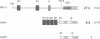Abstract
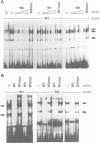
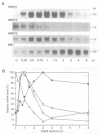
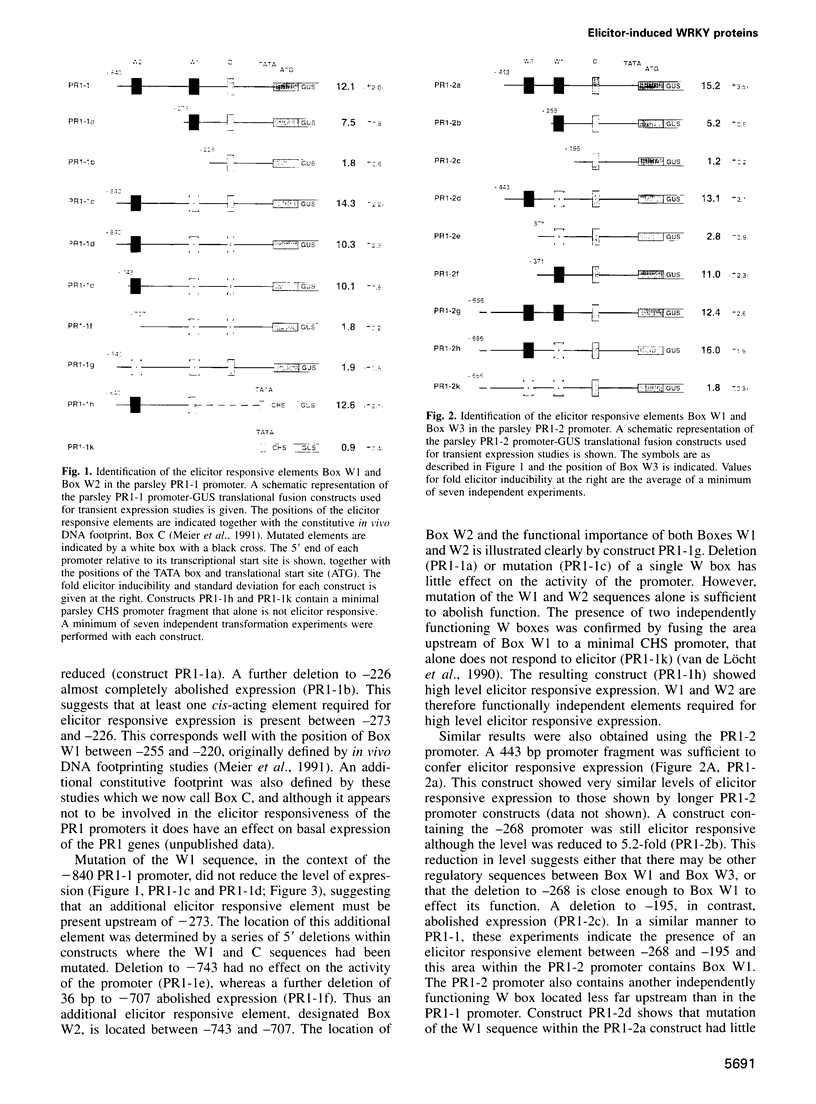
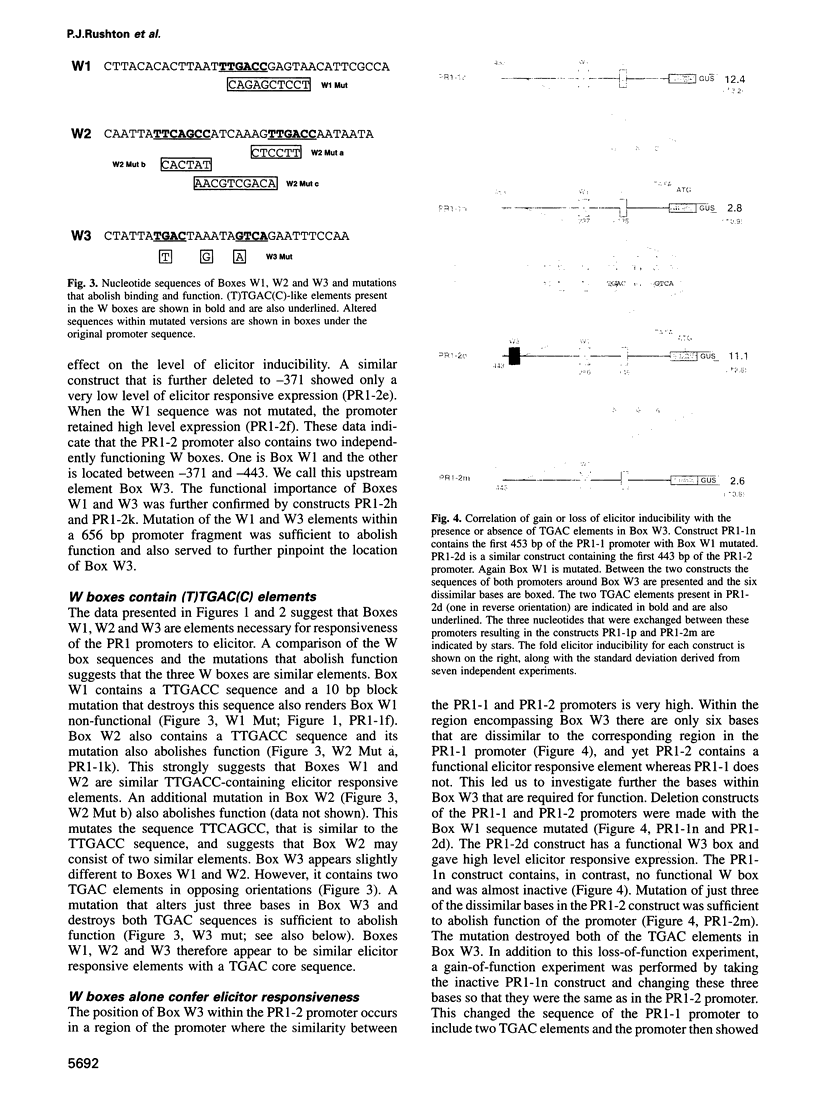
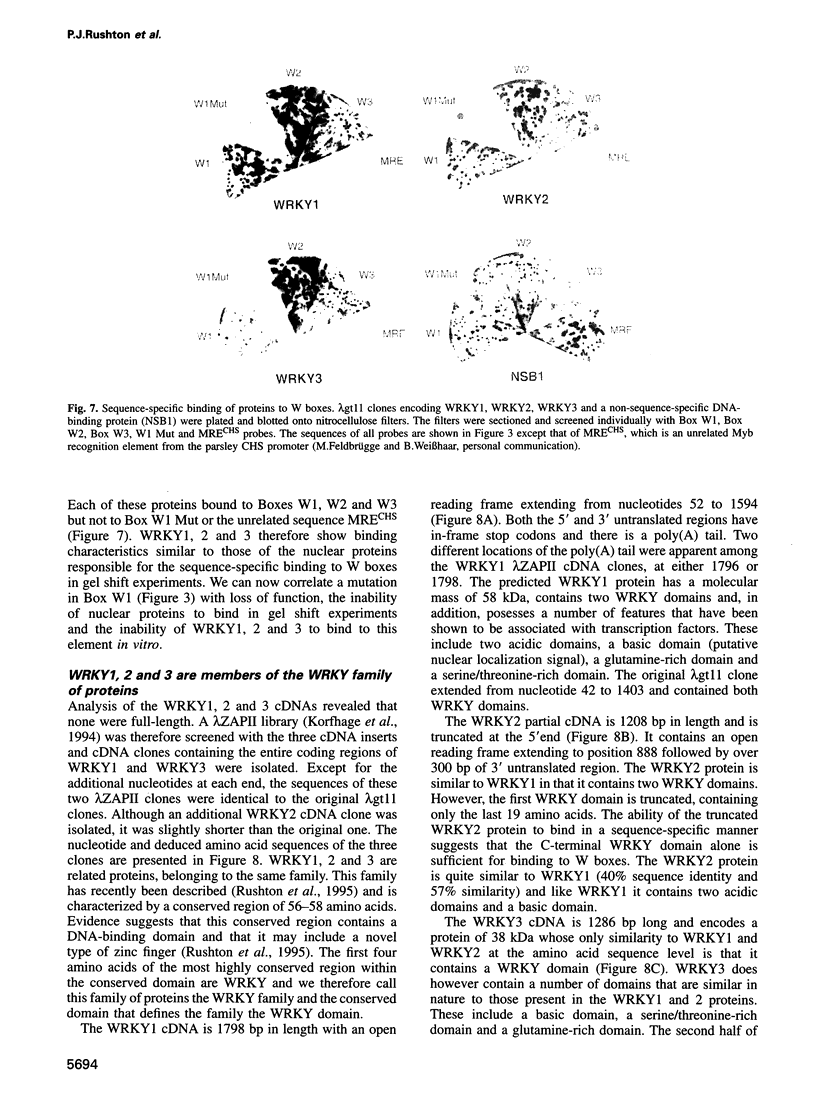
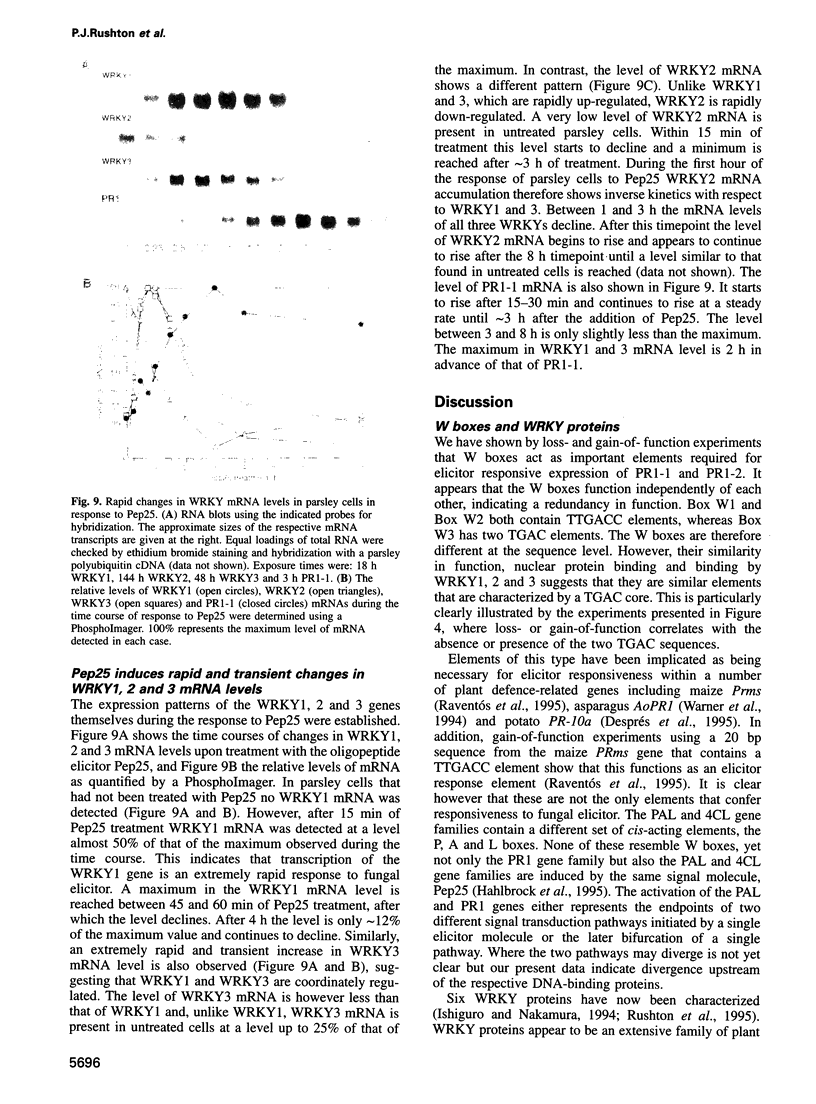
PR1 is a pathogenesis-related protein encoded in the parsley genome by a family of three genes (PR1-1, PR1-2 and PR1-3). Loss- and gain-of-function experiments in a transient expression system demonstrated the presence of two fungal elicitor responsive elements in each of the PR1-1 and PR1-2 promoters. These elements, W1, W2 and W3, contain the sequence (T)TGAC(C) and mutations that disrupt this sequence abolish function. Gel shift experiments demonstrated that W1, W2 and W3 are bound specifically by similar nuclear proteins. Three cDNA clones encoding sequence-specific DNA-binding proteins were isolated by South-Western screening and these proteins, designated WRKY1, 2 and 3, also bind specifically to W1, W2 and W3. WRKY1, 2 and 3 are members of the family of sequence-specific DNA-binding proteins, which we call the WRKY family. Treatment of parsley cells with the specific oligopeptide elicitor Pep25 induced a transient and extremely rapid increase in mRNA levels of WRKY1 and 3. WRKY2 mRNA levels in contrast showed a concomitant transient decrease. These rapid changes in WRKY mRNA levels in response to a defined signal molecule suggest that WRKY1, 2 and 3 play a key role in a signal transduction pathway that leads from elicitor perception to PR1 gene activation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong G. A., Weisshaar B., Hahlbrock K. Homodimeric and heterodimeric leucine zipper proteins and nuclear factors from parsley recognize diverse promoter elements with ACGT cores. Plant Cell. 1992 May;4(5):525–537. doi: 10.1105/tpc.4.5.