 medium was then added to Huh7 cells. After 4 h, The Huh7 cells were collected for protein analysis.
2.10 Detection of PCSK9 in different lipoprotein fractions after serum lipoprotein separation by ultracentrifugation
Lipoproteins (VLDL, IDL, LDL, and HDL) were isolated from pooled hamster serum samples of HFD or NCD group by sequential flotation ultracentrifugation [28,29]. Briefly, the initial density of serum was 1.006 g/mL. To isolate VLDL + IDL, solid KBr was added to serum to increase the density to 1.025 g/mL and the serum samples were then centrifuged at 150,000g for 16 h. The upper mobile layer was collected as VLDL + IDL. The remaining part was resuspended and solid KBr was added to adjust the density to 1.063 g/mL. Serum samples were then centrifuged at 150,000g for 24 h and the top faction was collected as LDL. Finally, solid KBr was added to the remaining part to increase the density to 1.210 g/mL and samples were centrifuged at 150,000g for 40 h. The upper layer was collected as HDL. Equal volume of VLDL + IDL, LDL or HDL fractions of NCD and HFD were examined for the presence of PCSK9 by Western blotting with anti-hamster PCSK9 antibody.
2.11 Statistical analysis
Values are presented as mean ± SEM. Significant differences between diet groups were assessed by two-tailed Student t-test (nonparametric Mann Whitney test). Statistical significance is displayed as p < 0.05 (one asterisk), p < 0.01 (two asterisks) or p < 0.001 (three asterisks).
3. Results
3.1 HFD feeding elevated serum LDL-C and reduced circulating PCSK9 levels without impacting on hepatic LDLR protein abundance in mice
Various genetically engineered mouse models with PCSK9 knockdown and overexpression have been widely used to study PCSK9-mediated hepatic LDLR degradation [30-34]. To examine the effect of fructose diet on serum PCSK9 and hepatic LDLR levels in normal mice, male C57BL/6J mice were fed a HFD or a NCD for three weeks. Measurement of individual serum samples shows that HFD feeding elevated serum total cholesterol (TC) by 31% (p<0.01), non-HDL-C by 42% (p<0.01) and HDL-C by 27% (p<0.01) as compared with those in the mice fed a NCD (Fig. 1A). Next, we performed HPLC analysis of profiles of lipoprotein-cholesterol (Fig. 1B) and triglyceride (TG) (Fig. 1C) in pooled serum samples of NCD and HFD groups. The results showed a 29% increase in total cholesterol and a prominent increase of 68% in LDL-associated cholesterol by HFD feeding. Total serum TG as well as TG associated with VLDL, LDL and HDL fractions were actually decreased in HFD fed mice. Further analysis of cholesterol and TG contents in liver tissues revealed that the decrease in serum TG was accompanied by a significant increase of 53% (p<0.001) in hepatic TG level in HFD fed mice as compared to NCD fed mice, while the hepatic TC level did not change (Fig. 1D). Importantly, in opposite to the increased serum cholesterol levels, serum PCSK9 levels were significantly reduced in HFD-fed mice than that of control mice (Fig. 1F) while serum insulin levels were unchanged (Fig. 1E).
Figure 1. Serum LDL-C and PCSK9 levels were inversely correlated in mice fed a HFD.
Male C57BL/6J mice fed a HFD (n=8) or a NCD (n=8) for three weeks were euthanized after a 4-h fast. Serum and liver samples were collected. (A) Serum TC and HDL-C of individual mouse were measured after three weeks on NCD or HFD. The concentrations of non-HDL-C were derived after subtraction of HDL-C from total cholesterol. (B, C) The profiles of lipoproteincholesterol and triglyceride of pooled mouse serum samples from HFD and NCD groups were analyzed using HPLC. (D) Hepatic TC and TG levels were measured in individual mouse liver samples. (E) Individual mouse serum insulin levels were quantified by a mouse/rat insulin ELISA kit. (F) Individual mouse serum PCSK9 levels were quantified by a mouse PCSK9 ELISA kit. (G) Real-time PCR analysis of mouse liver mRNA levels of indicated genes in HFD and NCD groups. (H) 50 μg of homogenate proteins of individual mouse liver samples were resolved by SDS-PAGE. LDLR and PCSK9 were detected by Western blot. The protein abundance of LDLR or PCSK9 was quantified with the Alpha View Software with normalization by signals of β-actin. Values are the mean ± SEM of 8 samples per group. *p < 0.05, **p < 0.01 and ***p < 0.001 as compared to NCD group.
To seek a clear understanding of the reducing effect of HFD on serum PCSK9 in mice, hepatic mRNA and protein levels of PCSK9 were analyzed by qPCR and Western blot. Fig. 1G shows that HFD feeding reduced PCSK9 mRNA levels by 44% (p<0.05). We also measured mRNA levels of three additional SREBP2-target genes and showed that HMGCR was downregulated significantly while a tendency in lowering LDLR and SREBP2 was detected but that was not statistically significant. HFD feeding did not affect the mRNA levels of SREBP1 and FASN. Furthermore, HFD feeding did not change the mRNA level of HNF1α, a critical transactivator for PCSK9 gene expression in addition to SREBP2 [35,36]. The gene product of IDOL is implicated for degrading LDLR protein in extra hepatic tissues [37] and the SORT1 gene product [38] is recently shown to affect PCSK9 secretion. We did not observe any changes in their hepatic mRNA expressions by HFD feeding.
Protein levels of PCSK9 and LDLR in mouse liver homogenates were individually assessed by Western blotting and the signals were quantified (Fig. 1H). Similar to the reduction of circulating PCSK9, hepatic PCSK9 levels in whole liver homogenates were 20% lower (p<0.001) in HFD-fed mice as compared with the mice fed NCD. Surprisingly, despite an approximate 50% reduction in serum PCSK9, hepatic LDLR protein levels were not increased, instead, we observed a slight reduction in LDLR protein levels in HFD group without reaching a statistical significance. Altogether, these results demonstrated that HFD feeding for three weeks induced a modest dyslipidemia, and the TC and LDL-C are inversely correlated with endogenous serum PCSK9 levels in mice.
