Abstract
Medication adherence is highly predictive of health outcomes across chronic conditions, particularly HIV/AIDS. Depression is consistently associated with worse adherence, yet few studies have sought to understand how depression relates to adherence. This study tested three components of behavioral depression theory—goal-directed activation, positive reinforcement, and environmental punishment—as potential indirect effects in the relation between depressive symptoms and medication nonadherence among low-income, predominantly African American substance users (n = 83). Medication nonadherence was assessed as frequency of doses missed across common reasons for nonadherence. Non-parametric bootstrapping was used to evaluate the indirect effects. Of the three intermediary variables, there was only an indirect effect of environmental punishment; depressive symptoms were associated with greater nonadherence through greater environmental punishment. Goal-directed activation and positive reinforcement were unrelated to adherence. Findings suggest the importance of environmental punishment in the relation between depression and medication adherence and may inform future intervention efforts for this population.
Keywords: Depression, HIV/AIDS, Medication adherence, Substance use, Behavioral theory
Introduction
Medication adherence is of utmost importance across numerous chronic medical conditions, and arguably most impactful on health outcomes in HIV/AIDS. Indeed, anti-retroviral therapy (ART) for the treatment of HIV/AIDS has substantially improved clinical outcomes (Crum et al., 2006). However, positive health outcomes require consistent ART use and high levels of ART adherence (García et al., 2002). For this reason, there is great value in understanding the factors associated with suboptimal adherence.
One of the most prevalent and powerful predictors of nonadherence across chronic medical conditions is depression (DiMatteo et al., 2000). In HIV, recent meta-analyses and reviews of the relationship between depression and ART adherence (n = 42,366; 111 studies) have been definitive in the effects of depression on adherence (Gonzalez et al., 2011; Uthman et al., 2014). These meta-analyses included studies conducted in both higher-income areas (e.g., US, countries in Western Europe, Canada, Hong Kong, Australia) and lower-income countries (e.g., Ethiopia, South Africa, India, Peru, Uganda, Nigeria, Kenya). Depression as a barrier to adherence among HIV-positive individuals has received significant empirical and clinical attention given (1) its high prevalence among individuals with HIV/AIDS (e.g., Asch et al., 2003; Bing et al., 2001) and (2) the link between depression and later HIV disease progression (Gore-Felton & Koopman, 2008; Leserman et al., 2002). Among urban, low-income substance users living with HIV, rates of major depression have been shown to reach 72 % (Berger-Greenstein et al., 2007). Even at sub-threshold levels, depressive symptoms have a strong relationship with nonadherence; in a sample of substance users in methadone maintenance, a one-point increase in clinician-rated depressive symptoms (on the seven-point depression Clinical Global Impression Scale) was associated with a 75 % increase in the odds of ART nonadherence. Thus, even a moderate depression rating according to this scale would indicate almost a fivefold increase in the odds of nonadherence as compared to when no depressive symptoms are present (Gonzalez et al., 2011).
Despite the focus on depression as a reliable and powerful factor associated with medication nonadherence across chronic health conditions and among substance users living with HIV specifically, few studies have examined factors that may account for the relation between depression and medication nonadherence. It is particularly important to develop behavioral interventions to address improvements in medication adherence in populations most affected by depression, such as urban, low-income substance users (Berger-Greenstein et al., 2007). As such, the current study drew from longstanding behavioral theories of depression (Lewinsohn, 1974; Ferster, 1973) to identify key components that have particular relevance to medication adherence. These theories suggest that depression is characterized by: (1) lower levels of goal-directed activation (i.e., individuals engage in fewer pleasant activities or activities of mastery; Lewinsohn & Graf, 1973; Lewinsohn & Libet, 1972); (2) less positive reinforcement available in one’s environment (i.e., due to fewer social supports, other resources); and (3) greater experience of perceived punishment in one’s environment (i.e., greater experience of negative or aversive consequences).
Although these constructs have not been tested in relation to medication nonadherence specifically, there is evidence from the medication adherence literature suggesting the relevance of these constructs to adherence, and in particular to the relation between depression and adherence. First, regarding goal-directed activation, previous research has demonstrated that “patterns of regular behaviors and activities” (Wagner & Ryan, 2004), including changes in daily routine and ability to fit a regimen into a daily routine, have consistently been identified as important factors related to medication adherence, including ART, other forms of medication, and even placebos (Chesney et al., 2000; Gifford et al., 2000; Roberts, 2000; Wagner & Ryan, 2004). Second, various lines of evidence suggest that positive reinforcement in one’s environment may be important to inspire continued motivation for self-care behaviors (Berger-Greenstein et al., 2007; Holzemer et al., 1999; Ryan & Wagner, 2003). Third, regarding environmental punishment, perception of being exposed to punishing or aversive experiences is associated with the belief that behavioral choices will not lead to subsequent reinforcement (Hiroto, 1974; Rotter, 1966), such as positive health benefits of adherence. Similar constructs (e.g., external locus of control; Rotter, 1975) have shown that such beliefs are associated with poor adherence across chronic conditions, such as diabetes (Schlenk & Hart, 1984), hypertension (Stanton, 1987), and HIV (Aversa & Kimberlin, 1996; Evans et al., 2000). Finally, focusing on behavioral factors has high potential for applicability to medication nonadherence, given that the most commonly cited reasons for medication nonadherence are behavioral (e.g., Chesney, 2003; Palmer et al., 2003).