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers A. R., Ebel J., Valent B., Albersheim P. Host-Pathogen Interactions: X. Fractionation and Biological Activity of an Elicitor Isolated from the Mycelial Walls of Phytophthora megasperma var. sojae. Plant Physiol. 1976 May;57(5):760–765. doi: 10.1104/pp.57.5.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangl J. L., Hauffe K. D., Lipphardt S., Hahlbrock K., Scheel D. Parsley protoplasts retain differential responsiveness to u.v. light and fungal elicitor. EMBO J. 1987 Sep;6(9):2551–2556. doi: 10.1002/j.1460-2075.1987.tb02543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehesh K., Hung H., Tepperman J. M., Quail P. H. GT-2: a transcription factor with twin autonomous DNA-binding domains of closely related but different target sequence specificity. EMBO J. 1992 Nov;11(11):4131–4144. doi: 10.1002/j.1460-2075.1992.tb05506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despres C., Subramaniam R., Matton D. P., Brisson N. The Activation of the Potato PR-10a Gene Requires the Phosphorylation of the Nuclear Factor PBF-1. Plant Cell. 1995 May;7(5):589–598. doi: 10.1105/tpc.7.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A., Mayer J. E., Hahlbrock K. Fungal elicitor triggers rapid, transient, and specific protein phosphorylation in parsley cell suspension cultures. J Biol Chem. 1990 Apr 15;265(11):6360–6368. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Gerber H. P., Seipel K., Georgiev O., Höfferer M., Hug M., Rusconi S., Schaffner W. Transcriptional activation modulated by homopolymeric glutamine and proline stretches. Science. 1994 Feb 11;263(5148):808–811. doi: 10.1126/science.8303297. [DOI] [PubMed] [Google Scholar]