3.2 HFD feeding induced dyslipidemia in hamsters by elevating serum PCSK9 levels and reducing hepatic LDLR protein content
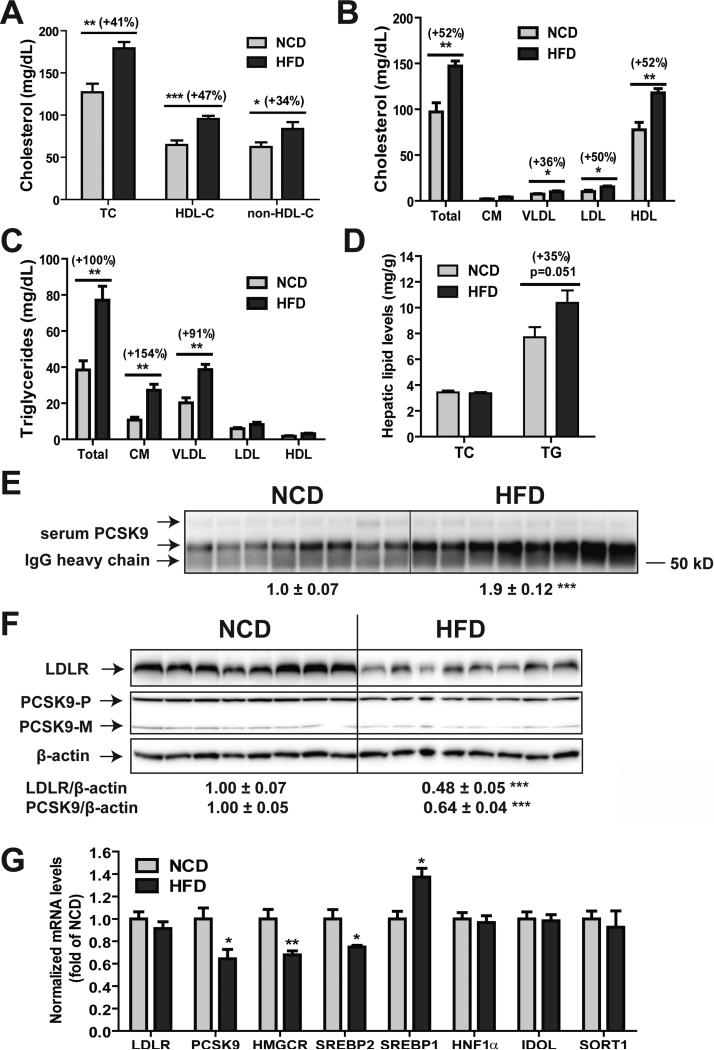
Hamsters were fed a HFD for three weeks, which increased plasma TC by 41% (p < 0.01), non-HDL-C by 34% (p<0.01) and HDL-C by 47% (p<0.001) as compared with those in the hamsters fed a NCD (Fig. 2A). Additional HPLC analysis of individual lipoprotein cholesterol profiles in serum samples of NCD and HFD groups largely confirmed the elevation of serum cholesterol levels by HFD feeding. Different from mice, feeding hamsters HFD for three weeks greatly elevated serum triglyceride levels, particularly in chylomicron and VLDL fractions without a statistically significant accumulation in hepatic TG (Fig. 2D).
Figure 2. Elevation of hamster serum PCSK9 and LDL-C and reduction of hepatic LDLR in response to HFD feeding.
Hamsters fed a HFD (n=8) or a NCD (n=8) for three weeks were euthanized after overnight fast and serum and liver samples were collected. (A) Serum TC and HDL-C of individual hamster were measured after three weeks on NCD or HFD. The concentrations of non-HDL-C were derived after subtraction of HDL-C from TC. (B, C) Profiles of lipoprotein-cholesterol and triglyceride of pooled hamster serum samples from HFD and NCD groups were analyzed using HPLC. (D) Hepatic TC and TG levels were measured in individual hamster liver samples. (E) 15 μL of individual hamster serum samples were used for PCSK9 IP and Western blotting. The intensity of PCSK9 bands was quantified with the Alpha View Software. (F) 50 μg of homogenate proteins of individual hamster liver samples were assessed for PCSK9 and LDLR protein levels by Western blot and the signals were quantified as described in Fig. 1. (G) Real-time PCR analysis of hamster liver mRNA levels of indicated genes in HFD and NCD groups. Values are the mean ± SEM of 8 samples per group. *p < 0.05, **p < 0.01 and ***p < 0.001 as compared to NCD group.
We performed IP of individual serum samples obtained from both diet groups and the amount of PCSK9 in immunoprecipitates was quantified by Western blotting. Serum PCSK9 levels in HFD group were significantly increased by 1.9-fold (p<0.001) as compared with the NCD group (Fig. 2E).
Next, we performed Western blotting using individual liver homogenates to analyze hepatic LDLR and PCSK9 protein levels. We observed a significant reduction in LDLR protein content (-52%, p<0.001) in HFD-fed hamsters as compared with the NCD group (Fig. 2F). In opposite to the increase in serum PCSK9, hepatic PCSK9 levels were 36% lower (p<0.001) in HFD group than the NCD group.
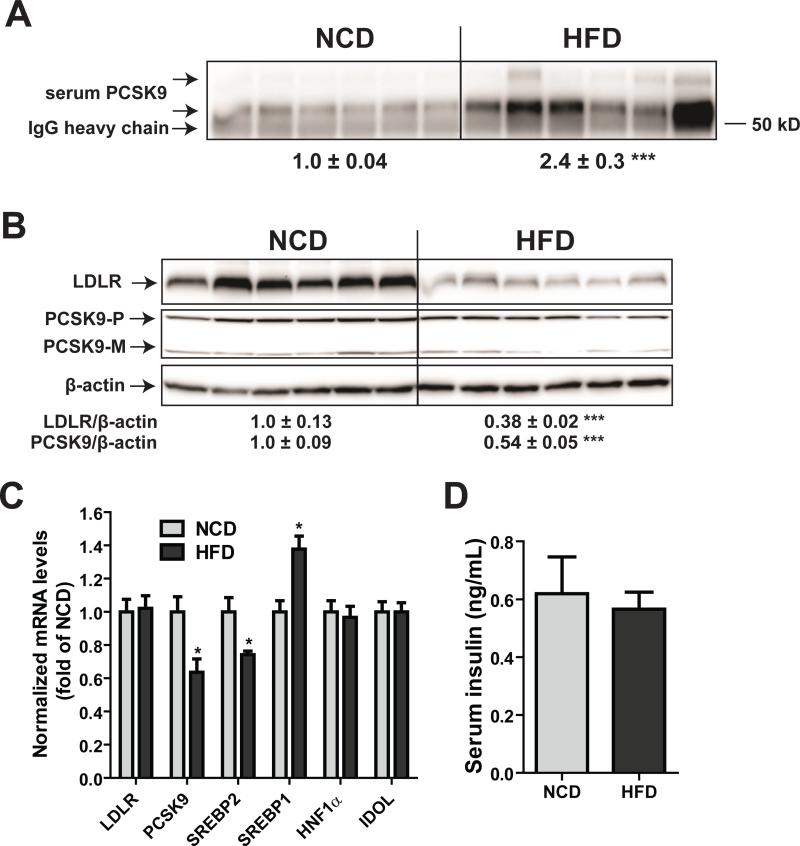
Hepatic gene expression analysis by real-time qPCR further confirmed the reduced mRNA expression of PCSK9 (-36%, p<0.05) along with other SREBP2-target genes (HMGCR and SREBP2) in HFD-fed hamsters as compared with those in NCD (Fig. 2G). In contrast to the reduction in SREBP2 target genes, we detected a significant increase in SREBP1c mRNA levels in liver samples of HFD group, which is in agreement with a previous report demonstrating the upregulation of hamster SREBP1c gene transcription in response to high fructose feeding [25]. Similar to the mouse study, we did not observe any changes in the hepatic mRNA expression of IDOL or SORT1. To confirm these new findings, the diet experiment was repeated by using another cohort of hamsters (Fig. 3 A-D). Again, we observed increased serum PCSK9, reduced liver LDLR protein, and decreased PCSK9 mRNA levels without significant lowering of LDLR mRNA levels or serum insulin levels in response to HFD feeding.