In sum, although the specific components of behavioral theories of depression have not been tested in relation to medication adherence, numerous lines of evidence suggest their relevance. As such, in the current study we tested the role of each of these three components as indirect effects in the relation between depressive symptoms and medication nonadherence. We hypothesized that lower levels of goal-directed activation, lower levels of positive reinforcement, and higher levels of environmental punishment would each be related to greater medication nonadherence, and further, that each would be a significant indirect effect in the relation between depressive symptoms and medication adherence.
Methods
Participants and procedure
Low-income HIV-positive substance users were recruited given the high rates of depressive symptoms and medication nonadherence commonly identified in this population (Berger-Greenstein et al., 2007). Participants were recruited from a large, urban residential substance abuse treatment center in the Northeast United States. Patients were referred by government agencies or mandated to treatment by the court system. Patients were required to have completed full detoxification and have a negative urine drug screen upon admission to the treatment facility. During this time period, all patients in the facility received a standard intake interview that included an assessment of medical history and daily medication use, which was verified with center records and used to determine study eligibility.
Patients were eligible if they were HIV-positive and were prescribed a medication regimen that required daily dosing. Patients were excluded if they were not prescribed any daily medication, which was necessary to assess medication adherence. Eligible patients were provided detailed information about study procedures and those interested provided informed consent. The importance of maintaining patient confidentiality and privacy was stressed throughout the study screening session. Treatment center staff members were not made aware of patients’ study participation or refusal, and participation in the study did not affect patients’ status in treatment. All structured clinical interviews and assessments were conducted by trained post-baccalaureate and pre-doctoral clinical psychology graduate students (six total). All study procedures were approved by the University Institutional Review Board.
Ninety-two participants were approached for the current study. Three declined participation (3.3 %), and six were excluded because they were not prescribed any daily medication regimen (6.5 %). Of the final sample (n = 83), 95.2 % were African American, 71.1 % heterosexual, 45.8 % female, and the mean age was 45.20 (SD 7.89). Patients were in substance abuse treatment for a mean of 13.76 days (SD 8.47) at the time of the study assessment.
Assessment measures
Demographics form assessed age, race/ethnicity, sexual orientation, education level, marital status, employment status, and annual household income.
The Structured Clinical Interview for the DSM-IV (SCID-IV; First & Gibbon, 2004) was used to assess current DSM-IV Axis-I and II psychopathology (past month for mood disorders and anxiety disorders, past year for substance use disorders) and administered by a trained clinician.
The Hamilton Depression Rating Scale—7 item version (HAMD-7; Maier & Phillip, 1985) was used to assess depressive symptoms. The HAMD-7 is a clinician-rated measure of severity of depressive symptoms. Clinicians rate each of the 7 items on a scale of 0–4 with higher scores indicating increased depression severity. Previous research has shown that the measure has strong internal consistency (α = 0.84) and excellent convergent validity with the Montgomery-Asberg Depression Rating Scale (MADRS) (r = .90; McIntyre et al., 2005).
The Behavioral Activation for Depression Scale (BADS; Kanter et al., 2007) was used to measure goal-directed activation. The BADS is a 25-item self-report measure of the frequency of activation as outlined in behavioral theories of depression, with one subscale specifically (“activation”) assessing goal-directed activity level. Example items include “I engaged in a wide and diverse array of activities” and “I did something that was hard to do but it was worth it”). Higher scores indicate greater activation. The BADS has been demonstrated to have strong internal consistency (α = 0.92) and good test–retest reliability (r = .74) in depressed and non-depressed samples (Kanter et al., 2007, 2009). Internal consistency for the activation subscale was good in this sample (α = 0.80).
The Reward Probability Index (RPI; Carvalho et al., 2011) was used to measure positive reinforcement and environmental punishment and was developed specifically in line with Lewinsohn’s model of depression. The RPI has 20 items and a two-facture structure, which includes “Reward Probability” and “Environmental Suppressors.” The Reward Probability subscale consists of 11 items related to the number of potential reinforcers and an individual’s ability to obtain reinforcement through instrumental behaviors. Example items in this subscale include “It is easy to find good ways to spend my time” and “I have the abilities to obtain pleasure in life.” Higher scores indicate greater probability of reward in the environment. The Environmental Suppressors subscale includes 9 items that assess the presence of aversive stimuli in the environment. Example items include “I have had many unpleasant experiences” and “It seems like bad things always happen to me.” Items in this subscale are reverse scored, and higher scores on this subscale indicate lower levels of environmental punishment. The RPI has been demonstrated to have strong internal consistency (α = 0.90) and test–retest reliability (r = .69). In the current sample, internal consistency for the two subscales ranged from acceptable to good (Environmental Suppressors: α = 0.70; Reward Probability: α = 0.86).
The ACTG Adherence to Antiretroviral Medication Questionnaire (Chesney et al., 2000) was used to assess self-reported medication adherence. Specifically, medication adherence was assessed for all daily medications prescribed (ART, psychotropic medications, cardiovascular medications, diabetes medications, anticonvulsants, and hormones). Participants provide a list of all daily medications prescribed in the 4 days prior to the study assessment and the number of doses taken. Ratios were calculated of the number of doses missed versus doses prescribed over the past 4 days for all daily medications.