- Hahlbrock K., Scheel D., Logemann E., Nürnberger T., Parniske M., Reinold S., Sacks W. R., Schmelzer E. Oligopeptide elicitor-mediated defense gene activation in cultured parsley cells. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4150–4157. doi: 10.1073/pnas.92.10.4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Karin M. The regulation of transcription by phosphorylation. Cell. 1992 Aug 7;70(3):375–387. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- Ishiguro S., Nakamura K. Characterization of a cDNA encoding a novel DNA-binding protein, SPF1, that recognizes SP8 sequences in the 5' upstream regions of genes coding for sporamin and beta-amylase from sweet potato. Mol Gen Genet. 1994 Sep 28;244(6):563–571. doi: 10.1007/BF00282746. [DOI] [PubMed] [Google Scholar]
- Jefferson R. A., Kavanagh T. A., Bevan M. W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987 Dec 20;6(13):3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawalleck P., Plesch G., Hahlbrock K., Somssich I. E. Induction by fungal elicitor of S-adenosyl-L-methionine synthetase and S-adenosyl-L-homocysteine hydrolase mRNAs in cultured cells and leaves of Petroselinum crispum. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4713–4717. doi: 10.1073/pnas.89.10.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombrink E., Hahlbrock K. Responses of cultured parsley cells to elicitors from phytopathogenic fungi : timing and dose dependency of elicitor-induced reactions. Plant Physiol. 1986 May;81(1):216–221. doi: 10.1104/pp.81.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korfhage U., Trezzini G. F., Meier I., Hahlbrock K., Somssich I. E. Plant homeodomain protein involved in transcriptional regulation of a pathogen defense-related gene. Plant Cell. 1994 May;6(5):695–708. doi: 10.1105/tpc.6.