Figure 3. Reduction of hepatic LDLR protein and elevation of serum PCSK9 in HFD-fed hamsters.
Hamsters fed a HFD (n=6) or a NCD (n=6) for four weeks were euthanized after overnight fast and serum and liver samples were collected. (A) 15 μL of individual hamster serum samples were used for PCSK9 IP and Western blotting. The intensity of PCSK9 bands was quantified with the Alpha View Software. (B) 50 μg of homogenate proteins of individual hamster liver samples were assessed for PCSK9 and LDLR protein levels by Western blot and the signals were quantified. (C) Real-time PCR analysis of hamster liver mRNA levels of indicated genes in HFD and NCD groups. (D) Individual hamster serum insulin levels were quantified by a mouse/rat insulin ELISA kit. Values are the mean ± SEM of 6 hamsters per group. *p < 0.05 and ***p < 0.001 as compared to the NCD group.
These results from two independent diet studies clearly demonstrate that HFD feeding increased serum PCSK9 and LDL-C levels with reduced hepatic LDLR protein abundance in hamsters. This positive correlation between serum PCSK9 levels and LDL-C concentration in hamsters is similar to previously reported human situations [6,7].
3.3 HFD decreases the clearance rate of circulating PCSK9 in hamsters
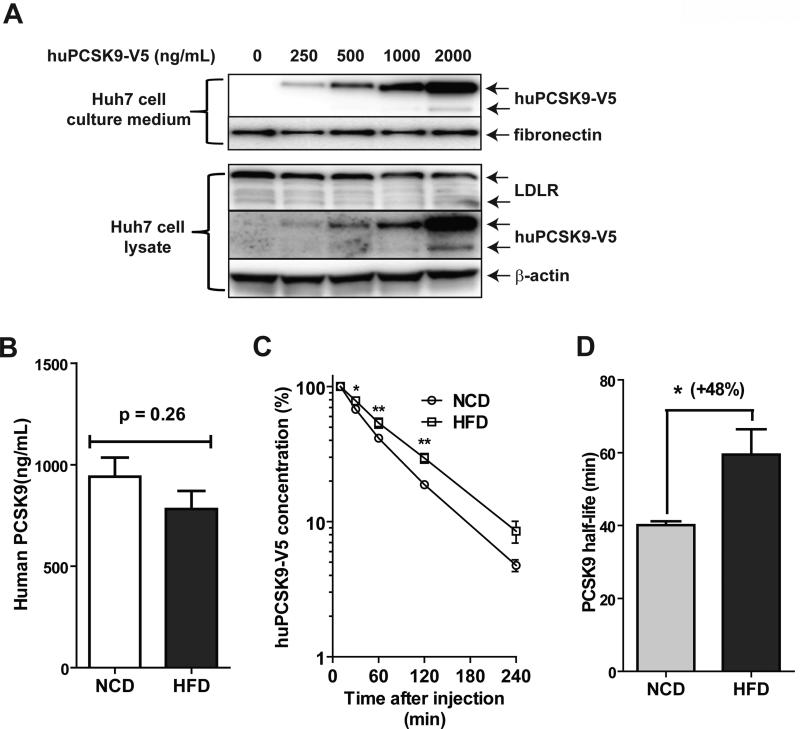
We wanted to know whether HFD feeding affected the interactive relationship between circulating PCSK9 and hepatic LDLR. Purified human PCSK9 tagged with V5 at different concentrations was added into Huh7 medium and its levels in culture medium and in cell lysates were quantified by Western blotting using anti-V5 antibody. Fig. 4A shows that the dose-dependent increases in recombinant PCSK9 in the culture medium led to a gradual decrease in LDLR protein abundance in Huh7 cells, and thereby confirming its function in causing LDLR degradation.
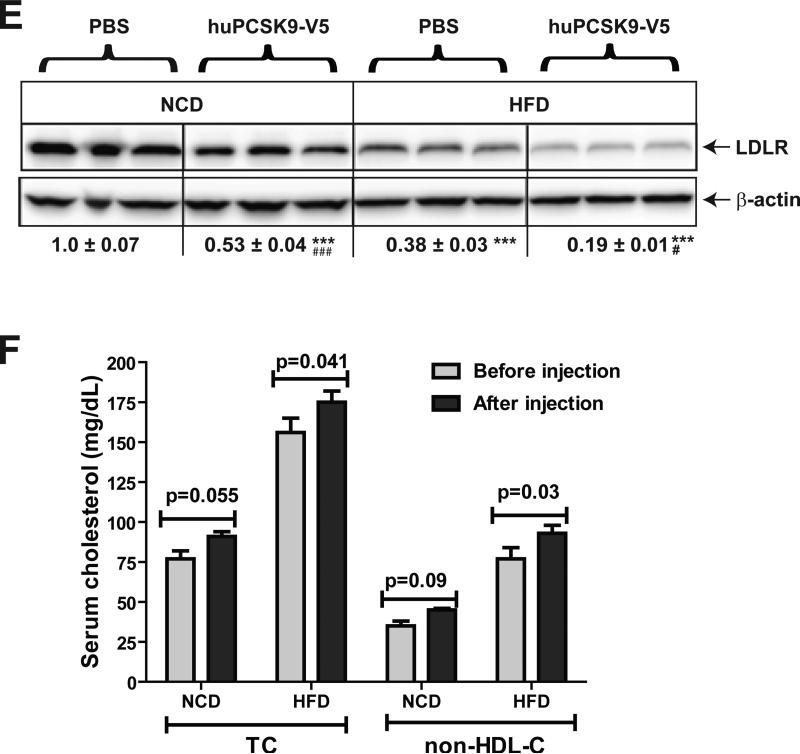
Figure 4. Determination of serum PCSK9 clearance rates in hamsters fed a HFD versus a NCD.