The ACTG was also used to assess frequency of doses missed across a range of reasons as a second indicator of self-reported nonadherence. Querying for reasons for nonadherence has been recommended as a way to minimize potential biases of self-report when assessing adherence, including inaccurate recall and social desirability (Simoni et al., 2006), and this measure has been used previously as a main adherence outcome (O’Cleirigh et al., 2007; DiIorio et al., 2009). Participants were presented a list of 14 reasons why people may ever miss taking their medications and were asked “how often have you missed taking your medications because you…” for each reason. Participants rate responses on a four-point scale (never, rarely, sometimes, often) to indicate the frequency of nonadherence due to each of the 14 reasons. The responses were summed to create a total score of frequency of doses missed across commonly endorsed reasons for medication nonadherence, which follows prior research (O’Cleirigh et al., 2007; DiIorio et al., 2009). Higher scores indicating greater frequency of doses missed across reasons for non-adherence. A previous factor analysis of the 14-item ACTG reasons for nonadherence questionnaire identified five items that loaded together on a single factor (i.e., “were away from home” “were busy with other things”; “simply forgot”; “had a change in daily routine; and “fell asleep/slept through dose time”), which was conceptualized as unintentional “logistical problems” related to nonadherence (α = 0.86 for factor). All analyses were conducted examining nonadherence across (1) all reasons and (2) “logistical/unintentional” reasons separately. Internal consistency for the nonadherence reasons subscale was excellent α = 0.90.
CD4 count was used as a biological indicator of health status given its relevance to depression in prior trials (Burack et al., 1993; Ickovics et al., 2001; Leserman, 2008). This was obtained from participants’ medical records of the most recent blood work appointment within a 90-day window from the assessment (60 days prior, 30 days after). Participants also self-reported years since HIV diagnosis and basic health care-related characteristics (i.e., whether they had a primary care physician [PCP] and a health insurance plan). All health status measures were considered as potential covariates.
Statistical analyses
We first conducted a correlation matrix to examine the relation among study variables (see Table 2). Next, we utilized non-parametric bootstrapping to examine the indirect effects of goal-directed activation, positive reinforcement, and environmental punishment separately in all three aims. Frequency of doses missed across all reasons for nonadherence was the primary dependent variable. We also examined whether any results changed when including the logistical/unintentional reasons factor as the outcome variable. Non-parametric bootstrapping is recommended for small samples, because there are no assumptions about the shape of the sampling distribution of the indirect effect (Preacher & Hayes, 2004). Bootstrapping is based on resampling with replacement, which is done many times to generate an empirical approximation of the sampling distribution of the indirect effect (Hayes, 2009). Non-parametric bootstrapping analyses conducted were based upon 5,000 bootstrapped samples (recommended by Hayes, 2009), and we used the INDIRECT SPSS Macro developed by Preacher and Hayes (2008). Indirect effects are significant if the 95 % bias-corrected or percentile-based confidence intervals (CIs) for the indirect effect do not include 0 (Preacher & Hayes, 2004, 2008). Covariates were selected based upon their relation to the dependent variable (Tabachnik & Fidell, 2007), including all variables listed in Table 1 as well as medication status (class of medication taken, ART status).
Table 2.
Correlation matrix of depressive symptoms, goal-directed activation, positive reinforcement, environmental punishment and frequency of doses missed across reasons for medication nonadherence
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| 1. HAMD-depression | – | −0.19 | −0.29** | −0.30** | 0.30** | 0.16 |
| 2. BADS-activation | – | 0.39** | 0.28* | −0.04 | 0.01 | |
| 3. RPI-positive reinforcement | – | 0.22* | −0.14 | −0.06 | ||
| 4. RPI-punishment | – | −0.40** | −0.29** | |||
| 5. ACTG-reasons for nonadherence (total) | – | 0.87*** | ||||
| 6. ACTG-reasons for nonadherence (logistical/unintentional) | – |
HAMD Hamilton Depression Rating Scale (higher scores = greater depressive symptoms), BADS Behavioral Activation for Depression Scale (Activation subscale; higher scores = greater activation), RPI Reward Probability Index (Positive reinforcement: higher scores = greater reinforcement; Punishment: higher scores = lower punishment), ACTG AIDS Clinical Trials Group (higher scores = greater frequency of missed doses across reasons for nonadherence) for all reasons (5) and logistical/unintentional factor only (6)
p < .001;
p < .01;
p < .05
Table 1.