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanahan M. B., Ho T. H., Rogers S. W., Rogers J. C. A gibberellin response complex in cereal alpha-amylase gene promoters. Plant Cell. 1992 Feb;4(2):203–211. doi: 10.1105/tpc.4.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landt O., Grunert H. P., Hahn U. A general method for rapid site-directed mutagenesis using the polymerase chain reaction. Gene. 1990 Nov 30;96(1):125–128. doi: 10.1016/0378-1119(90)90351-q. [DOI] [PubMed] [Google Scholar]
- Lois R., Dietrich A., Hahlbrock K., Schulz W. A phenylalanine ammonia-lyase gene from parsley: structure, regulation and identification of elicitor and light responsive cis-acting elements. EMBO J. 1989 Jun;8(6):1641–1648. doi: 10.1002/j.1460-2075.1989.tb03554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matton D. P., Prescott G., Bertrand C., Camirand A., Brisson N. Identification of cis-acting elements involved in the regulation of the pathogenesis-related gene STH-2 in potato. Plant Mol Biol. 1993 May;22(2):279–291. doi: 10.1007/BF00014935. [DOI] [PubMed] [Google Scholar]
- Meier I., Hahlbrock K., Somssich I. E. Elicitor-inducible and constitutive in vivo DNA footprints indicate novel cis-acting elements in the promoter of a parsley gene encoding pathogenesis-related protein 1. Plant Cell. 1991 Mar;3(3):309–315. doi: 10.1105/tpc.3.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Nürnberger T., Nennstiel D., Jabs T., Sacks W. R., Hahlbrock K., Scheel D. High affinity binding of a fungal oligopeptide elicitor to parsley plasma membranes triggers multiple defense responses. Cell. 1994 Aug 12;78(3):449–460. doi: 10.1016/0092-8674(94)90423-5. [DOI] [PubMed] [Google Scholar]