V5-tagged human PCSK9 was expressed in HEK293 cells and purified as described in methods. (A) Indicated doses of purified human PCSK9 were added to Huh7 medium for 4 h. Culture medium and total cell lysates were analyzed for V5-tagged human PCSK9 and LDLR protein levels by Western blotting using anti-V5 antibody and anti-LDLR antibody, respectively. (B-D) Purified human PCSK9 was injected into hamsters fed a NCD (n=4) or a HFD (n=4) for 3 weeks. Serum samples were collected before and at different time points after human PCSK9 injection (10 min, 30 min, 1 h, 2 h, and 4 h). Human PCSK9 levels in hamster serum samples were measured using a human PCSK9 ELISA kit. Human PCSK9 concentrations in hamster serum after 10 min injection are presented in B. In C, the amount of human PCSK9 at 10 min was set to 100, the concentrations at different time point was plotted against time after injection, and fitted to an exponential decay curve. The half-life (T1/2) of human PCSK9 in hamsters after injection was calculated and presented in D. (E) 4 h after injection of human PCSK9, hamsters were euthanized and LDLR protein levels in liver tissues were analyzed by Western blotting and the signals were quantified. (F) TC and HDL-C in serum samples of before and after 4 h of PCSK9 injection were measured. The concentrations of non-HDL-C were derived after subtraction of HDL-C from total cholesterol. Values are the mean ± SEM of 4 samples per group. *p < 0.05, **p < 0.01 and ***p < 0.001 as compared to NCD group. #p < 0.05 and ###p < 0.001 as compared to groups injected with PBS.
We then injected human PCSK9 into hamsters, previously maintained on a NCD or a HFD for three weeks. Fig. 4B shows that immediately after injection, human PCSK9 reached to comparable serum levels in NCD and HFD fed hamsters (940 ± 94 ng/mL in NCD, 781 ± 90 ng/mL in HFD). However, the clearance rate of injected human PCSK9 was significantly lower in HFD fed hamsters than hamsters fed NCD (Fig. 4C). The calculated half-life of injected human PCSK9 was increased from 40 min in NCD-fed hamsters to 59 min (p<0.05) in HFD-fed animals (Fig 4D). At the end of experimentation, hamsters were euthanized and livers were collected to measure LDLR protein levels (Fig. 4E). The liver LDLR protein levels were substantially lower in HFD group as compared to NCD group, consistent with the data presented above. Administration of human PCSK9 reduced liver LDLR protein abundance in both NCD and HFD hamster groups. Quantification of cholesterol levels in serum samples collected before and after 4-h of injection of human PCSK9 (Fig. 4F) demonstrated that downregulation of hepatic LDLR by PCSK9 treatment was accompanied by a transient elevation of serum TC and non-HDL-C levels in both diet groups. The increases in TC and non-HDL-C levels achieved statistical significance only in HFD fed and PCSK9 treated hamsters. Altogether, these data provide additional evidence for a reduced hepatic LDLR function under HFD feeding, which might explain the elevation of serum PCSK9 in the absence of increased PCSK9 liver synthesis.
3.4 Characterization of serum hamster PCSK9 molecular species
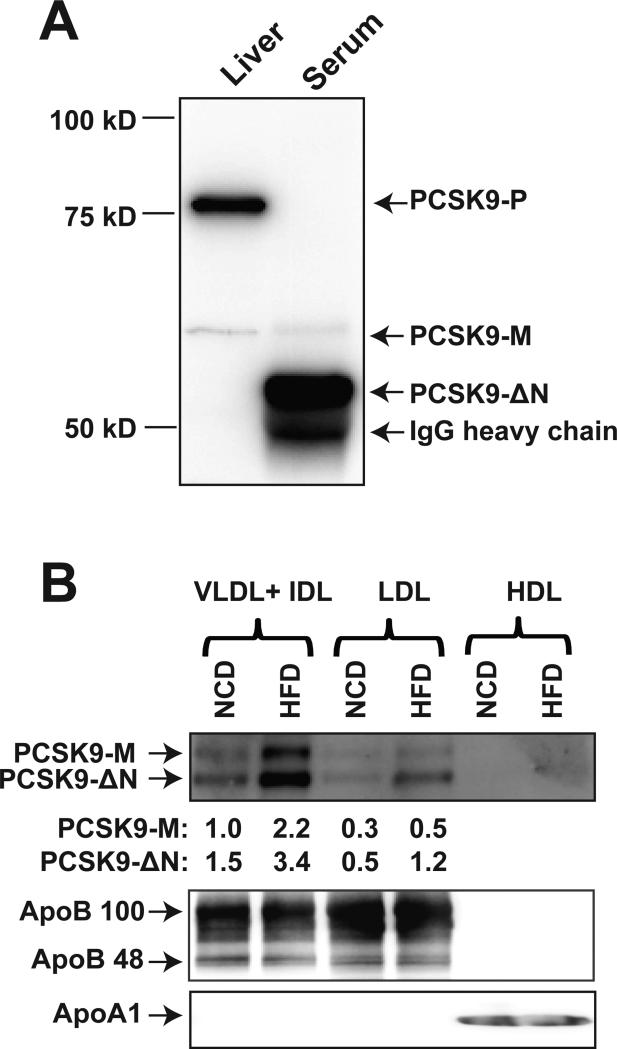
It has been shown that in hepatocytes, furin and PC5/6A cleave human PCSK9 at Arg218-Gln219 to generate PCSK9-ΔN218 (~53 kDa) that circulates in blood along with the intact PCSK9 form as a minor component [3]. The data presented in Fig. 2&3 show that unlike humans, hamsters have low levels of the mature form of PCSK9 (~62 kDa) and that the majority of serum PCSK9 detected by IP-Western blotting had an approximate molecular weight of 53 kDa, corresponding to the truncated human PCSK9-ΔN218. Protein sequence comparison between hamster PCSK9 (698 amino acid) and human PCSK9 (692 amino acid) shows that the prosegment cleavage site and the further N-segment furin cleavage site are identical (Supplemental Figure I), suggesting that the abundant 53 kDa is likely the truncated hamster PCSK9-ΔN224, equivalent to human PCSK9-ΔN218. We evaluated the relative levels of various serum and hepatic forms of PCSK9 in hamsters (Fig. 5A). The major precursor form of PCSK9 (PCSK9-P, 75 kDa) and a minor mature PCSK9 (PCSK9-M, 62 kDa) were the only two PCSK9 forms (bands) being detected in hamster liver, whereas the abundant molecular species of PCSK9 in hamster serum detected by IP was the 53 kDa, possibly the PCSK9-ΔN224 form.
Figure 5. Analysis of hepatic and circulating forms of hamster PCSK9 and PCSK9 association with lipoprotein particle types of hamster serum.
(A) Hamster liver protein homogenates and serum PCSK9 IP sample were subjected to SDS-PAGE and different forms of hamster PCSK9 were detected by Western blotting using anti-hamster PCSK9 antibody. (B) Different lipoprotein fractions were isolated from pooled hamster serum samples of NCD and HFD groups by sequential flotation of ultracentrifugation. 5 μL of VLDL + IDL, LDL or HDL fractions were analyzed for amounts of PCSK9, ApoB or ApoA1 by Western blotting. The data shown are representative of two separate experiments with similar results.
In mouse and human plasmas, PCSK9 was found to partially associate with apoB containing lipoprotein particles, mostly LDL [31,40]. To determine whether the N-segment truncation affects hamster PCSK9 association with lipoproteins, we fractioned pooled sera samples from NCD and HFD fed hamsters. Fig. 5B shows that the amount of the lipoprotein-associated PCSK9 (53 kDa and 60 kDa) were found both in VLDL+IDL fraction and LDL fraction. Feeding hamster HFD increased PCSK9 levels without affecting the relative distribution of PCSK9 forms between VLDL+IDL and LDL fractions. In agreement with the existing literature, no PCSK9 was detected in the HDL fraction.