Demographic information, Axis I and II psychopathology, health status and health care-related factors for total sample and by ART status
| Overall (n = 83) | On ART (n = 57) | Not on ART (n = 26) | Statistic | p value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age [mean (SD)] | 45.2 (7.9) | 46.3 (7.9) | 43.1 (9.4) | t(80) = 1.98 | .051 |
| Gender (%) | χ2(2) = 3.21 | .20 | |||
| Female | 45.8 | 40.4 | 57.7 | ||
| Male | 50.6 | 56.1 | 38.5 | ||
| Transgender | 7.2 | 5.3 | 11.5 | ||
| Marital status (%) | χ2(2) = 1.37 | .50 | |||
| Married | 7.2 | 7.0 | 7.7 | ||
| Separated/divorced | 24.0 | 28.1 | 15.3 | ||
| Single | 63.9 | 59.6 | 73.1 | ||
| Race/ethnicity (%) | χ2(2) = 1.37 | .51 | |||
| Black | 95.2 | 94.7 | 96.2 | ||
| Hispanic | 2.4 | 3.5 | 0.0 | ||
| White | 1.2 | 1.8 | 0.0 | ||
| Heterosexual (%) | 71.1 | 73.7 | 65.4 | χ2(1) = 0.28 | .60 |
| ≤High school/GED (%) | 78.3 | 80.6 | 72.9 | χ2(1) = 0.41 | .52 |
| Total annual income < $10,000 (%) | 77.1 | 82.5 | 65.4 | χ2(1) = 2.12 | .15 |
| Unemployed (%) | 90.4 | 89.5 | 92.3 | χ2(1) = 0.95 | .33 |
| Psychopathology* | |||||
| Current MDD (%) | 16.9 | 15.8 | 19.2 | χ2(1) = 0.06 | .81 |
| Crack cocaine dependence (past year) (%) | 50.6 | 45.6 | 61.5 | χ2(1) = 1.73 | .19 |
| Alcohol dependence (past year) (%) | 28.9 | 26.3 | 34.6 | χ2(1) = 0.39 | .53 |
| Opioid (heroin) dependence (past year) (%) | 21.7 | 21.1 | 23.1 | χ2(1) = 0.02 | .97 |
| ASPD (%) | 25.3 | 24.6 | 26.9 | χ2(1) = 0.04 | .85 |
| Health status | |||||
| CD4 count [mean (SD)] | 451.19 (246.87) | 408.33 (217.58) | 524.15 (246.33) | χ2(23) = 24.00 | .40 |
| Years since HIV diagnosis [mean (SD)] | 11.48 (7.31) | 11.44 (6.92) | 11.60 (8.75) | χ2(69) = 70.00 | .44 |
| Health care-related factors | |||||
| Has a PCP (%) | 96.4 | 98.2 | 92.3 | χ2(1) = 1.81 | .18 |
| Has a health-insurance plan (%) | 96.4 | 96.5 | 96.2 | χ2(1) = 0.01 | .94 |
MDD major depressive disorder (current defined as past month), ASPD antisocial personality disorder, PCP primary care physician
All Axis I and II diagnoses with ≥15 % prevalence in current sample are listed
Results
Medication characteristics
Participants were taking an average of 5.82 pills per day (SD 3.48). 68.7 % (n = 57) of the current sample was taking ART, 38.6 % of the sample was taking cardiovascular drugs, 41.0 % antidepressant medication, 15.7 % anticonvulsant medication, 12.0 % diabetes medication, and 3.6 % daily hormones. Participants on ART were taking an average of 3.16 antiretroviral pills per day (SD 1.74). See Table 1 for additional demographic and clinical information for the total sample and by ART status. Mean adherence rates ranged from 94.46 to 97.72 % in the past 4 days; daily medication adherence rates were 97.72 % yesterday, 94.46 % 2 days ago, 97.02 % 3 days ago, and 96.85 % 4 days ago. There was little variability in rates of nonadherence over the past 4 days, and more specifically a ceiling in rates of nonadherence was present. All adherence ratios were highly skewed (skewness statistics<−4.5 for all ratios), which was not improved following transformations. Thus it was not possible to use these ratios in analyses. However, there was greater variability in our second indicator of adherence: frequency of doses missed across reasons for nonadherence. Frequency of doses missed across reasons for nonadherence was in the normal range for skew and kurtosis and was not transformed. Responses ranged from 0 to 36, and the mean of the total score in the current sample was 12.01 (SD 9.32).
Correlations between variables
Table 2 shows the correlations amongst depressive symptoms, goal-directed activation, positive reinforcement, environmental punishment, and medication nonadherence (frequency of doses missed across all reasons for nonadherence and logistical/unintentional reasons only). Higher depressive symptoms (r = .30, p < .01) and greater environmental punishment (−0.40; p < .01) were significantly associated with greater frequency of doses missed across all reasons for nonadherence. Depressive symptoms unrelated to frequency of doses missed when reasons were restricted to only those that were logistical/unintentional.
Identification of covariates
All variables listed in Table 1 were tested in relation to the primary dependent variable (frequency of doses missed across reasons endorsed for nonadherence). The only variables significantly related to greater frequency of doses missed across reasons were sexual orientation and crack/cocaine dependence; individuals who identified as homosexual or bisexual reported significantly greater frequency of doses missed across reasons compared to heterosexual individuals (t(74) = 3.18, p < .005), and individuals with crack/cocaine dependence also reported significantly greater frequency of doses missed across reasons compared to individuals without crack/cocaine dependence (t(71) = −3.01 p < .005). All other relations were non-significant (all p’s > 0.15). We examined type of medication being taken and ART status as potential covariates; however, neither was significantly correlated with the dependent variable (all p’s > 0.6). Given the majority of the sample was African American, we also ran all analyses only including African American individuals (n = 79), and this did not affect the significance or parameters of any results reported below.