- Papavassiliou A. G., Treier M., Chavrier C., Bohmann D. Targeted degradation of c-Fos, but not v-Fos, by a phosphorylation-dependent signal on c-Jun. Science. 1992 Dec 18;258(5090):1941–1944. doi: 10.1126/science.1470918. [DOI] [PubMed] [Google Scholar]
- Raikhel N. Nuclear targeting in plants. Plant Physiol. 1992 Dec;100(4):1627–1632. doi: 10.1104/pp.100.4.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raventós D., Jensen A. B., Rask M. B., Casacuberta J. M., Mundy J., San Segundo B. A 20 bp cis-acting element is both necessary and sufficient to mediate elicitor response of a maize PRms gene. Plant J. 1995 Jan;7(1):147–155. doi: 10.1046/j.1365-313x.1995.07010147.x. [DOI] [PubMed] [Google Scholar]
- Rushton P. J., Hooley R., Lazarus C. M. Aleurone nuclear proteins bind to similar elements in the promoter regions of two gibberellin-regulated alpha-amylase genes. Plant Mol Biol. 1992 Sep;19(6):891–901. doi: 10.1007/BF00040522. [DOI] [PubMed] [Google Scholar]
- Rushton P. J., Macdonald H., Huttly A. K., Lazarus C. M., Hooley R. Members of a new family of DNA-binding proteins bind to a conserved cis-element in the promoters of alpha-Amy2 genes. Plant Mol Biol. 1995 Nov;29(4):691–702. doi: 10.1007/BF00041160. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somssich I. E., Schmelzer E., Kawalleck P., Hahlbrock K. Gene structure and in situ transcript localization of pathogenesis-related protein 1 in parsley. Mol Gen Genet. 1988 Jul;213(1):93–98. doi: 10.1007/BF00333403. [DOI] [PubMed] [Google Scholar]
- Stone J. M., Walker J. C. Plant protein kinase families and signal transduction. Plant Physiol. 1995 Jun;108(2):451–457. doi: 10.1104/pp.108.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tague B. W., Goodman H. M. Characterization of a family of Arabidopsis zinc finger protein cDNAs. Plant Mol Biol. 1995 May;28(2):267–279. doi: 10.1007/BF00020246. [DOI] [PubMed] [Google Scholar]