3.5 Hamster PCSK9-ΔN224 is functional in mediating hepatic LDLR degradation
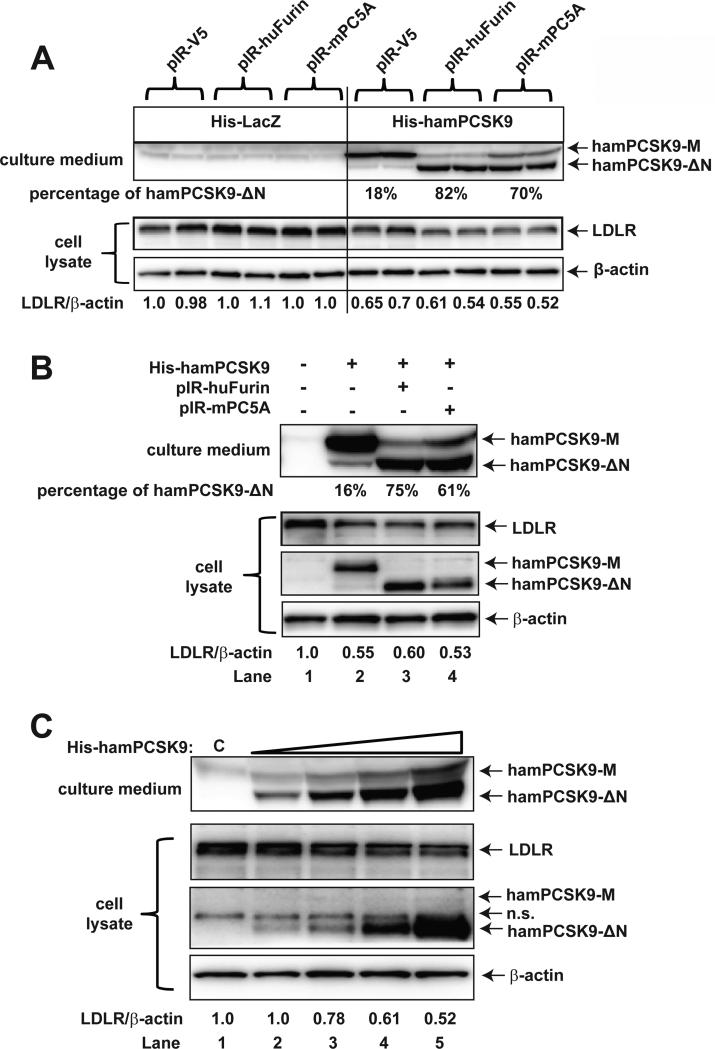
There have been conflicting reports with regard to the functions of human PCSK9-ΔN218. Some studies reported that the N-segment truncated PCSK9 form is functionally inactive [2,3], whereas another study reported the PCSK9-ΔN218 and the mature form of PCSK9 exhibit comparable functional activities [4]. Given that hamster ~53 kDa species is the relatively abundant circulatory form of PCSK9, we assessed its function in causing the degradation of hepatic LDLR. We performed in vitro experiments using Huh7 cells transiently overexpressing hamster PCSK9 alone or in combination with furin/PC5A (Fig. 6A). Western blotting of the culture medium from transfected Huh7 cells demonstrated that coexpression of hamster PCSK9 with either furin or PC5A increased the 53 kDa form to 82% and 70% of total PCSK9 species respectively, compared to 18% of the total PCSK9 species in the absence of furin or PC5A coexpression, suggesting that the hamster serum 53 kDa form is indeed the furin/PC5A enzymatic cleavage product. Importantly, the corresponding LDLR protein levels were reduced to similar extents in Huh7 cells expressing hamster PCSK9 without or with furin/PC5A coexpression. These results suggest that the truncated hamster PCSK9 is capable of inducing hepatic LDLR degradation.
Figure 6. Assessment of intrinsic activity of hamster mature and N-segment truncated forms of PCSK9 in hepatic cells.
(A) Huh7 cells were co-transfected with His-HamPCSK9 and pIR-huFurin/pIR-mPC5A plasmids. His-LacZ and pIR-V5 were used as control plasmids. Media and cell lysates were collected 48 h after transfection. Anti-hamster PCSK9 antibody, which does not recognize endogenous human PCSK9, was used to detect transfected hamster PCSK9. Data shown are representative of 3-4 separate transfection experiments with similar results. (B) HEK293 cells were co-transfected with His-HamPCSK9 and pIR-huFurin/pIR-mPC5A plasmids. HEK293 medium were collected 48 h after transfection and was added to naïve Huh7 cells. After 4 h incubation, medium was removed and Huh7 cells were washed with cold PBS and lysed for Western blot analysis. Hamster PCSK9 levels in culture medium and cell lysate were detected by Western blotting using anti-hamster PCSK9 antibody. Data shown are representative of 3-4 separate transfection experiments with similar results. (C) HEK293 cells were co-transfected with His-HamPCSK9 and pIR-huFurin. HEK293 medium was collected 48 h after transfection and different amounts of the conditioned medium were added to naïve Huh7 cells (lanes 2-5; untransfected HEK293 cell medium was added to lane 1 as control). After 4 h incubation, medium was removed and Huh7 cells were washed with cold PBS and lysed for Western blot analysis. Hamster PCSK9 levels in culture medium and cell lysate were detected by Western blotting using anti-hamster PCSK9 antibody. N.S., nonspecific band.
To provide additional support to this conclusion, medium from HEK293 cells transfected with hamster PCSK9 without or with furin or PC5A were collected and added to naïve Huh7 cells. Without co-transfection with furin or PC5A, only 16% of total hamster PCSK9 species in the HEK293 medium was in the ΔN form; with co-transfection of furin or PC5A, 75% or 61% of total hamster PCSK9 species secreted in HEK293 medium was in the ΔN form (Fig. 6B upper panel). When equal amounts of HEK293 conditioned medium were added to naïve Huh7 cells, the PCSK9-containing media displayed similar levels of efficacy in suppressing LDLR protein levels in Huh7 cells (Fig. 6B, lower panel). This was correlated with similar levels of intracellular hamster PCSK9 no matter in mature form (lane 2) or the N-segment truncated form (lanes 3 and 4). These results further showed that both the mature and the ΔN form of hamster PCSK9 are endocytosed to the same extent by Huh7 cells. In addition, to demonstrate a dose-dependent effect of the cleaved hamster PCSK9 on LDLR protein, HEK293 cells were cotransfected with His-HamPCSK9 and pIR-huFurin. HEK293 medium was collected 48h after transfection and different amounts of the medium were added to naïve Huh7 cells for 4 h. Fig. 6C shows that HEK293 medium contained predominantly cleaved hamster PCSK9, and the increasing concentration of hamster PCSK9-ΔN in the culture medium led to a corresponding decrease in LDLR protein levels in Huh7 cells. We also observed increased accumulation of the cleaved hamster PCSK9 in Huh7 cell lysates. Taken together, these data provide additional support to our conclusion that both the mature form and the furin-cleaved form of hamster PCSK9 interact with hepatic LDLR and that both forms are functionally active in promoting LDLR degradation.