Indirect effects
Goal-directed activation
We first tested the indirect effect of goal-directed activation in the relation between depressive symptoms and medication nonadherence (frequency of doses missed across all reasons for nonadherence). Results indicated that there was not a significant indirect effect of goal-directed activation [IE = −0.004, SE = 0.08; Bias-Corrected 95 %CI −0.23, 0.08]. Next, we tested the indirect effect of goal-directed activation in the relation between depressive symptoms and unintentional medication nonadherence (frequency of doses missed across logistical/unintentional reasons for nonadherence). Results indicated that there was not a significant indirect effect of goal-directed activation in this relationship [IE = −0.02, SE = 0.04; Bias-Corrected 95 %CI −0.13, 0.03].
Positive reinforcement
Second, we tested the indirect effect of positive reinforcement in the relation between depressive symptoms and medication nonadherence (frequency of doses missed across all reasons for nonadherence). Results indicated that there was not a significant indirect effect of positive reinforcement [IE = 0.05, SE = 0.11; Bias-Corrected 95 %CI −0.13, 0.33]. Next, we tested the indirect effect of positive reinforcement in the relation between depressive symptoms and unintentional medication nonadherence (frequency of doses missed across logistical/unintentional reasons for nonadherence). Results indicated that there was not a significant indirect effect of positive reinforcement in this relationship [IE = 0.002, SE = 0.05; Bias-Corrected 95 %CI −0.08, 0.11].
Environmental punishment
Finally, we tested the indirect effect of environmental punishment in the relation between depressive symptoms and medication nonadherence (frequency of doses missed across all reasons for nonadherence). Results indicated that there was a significant indirect effect of environmental punishment [IE = 0.17, SE = 0.12; Bias-Corrected 95 %CI 0.005, 0.52]. Specifically, individuals with higher levels of clinician-rated depressive symptoms had greater experiences of environmental punishment, and through higher levels of environmental punishment, reported greater frequency of missed medication doses across reasons for medication nonadherence (see Fig. 1 for a depiction of these results). Next, we tested the indirect effect of environmental punishment in the relation between depressive symptoms and unintentional medication nonadherence (frequency of doses missed across logistical/unintentional reasons for nonadherence). Results indicated that there was not a significant indirect effect of environmental punishment when the dependent variable was restricted to unintentional/logistical reasons for nonadherence [IE = 0.05, SE = 0.05; Bias-Corrected 95 %CI −0.02, 0.20].
Fig. 1.
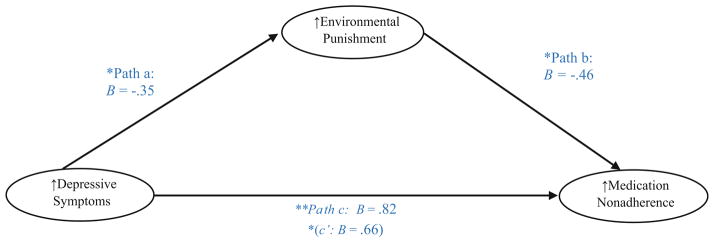
Indirect effect of environmental punishment in the relation between depressive symptoms and frequency of missed doses across all reasons for medication nonadherence. *<0.05; **<0.01. Bootstrapping results: Indirect Effect (IE) = 0.17, SE = 0.12; Bias-corrected 95 % CI 0.005, 0.52. Depressive symptoms Hamilton Depression Rating Scale (higher scores = greater depressive symptoms); environmental punishment Reward Probability Index (higher scores = lower punishment); medication adherence AIDS Clinical Trials Group (ACTG) assessment of frequency of doses missed across all reasons for nonadherence (higher scores = greater nonadherence)
Discussion
The current study sought to apply behavioral theories of depression (Ferster, 1973; Lewinsohn, 1974) to inform the understanding of how depression may relate to medication nonadherence. Related lines of research from the adherence literature suggested that each of the three components of behavioral theories of depression—goal directed activation, positive reinforcement, and environmental punishment—may be relevant to medication nonadherence, although this had not been explicitly tested. Of the three key components of behavioral theories of depression, only environmental punishment was significantly related to medication nonadherence when assessed as the frequency of doses missed across all commonly endorsed reasons for medication nonadherence. Goal-directed activation and positive reinforcement were unrelated to medication non-adherence.
The finding that environmental punishment may play an important role in the relation between depressive symptoms and nonadherence fits with the theoretical framework of external locus of control of reinforcement, particularly as applied to health behavior (Wallston et al., 1978). It has been suggested that perceptions of punishment and external locus of control are overlapping constructs (Hiroto, 1974; Rotter, 1966), and as such, although we did not assess external locus of control directly, it may be useful to draw from this well-established framework to interpret the current study findings. The locus of control theoretical framework suggests that the degree to which individuals expect that a particular outcome is contingent on their own behavior or personal characteristics is strongly related to the likelihood of engaging in that behavior (Rotter, 1975). Individuals with an external locus of control are less likely to perceive direct benefits from healthy behavior and rather are more likely to report aversive consequences (i.e., environmental punishment) as a result of attempting healthy behavior and a lack of control over managing their health condition (Rotter, 1966; Wallston et al., 1978). The current study’s findings fit with previous research demonstrating that an external locus of control has been strongly implicated in depression, in particular in relation to symptoms of depression related to avolition (Benassi et al., 1988), substance use (Newcomb & Harlow, 1986), and persistence with self-care across numerous chronic conditions, such as diabetes (Schlenk & Hart, 1984), hypertension (Stanton, 1987), as well as HIV/AIDS (Aversa & Kimberlin, 1996; Evans et al., 2000).