- Warner S. A., Gill A., Draper J. The developmental expression of the asparagus intracellular PR protein (AoPR1) gene correlates with sites of phenylpropanoid biosynthesis. Plant J. 1994 Jul;6(1):31–43. doi: 10.1046/j.1365-313x.1994.6010031.x. [DOI] [PubMed] [Google Scholar]
- Weisshaar B., Armstrong G. A., Block A., da Costa e Silva O., Hahlbrock K. Light-inducible and constitutively expressed DNA-binding proteins recognizing a plant promoter element with functional relevance in light responsiveness. EMBO J. 1991 Jul;10(7):1777–1786. doi: 10.1002/j.1460-2075.1991.tb07702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa S. A novel DNA-binding domain that may form a single zinc finger motif. Nucleic Acids Res. 1995 Sep 11;23(17):3403–3410. doi: 10.1093/nar/23.17.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Loh Y. T., Bressan R. A., Martin G. B. The tomato gene Pti1 encodes a serine/threonine kinase that is phosphorylated by Pto and is involved in the hypersensitive response. Cell. 1995 Dec 15;83(6):925–935. doi: 10.1016/0092-8674(95)90208-2. [DOI] [PubMed] [Google Scholar]
- da Costa e Silva O., Klein L., Schmelzer E., Trezzini G. F., Hahlbrock K. BPF-1, a pathogen-induced DNA-binding protein involved in the plant defense response. Plant J. 1993 Jul;4(1):125–135. doi: 10.1046/j.1365-313x.1993.04010125.x. [DOI] [PubMed] [Google Scholar]
- van de Löcht U., Meier I., Hahlbrock K., Somssich I. E. A 125 bp promoter fragment is sufficient for strong elicitor-mediated gene activation in parsley. EMBO J. 1990 Sep;9(9):2945–2950. doi: 10.1002/j.1460-2075.1990.tb07486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]