4. Discussion
In this current study, we examined the effects of dietary administration of fructose enriched diet on serum cholesterol and PCSK9 levels and hepatic expression of LDLR protein in two different animal models mice and hamsters. As summarized in Table 2, our results provide the first evidence that while HFD induced mild dyslipidemia in both mice and hamsters, it differentially modulated serum PCSK9 and hepatic LDLR expression. In mice, increased serum cholesterol concentrations were inversely associated with serum PCSK9 levels, whereas HFD feeding caused a co-elevation of TC and PCSK9 serum concentrations in hamsters.
Table 2.
Comparisons of fructose-diet induced changes of lipid levels and PCSK9/LDLR expression levels in mice and hamsters
| Mouse | Hamster | |
|---|---|---|
| Serum TC | ↑ | ↑ |
| Liver TC | - | - |
| Serum TG | ↓ | ↑ |
| Liver TG | ↑ | - |
| Serum PCSK9 | ↓ | ↑ |
| Liver PCSK9 protein | ↓ | ↓ |
| Liver PCSK9 mRNA | ↓ | ↓ |
| Liver LDLR protein | ↓ | ↓ |
| Liver LDLR mRNA | - | - |
Since the discovery of PCSK9 in 2003, various genetic engineered mouse models have been extensively utilized to demonstrate the function of circulating PCSK9 in mediating the degradation of hepatic LDLR protein and the consequential impact on plasma cholesterol levels. In PCSK9 knockout mice, liver LDLR protein was elevated nearly 3-fold and the plasma cholesterol levels fell by half [30]. Conversely, overexpression of PCSK9 in transgenic mice [34,40), as well as in mice infected with adenovirus expressing human PCSK9 [32,41,42], led to severe reductions of liver LDLR protein and hyperlipidemia. However, relative to the large number of PCSK9 studies of genetic manipulations, limited studies have been reported to examine the interactive relationship between endogenous PCSK9 and hepatic LDLR protein in normal mice under different nutritional conditions. In the present study, we observed that serum PCSK9 levels in mice fed a HFD were significantly reduced. Since the mRNA and protein levels of hepatic PCSK9 were both lower in HFD-fed mice than the NCD-fed control mice, the transcriptional suppression of PCSK9 by HFD is likely responsible for the reduced serum PCSK9 concentrations. Interestingly, despite approximate 50% reduction in serum PCSK9, liver LDLR protein levels were not increased; instead it showed a trend of slight reduction. Thus, our results suggest that in mice, LDLR protein levels are not sensitive to changes in endogenous serum PCSK9 levels. The inverse relationship between serum PCSK9 and LDL-C levels were also observed in our recent study of C57BL/6J mice fed a high-cholesterol diet [26].
In opposite to mice, in hamsters, we consistently observed a positive relationship between LDL-C and PCSK9 serum levels from two independent diet studies. Moreover, the increase in serum PCSK9 levels was accompanied with marked reductions of hepatic LDLR protein in both hamster cohorts in response to HFD feeding. Interestingly, the rise in circulating PCSK9 levels did not correspond to increased hepatic synthesis of PCSK9. Our hepatic gene expression analysis showed that HFD lowered the mRNA levels of PCSK9 and other SREBP2-target genes (HMGCR, SREBP2), while increasing SREBP1c gene expression in hamster liver. The inducing effect of HFD on transcription of lipogenic genes through activation of SREBP1c has been reported in other animal studies [14,18], our current study further linked fructose diet to deregulated SREBP2 pathway in hamsters. In mice, we did not observe elevated hepatic SPREBP1c mRNA levels in HFD group. It is unclear that the lack of induction of SREBP1c is related to the reduced serum PCSK9 level in HFD fed mice. Further studies are required to elucidate whether SREBP1c plays a role in HFD-induced reduction of serum PCSK9 in mice.
The opposed changes in serum and hepatic PCSK9 levels in HFD-fed hamsters led us to examine the serum clearance of PCSK9 in hamsters fed the two different diets. The approach of injecting human PCSK9 into mice has been successfully utilized to study the dynamics of interactive relationship of serum PCSK9 and hepatic LDLR in mice overexpressing PCSK9 [33]. By employing a similar strategy, we demonstrate that the serum clearance rate of injected human PCSK9 was significantly decreased in HFD fed hamsters. This piece of data provides additional support to a reduced LDLR function by HFD feeding, and may explain the excessive accumulation of serum PCSK9 in response to HFD feeding. Our results suggest that the elevated serum PCSK9 levels resulted in enhanced liver LDLR protein degradation, which, in turn resulted in the reduced removal of serum cholesterol via LDLR/endocytic pathway. Recent studies have identified sortilin, encoded by the hypercholesterolemia-risk gene SORT1, as a high-affinity sorting receptor for the PCSK9 [38]. Increased expression of sortilin in liver was correlated with higher plasma PCSK9 levels and reduced hepatic PCSK9 content. We measured liver SORT1 mRNA levels in HFD and NCD groups and did not detect a difference, suggesting that sortilin is unlikely to play a role in HFD-mediated elevation of serum PCSK9 levels.
Our studies demonstrate that the 53 kDa, possibly representing the N-segment furin cleaved mature PCSK9, is the abundant form of PCSK9 in hamster species, which is different from the dominant mature form of 62 kDa reported for human plasma and in mouse plasma. This raises an important question of whether this truncated PCSK9 is functionally active in causing the hamster hepatic LDLR degradation. Thorough co-expression of hamster PCSK9 with furin or PC5A, we demonstrate that the 53 kDa truncated PCSK9 species is capable of down-regulating LDLR protein levels in cultured hepatic cells, which were evident by the similar levels of endocytosed hamster PCSK9-M and hamster PCSK9-ΔN in Huh7 cell lysates and the similar extent of reduction of LDLR protein levels in Huh7 cells by the two forms of hamster PCSK9. During the preparation of this manuscript, a new study reported that furin-cleaved recombinant human PCSK9 (PCSK9-ΔN218) did not inhibit LDL uptake in HepG2 cells [43]. The discrepancy between our study and that new report could be caused by different experimental conditions or the difference in species, i.e. human versus hamster.
Because we did not separate the 53 kDa truncated PCSK9 species from the 62 kDa of hamster PCSK9 in the cell culture studies, we could not absolutely rule out the possibility that the small portion of the 62 kDa mature form of hamster PCSK9 added to the Huh7 cell culture medium was more functional than the truncated hamster PCSK9 and had higher contribution to the reduced LDLR protein in Huh7 cells. Future investigations using purified hamster truncated PCSK9 will be required to definitely address this question.