Environmental punishment was only a significant indirect effect in the model when medication adherence was examined across all reasons for nonadherence. When assessed using a single factor—“unintentional/logistical” reasons for nonadherence—environmental punishment no longer was a significant indirect effect. Depressive symptoms were not significantly associated with frequency of doses missed for unintentional reasons. This may suggest that environmental punishment plays a stronger role in explaining the relationship between depressive symptoms and medication nonadherence when nonadherence is largely due to intentional reasons for missed doses (i.e., “wanted to avoid side effects,” “felt like the drug was toxic/harmful”). Individuals with depression who are more likely to anticipate environmental punishment may have reduced motivation to take medication and/or greater perceived negative consequences of medication use. The findings also suggest that depression may be more closely related nonadherence when missed doses are intentional as opposed to unintentional or more reflective of logistical reasons (e.g., simply forgetting, sleeping through dose time). However, this is contrary to recent evidence suggesting that depressive symptoms disrupt adherence through disruptions in lifestyle structure and routine (Magidson et al., 2013, 2014). There has been prior research on the differential aspects of depression in relation to medication adherence (e.g., Wagner et al., 2011), and greater empirical attention is needed to understand how depression may differentially impact unintentional versus intentional patterns of medication adherence.
Regarding the non-significant relations of goal-directed activation and positive reinforcement with frequency of doses missed across commonly endorsed reasons for medication nonadherence, it may be that these constructs are not as strongly related to medication nonadherence compared to environmental punishment. Alternatively, this may also reflect the setting in which the current study was conducted—a controlled environment with very little variability or flexibility regarding one’s schedule and few ways to obtain reinforcement from one’s environment—which may have made activation and reinforcement in one’s environment less relevant to medication adherence. In future work, recruiting participants from a community setting or an outpatient center where individuals have greater control over their schedule and opportunities for obtaining reinforcement may be useful.
Additionally, it is interesting to note that of the potential covariates, only crack cocaine dependence diagnosis in the past year and sexual orientation (self-identified homosexual or bisexual) were related to a greater frequency of doses missed across reasons for nonadherence. Recent crack cocaine use has been shown to be associated with suboptimal adherence to ART (Eldred et al., 1998; Sharpe et al., 2004), with adherence concerns for other types of medications among HIV-positive individuals (i.e., by providers in HIV patients’ medical records; Ingersoll, 2004), and with reduced insulin adherence in diabetes care (Warner et al., 1998). The finding that sexual orientation was associated with medication nonadherence in this predominantly African American sample fits with prior research that has pointed to high risk for poor HIV-related self care among African American men who have sex with men (MSM) (Bogart et al., 2010; Malebranche et al., 2004).
The current study recruited specifically low-income, HIV-positive substance users at high risk for poor HIV outcomes (i.e., resulting from ART nonadherence or not being on ART; Chander et al., 2009). Indeed, we found that almost one-third of our sample recruited was not taking ART, supporting the notion that this is a sample at high risk for poor HIV outcomes. Including more HIV-positive individuals not on ART in adherence research is important to understand unique barriers towards ART uptake facing this group. HIV-positive individuals often face other comorbid chronic conditions such as diabetes, Hepatitis C, hypertension, and arthritis; a study of patients seeking treatment at HIV clinics revealed 89 % had comorbid conditions, with a mean of 2.4 comorbid conditions per patient, and 81 % taking medications for conditions other than HIV infection (Shah et al., 2002). This is consistent with our sample, with almost 40 % of the sample taking cardiovascular medications and approximately the same percentage taking antidepressant medication, with an overall mean pill count of almost six pills per day. Adherence to multiple other forms of medication is likely challenging, and in particular, low-income, African American HIV-positive individuals have been shown to be least likely to be adherent to other forms of medication (i.e., to TB medication; Pablos-Méndez et al., 1997). This clearly underscores the importance of also understanding factors relevant to adherence to medications other than ART in this population.
One primary limitation of the current study is the single assessment time point, and as such causation or directionality cannot be inferred from findings. Although a longitudinal design is necessary for testing true mediation and causality (Maxwell & Cole, 2007), cross-sectional designs can still provide insight regarding potential indirect effects, which begin to address functional relations and may spur future empirical questions to be tested in larger longitudinal designs. Other limitations relate to the assessment of adherence. Our priority in recruiting a group at highest risk for poor outcomes also forced us to forgo precision in our adherence assessment (i.e., rather than only focusing on individuals on ART). Although not empirically supported in this sample, it is possible that actual rates of adherence differ across different medical conditions and medication types, which may also hold true across different classes of ART given differing dosing instructions and side effect profiles (e.g., Airoldi et al., 2010). It is also very likely that adherence to these other forms of medication (e.g., antidepressants), may have a direct effect on ART adherence (Horberg et al., 2008; Walkup et al., 2008). Although we were not powered in the current sample to run the primary analyses comparing individuals on ART versus not (Fritz & MacKinnon, 2007), this would be an important direction for future work using larger sample sizes to examine whether these results differ when comparing adherence to ART versus other medications.
Given how high rates of self-reported adherence were in the past 4 days—reaching almost 100 %—there was not sufficient variability in this measure to meaningfully analyze. The high rates of adherence in the past 4 days may reflect typical inflation of self-reported adherence behavior when asked to report on missed doses directly (Kalichman et al., 2009; Liu et al., 2001; Simoni et al., 2006) or actual high adherence rates common in the context of a controlled, substance abuse treatment setting (Hicks et al., 2007). This is in line with recent reviews that have shown HIV-positive substance users adhere to ART at comparable rates to HIV-positive individuals who do not use drugs (Malta et al., 2008) and that adherence interventions can be effective to improve adherence in the short-term for HIV-positive substance users (Binford et al., 2012).
Although not necessarily a perfect proxy for actual adherence behavior, querying for reasons for nonadherence has been suggested to be an effective strategy to minimize social desirability biases and other inaccuracies of self-reported adherence (Simoni et al., 2006), yet there are some significant limitations of this measure. It is unclear whether a greater frequency of doses missed across commonly endorsed reasons for nonadherence reflects actual missed doses. It may be that some individuals perceive greater barriers to adherence and report nonadherence across a range of reasons, whereas other individuals may consistently miss more doses but for a single reason. Although difficult in a controlled environment with little privacy, electronic pill caps (for ART as well as other medications) would have been a potentially more accurate, objective measure of adherence. Future studies must examine the accuracy of assessing reasons endorsed for nonadherence as a measure of missed doses by comparing it to objective measures of adherence. Finally, generalizing these findings to global resource-limited settings should be done with caution; although findings from recent meta-analyses suggest the relationship between depression and adherence is consistent across low- and high-income countries (Gonzalez, Batchelder et al., 2011; Uthman et al., 2014), and that there are not significant differences in rates of depression based upon country income level (Uthman et al., 2014).
Conclusions
Considered within the context of the limitations noted above, the findings do have important potential clinical implications. The identification of a potentially modifiable factor that plays an important role in the relation between depression and medication adherence may lend itself to future intervention development efforts. Findings point to the unique role of environmental punishment in the relation between depression and medication adherence. If findings continue to replicate, this may suggest adapting existing interventions for depression and adherence among substance users to focus more exclusively on reducing environmental punishment—or perceptions of punishment—and the impact environmental punishment may have on one’s perceived self-efficacy to adhere to medication regimens. Existing cognitive behavioral interventions for improving depressive symptoms and medication adherence among substance users with HIV/AIDS (Daughters et al., 2010; Magidson et al., 2013, 2014; Safren et al., 2012) may incorporate strategies to more directly address perceptions or experiences of environmental punishment that may interfere with adherence; this may assist with anticipating and normalizing the potential punishing experiences one may encounter when taking ART (side effects, stigma, etc.). These findings are a first step in improving the understanding of how depression may relate to medication nonadherence and the role environmental punishment plays in this relation among HIV-positive, predominantly African American substance users. Along with future replications and extensions of this work, these findings may spur efforts for continued refinement of clinical interventions for this population.
Acknowledgments
This study was supported by Grants R01DA022 974 (PI: Daughters) and R36DA034513 (PI: Magidson). Dr. Magidson’s work on this manuscript was also supported by T32MH093310 (PIs: Henderson and Fricchione). Dr. Safren is supported by Grant K24MH094214.
Footnotes
Conflict of interest Jessica F. Magidson, Alyson Listhaus, C.J. Seitz-Brown, Steven A. Safren, C.W. Lejuez, and Stacey B. Daughters declare that they have no conflict of interest.
Human and animal rights and informed consent All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
Contributor Information
Jessica F. Magidson, Email: jmagidson@partners.org, Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, One Bowdoin Square, 7th Floor, Boston, MA 02114, USA. University of Maryland, College Park, MD, USA
Alyson Listhaus, University of Maryland, College Park, MD, USA. Columbia University, New York, NY, USA.