A recent human study by Cariou et al. showed that a short exposure of healthy volunteers to a high-fructose diet elevated serum PCSK9 levels while LDL-C levels remained unchanged during the 4-days fructose diet. In our hamster study we observed significant elevations of both LDL-C and PCSK9 serum levels. The difference in the duration of the fructose diet between our hamster study (three weeks) and in that human study (4 days) could explain the different effects of fructose diet on serum cholesterol levels. It is well known that dietary consumption of excessive amounts of fructose leads to abnormalities in lipid and glucose homeostasis in humans [14,44]. Based upon our study results, we speculate that longer exposure to fructose diet could induce dyslipidemia in humans with elevated serum PCSK9 and reduced hepatic LDLR-mediated LDLC removal.
5. Conclusion
We have demonstrated that HFD feeding inversely modulates LDL-C and PCSK9 serum levels in mice without a significant impact on liver LDLR protein levels. However, HFD significantly elevates LDL-C and PCSK9 serum levels and reduces liver LDLR protein abundance in hamsters. This work underscores the influence of nutritional factors affecting PCSK9 metabolism in a species-specific manner.
Supplementary Material
Highlights.
High fructose diet (HFD) induced an inverse relationship between serum LDL-C and PCSK9 levels without impacting hepatic LDLR protein levels in mice
HFD feeding induces dyslipidemia in hamsters with elevated serum PCSK9 levels and reduced liver LDLR protein abundance
HFD feeding reduces serum clearance of PCSK9 in hamsters
Hamster is a better model than mouse to study the regulation of PCSK9-LDLR pathway by atherogenic diets
Acknowledgement
Authors wish to thank Dr. Khosrow Adeli for providing anti-ApoB antibody and the ApoB detection protocol and Ms. Yang Cortez for her technical assistance in fractionation and isolation of plasma lipoproteins. This study was supported by the Department of Veterans Affairs Office of Research and Development, Medical Research Service and by grants (1R01AT006336; 2R01HL33881) from National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
References
- 1.Horton JD, Cohen JC, Hobbs HH. Molecular biology of PCSK9: its role in LDL metabolism. Trends Biochem Sci. 2006;32:71–77. doi: 10.1016/j.tibs.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjannet S, Rhainds D, Hamelin J, Nassoury N, Seidah NG. The proprotein convertase (PC) PCSK9 is inactivated by furin and/or PC5/6A: functional consequences of natural mutations and post-translational modifications. J Biol Chem. 2006;281:30561–30572. doi: 10.1074/jbc.M606495200. [DOI] [PubMed] [Google Scholar]
- 3.Essalmani R, Susan-Resiga D, Chamberland A, et al. In vivo evidence that furin from hepatocytes inactivates PCSK9. J Biol Chem. 2011;286:4257–4263. doi: 10.1074/jbc.M110.192104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lipari MT, Li W, Moran P, et al. Furin-cleaved proprotein convertase subtilisin/kexin type 9 (PCSK9) is active and modulates low density lipoprotein receptor and serum cholesterol levels. J Biol Chem. 2012;287:43482–43491. doi: 10.1074/jbc.M112.380618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grefhorst A, McNutt MC, Lagace TA, Horton JD. Plasma PCSK9 preferentially reduces liver LDL receptors in Mice. J Lipid Res. 2008;49:1303–1311. doi: 10.1194/jlr.M800027-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambert G, Ancellin N, Charlton F, et al. Plasma PCSK9 concentration correlate with LDL and total cholesterol in diabetic patients and are decreased by fenofibrate treatment. Clinical Chemistrry. 2008;54:1038–1045. doi: 10.1373/clinchem.2007.099747. [DOI] [PubMed] [Google Scholar]
- 7.Lakoski SG, Lagace TA, Cohen JC, Horton JD, Hobbs HH. Genetic and metabolic determinants of plasma PCSK9 levels. J Clin Endocrinol Metab. 2009;94:2537–2543. doi: 10.1210/jc.2009-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seidah NG. PCSK9 as a therapeutic target of dyslipidemia. Expert Opin. Ther. Targets. 2009;13:19–28. doi: 10.1517/14728220802600715. [DOI] [PubMed] [Google Scholar]
- 9.Hooper AJ, Burnett JR. Anti-PCSK9 therapies for the treatment of hypercholesterolemia. Expert. Opin Biol Ther. 2013;13:429–435. doi: 10.1517/14712598.2012.748743. [DOI] [PubMed] [Google Scholar]
- 10.Graham MJ, Lemonidis KM, Whipple CP, et al. Antisense inhibition of proprotein convertase subtilisin/kexin type 9 reduces serum LDL in hyperlipidemic mice. J Lipid Res. 2007;48:763–767. doi: 10.1194/jlr.C600025-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Frank-Kamenetsky M, Grefhouse A, Anderson NN, et al. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholestertol in rodents and LDL cholesterol in nonhuman primates. Proc. Natl. Acad. Sci. USA. 2008;105:11915–11920. doi: 10.1073/pnas.0805434105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ling H, Burns TL, Hilleman DE. An update on the clinical development of proprotein convertase subtilisin kexin 9 inhibitors, novel therapeutic agents for lowering low-density lipoprotein cholesterol. Cardiovasc. Ther. 2014;32:82–88. doi: 10.1111/1755-5922.12056. [DOI] [PubMed] [Google Scholar]
- 13.Cariou B, Langhi C, Le BM, et al. Plasma PCSK9 concentrations during an oral fat load and after short term high-fat, high-fat high-protein and high-fructose diets. Nutr. Metab (Lond) 2013;10:4. doi: 10.1186/1743-7075-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tappy L, Le KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90:23–46. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- 15.Koo HY, Miyashita M, Cho BH, Nakamura MT. Replacing dietary glucose with fructose increases ChREBP activity and SREBP-1 protein in rat liver nucleus. Biochem Biophys Res Commun. 2009;390:285–289. doi: 10.1016/j.bbrc.2009.09.109. [DOI] [PubMed] [Google Scholar]
- 16.Saravanan M, Pandikumar P, Saravanan S, Toppo E, Pazhanivel N, Ignacimuthu S. Lipolytic and antiadipogenic effects of (3,3-dimethylallyl) halfordinol on 3T3-L1 adipocytes and High fat and fructose diet induced obese C57/BL6J mice. Eur. J Pharmacol. 2014 doi: 10.1016/j.ejphar.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Cheng Q, Zhang X, Wang O, et al. Anti-diabetic effects of the ethanol extract of a functional formula diet in mice fed with a fructose/fat-rich combination diet. J Sci Food Agric. 2015;95:401–408. doi: 10.1002/jsfa.6737. [DOI] [PubMed] [Google Scholar]
- 18.Nunes PM, Wright AJ, Veltien A, et al. Dietary lipids do not contribute to the higher hepatic triglyceride levels of fructose- compared to glucose-fed mice. FASEB J. 2014;28:1988–1997. doi: 10.1096/fj.13-241208. [DOI] [PubMed] [Google Scholar]
- 19.Dissard R, Klein J, Caubet C, et al. Long term metabolic syndrome induced by a high fat high fructose diet leads to minimal renal injury in C57BL/6 mice. PLoS. One. 2013;8:e76703. doi: 10.1371/journal.pone.0076703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Debosch BJ, Chen Z, Finck BN, Chi M, Moley KH. Glucose transporter-8 (GLUT8) mediates glucose intolerance and dyslipidemia in high-fructose diet-fed male mice. Mol. Endocrinol. 2013;27:1887–1896. doi: 10.1210/me.2013-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh AB, Kan CF, Shende V, Dong B, Liu J. A novel posttranscriptional mechanism for dietary cholesterol-mediated suppression of liver LDL receptor expression. J Lipid Res. 2014 doi: 10.1194/jlr.M049429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Briand F, Thieblemont Q, Muzotte E, Sulpice T. High-fat and fructose intake induces insulin resistance, dyslipidemia, and liver steatosis and alters in vivo macrophage-to-feces reverse cholesterol transport in hamsters. J Nutr. 2012;142:704–709. doi: 10.3945/jn.111.153197. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi AA, Webb J, Choi J, et al. Intestinal SR-BI is upregulated in insulin-resistant states and is associated with overproduction of intestinal apoB48-containing lipoproteins. Am. J Physiol Gastrointest. Liver Physiol. 2011;301:G326–G337. doi: 10.1152/ajpgi.00425.2010. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh J, Hayashi AA, Webb J, Adeli K. Postprandial dyslipidemia in insulin resistance: mechanisms and role of intestinal insulin sensitivity. Atheroscler. Suppl. 2008;9:7–13. doi: 10.1016/j.atherosclerosissup.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 25.Basciano H, Miller AE, Naples M, et al. Metabolic effects of dietary cholesterol in an animal model of insulin resistance and hepatic steatosis. Am. J Physiol Endocrinol. Metab. 2009;297:E462–E473. doi: 10.1152/ajpendo.90764.2008. [DOI] [PubMed] [Google Scholar]
- 26.Dong B, Singh AB, Kan FC, Liu J. CETP inhibitors downregulate hepatic LDL receptor and PCSK9 expression in vitro and in vivo through a SREBP2 dependent mechanism. Atherosclerosis. 2014;235:449–462. doi: 10.1016/j.atherosclerosis.2014.05.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu M, Dong B, Cao A, Li H, Liu J. Delineation of molecular pathways that regulate hepatic PCSK9 and LDL receptor expression during fasting in normolipidemic hamsters. Atherosclerosis. 2012;224:401–410. doi: 10.1016/j.atherosclerosis.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Havel RJ, EDER HA, BRAGDON JH. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955;34:1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faergeman O, Sata T, Kane JP, Havel RJ. Metabolism of apoprotein B of plasma very low density lipoproteins in the rat. J Clin Invest. 1975;56:1396–1403. doi: 10.1172/JCI108220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rashid S, Curtis DE, Garuti R, et al. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. PNAS. 2005;102:5374–5379. doi: 10.1073/pnas.0501652102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kosenko T, Golder M, Leblond G, Weng W, Lagace TA. Low density lipoprotein binds to proprotein convertase subtilisin/kexin type-9 (PCSK9) in human plasma and inhibits PCSK9-mediated low density lipoprotein receptor degradation. J Biol Chem. 2013;288:8279–8288. doi: 10.1074/jbc.M112.421370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lambert G, Jarnoux AL, Pineau T, et al. Fasting induces hyperlipidemia in mice overexpressing proprotein convertase subtilisin kexin type 9: lack of modulation of very-low-density lipoprotein hepatic output by the low-density lipoprotein receptor. Endocrinology. 2006;147:4985–4995. doi: 10.1210/en.2006-0098. [DOI] [PubMed] [Google Scholar]
- 33.Tavori H, Fan D, Blakemore JL, et al. Serum proprotein convertase subtilisin/kexin type 9 and cell surface low-density lipoprotein receptor: evidence for a reciprocal regulation. Circulation. 2013;127:2403–2413. doi: 10.1161/CIRCULATIONAHA.113.001592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaid A, Roubtsova A, Essalmani R, et al. Proprotein convertase subtilisin/kexin type 9 (PCSK9): hepatocyte-specific low-density lipoprotein receptor degradation and critical role in mouse liver regeneration. Hepatology. 2008;48:646–654. doi: 10.1002/hep.22354. [DOI] [PubMed] [Google Scholar]
- 35.Jeong HJ, Lee H-S, Kim K-S, Kim Y-K, Yoon D, Park SW. Sterol-dependent regulation of proprotein convertase subtilisin/kexin type 9 expression by sterol-regulatroy element binding protein-2. J Lipid Res. 2008;49:399–409. doi: 10.1194/jlr.M700443-JLR200. [DOI] [PubMed] [Google Scholar]
- 36.Li H, Dong B, Park SW, Lee HS, Chen W, Liu J. Hepatocyte nuclear factor 1alpha plays a critical role in PCSK9 gene transcription and regulation by the natural hypocholesterolemic compound berberine. J Biol. Chem. 2009;284:28885–28895. doi: 10.1074/jbc.M109.052407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zelcer N, Hong C, Boyadjian R, Tontonoz P. LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor. Science. 2009;325:100–104. doi: 10.1126/science.1168974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gustafsen C, Kjolby M, Nyegaard M, et al. The hypercholesterolemia-risk gene SORT1 facilitates PCSK9 secretion. Cell Metab. 2014;19:310–318. doi: 10.1016/j.cmet.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 39.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of the membrane bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 40.Sun H, Samarghandi A, Zhang N, Yao Z, Xiong M, Teng BB. Proprotein convertase subtilisin/kexin type 9 interacts with apolipoprotein B and prevents its intracellular degradation, irrespective of the low-density lipoprotein receptor. Arterioscler. Thromb. Vasc. Biol. 2012;32:1585–1595. doi: 10.1161/ATVBAHA.112.250043. [DOI] [PubMed] [Google Scholar]
- 41.Luo Y, Warren L, Xia D, et al. Function and distribution of circulating human PCSK9 expressed extrahepatically in transgenic mice. J Lipid Res. 2009;50:1581–1588. doi: 10.1194/jlr.M800542-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maxwell KN, Breslow JL. Adenoviral-mediated expression of Pcsk9 in mice results in a low-density lipoprotein receptor knockout phenotype. Proc Natl Acad Sci U.S.A. 2004;101:7100–7105. doi: 10.1073/pnas.0402133101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han B, Eacho PI, Knierman MD, et al. Isolation and Characterization of the Circulating Truncated Form of PCSK9. J Lipid Res. 2014 doi: 10.1194/jlr.M049346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simopoulos AP. Dietary omega-3 fatty acid deficiency and high fructose intake in the development of metabolic syndrome, brain metabolic abnormalities, and non-alcoholic fatty liver disease. Nutrients. 2013;5:2901–2923. doi: 10.3390/nu5082901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.