C. J. Seitz-Brown, University of Maryland, College Park, MD, USA
Steven A. Safren, Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, One Bowdoin Square, 7th Floor, Boston, MA 02114, USA
C. W. Lejuez, University of Maryland, College Park, MD, USA
Stacey B. Daughters, University of North Carolina, Chapel Hill, NC, USA
References
- Airoldi M, Zaccarelli M, Bisi L, Bini T, Antinori A, Mussini C, et al. One-pill once-a-day HAART: A simplification strategy that improves adherence and quality of life of HIV-infected subjects. Patient Preference and Adherence. 2010;4:115. doi: 10.2147/ppa.s10330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asch SM, Kilbourne AM, Gifford AL, Burnam MA, Turner B, Shapiro MF, et al. Underdiagnosis of depression in HIV. Journal of General Internal Medicine. 2003;18:450–460. doi: 10.1046/j.1525-1497.2003.20938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aversa SL, Kimberlin C. Psychosocial aspects of antiretroviral medication use among HIV patients. Patient Education and Counseling. 1996;29:207–219. doi: 10.1016/0738-3991(96)00910-x. [DOI] [PubMed] [Google Scholar]
- Benassi VA, Sweeney PD, Dufour CL. Is there a relation between locus of control orientation and depression? Journal of Abnormal Psychology. 1988;97:357–367. doi: 10.1037//0021-843x.97.3.357. [DOI] [PubMed] [Google Scholar]
- Berger-Greenstein JA, Cuevas CA, Brady SM, Trezza G, Richardson MA, Keane TM. Major depression in patients with HIV/AIDS and substance abuse. AIDS Patient Care and STDs. 2007;21:942–955. doi: 10.1089/apc.2006.0153. [DOI] [PubMed] [Google Scholar]
- Binford MC, Kahana SY, Altice FL. A systematic review of antiretroviral adherence interventions for HIV-infected people who use drugs. Current HIV/AIDS Reports. 2012;9:287–312. doi: 10.1007/s11904-012-0134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Archives of General Psychiatry. 2001;58:721. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- Bogart LM, Wagner GJ, Galvan FH, Klein DJ. Longitudinal relationships between antiretroviral treatment adherence and discrimination due to HIV-serostatus, race, and sexual orientation among African-American men with HIV. Annals of Behavioral Medicine. 2010;40:184–190. doi: 10.1007/s12160-010-9200-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burack JH, Barrett DC, Stall RD, Chesney MA, Ekstrand ML, Coates TJ. Depressive symptoms and CD4 lymphocyte decline among HIV-infected men. Journal of the American Medical Association (JAMA) 1993;270:2568–2573. [PubMed] [Google Scholar]
- Carvalho JP, Gawrysiak MJ, Hellmuth JC, McNulty JK, Magidson JF, Lejuez CW, et al. The Reward Probability Index (RPI): Design and validation of a scale measuring access to environmental reward. Behavior Therapy. 2011;42:249–262. doi: 10.1016/j.beth.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Chander G, Himelhoch S, Fleishman JA, Hellinger J, Gaist P, Moore RD, et al. ART receipt and viral suppression among HIV-infected patients with co-occurring mental illness and illicit drug use. AIDS Care. 2009;21:655–663. doi: 10.1080/09540120802459762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesney M. Adherence to ART regimens. AIDS Patient Care and STDs. 2003;17:169–177. doi: 10.1089/108729103321619773. [DOI] [PubMed] [Google Scholar]
- Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, et al. Self-reported adherence to antiretroviral medications among participants in the HIV clinical trials: The AACTG Adherence Instruments. AIDS Care. 2000;12:255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- Crum NF, Riffenburgh RH, Wegner S, Agan BK, Tasker SA, Spooner KM, et al. Comparisons of causes of death and mortality rates among HIV-infected persons: Analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. Journal of Acquired Immune Deficiency Syndromes (JAIDS) 2006;41:194–200. doi: 10.1097/01.qai.0000179459.31562.16. [DOI] [PubMed] [Google Scholar]
- Daughters SB, Magidson JF, Schuster RM, Safren SA. ACT HEALTHY: A combined cognitive-behavioral depression and medication adherence treatment for HIV-infected substance users. Cognitive and Behavioral Practice. 2010;17:309–321. doi: 10.1016/j.cbpra.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiIorio C, McCarty F, Depadilla L, Resnicow K, Holstad MM, Yeager K, et al. Adherence to antiretroviral medication regimens: A test of a psychosocial model. AIDS and Behavior. 2009;13:10–22. doi: 10.1007/s10461-007-9318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: Meta-analysis of the effects of anxiety and depression on patient adherence. Archives of Internal Medicine. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- Eldred LJ, Wu AW, Chaisson RE, Moore RD. Adherence to antiretroviral and pneumocystis prophylaxis in HIV disease. JAIDS. 1998;18:117–125. doi: 10.1097/00042560-199806010-00003. [DOI] [PubMed] [Google Scholar]
- Evans S, Ferrando SJ, Rabkin JB, Fishman B. Health locus of control, distress, and utilization of protease inhibitors among HIV-positive men. Journal of Psychosomatic Research. 2000;49:157–162. doi: 10.1016/s0022-3999(00)00157-4. [DOI] [PubMed] [Google Scholar]
- Ferster CB. A functional analysis of depression. American Psychologist. 1973;28:857–870. doi: 10.1037/h0035605. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M. Structured clinical interview for DSM-IV axis I disorders (SCID-I) and the structured clinical interview for DSM-IV Axis II disorders (SCID-II) In: Hilsenroth MJ, Segal DL, editors. Comprehensive handbook of psychological assessment, Vol. 2: Personality assessment. Hoboken, NJ: Wiley; 2004. [Google Scholar]
- Fritz MS, MacKinnon DP. Required sample size to detect the mediated effect. Psychological Science. 2007;18:233–239. doi: 10.1111/j.1467-9280.2007.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García DOP, Knobel H, Carmona A, Guelar A, López-Colomés JL, Caylà JA. Impact of adherence and highly active antiretroviral therapy on survival in HIV-infected patients. JAIDS. 2002;30:105–110. doi: 10.1097/00042560-200205010-00014. [DOI] [PubMed] [Google Scholar]
- Gifford A, Bormann J, Shively M. Predictors of self-reported adherence and plasma HIV concentrations in patients on multidrug antiretroviral regimens. JAIDS. 2000;23:386–395. doi: 10.1097/00126334-200004150-00005. [DOI] [PubMed] [Google Scholar]
- Gonzalez J, Batchelder AW, Psaros C, Safren S. Depression and HIV/AIDS treatment nonadherence: A review and meta-analysis. JAIDS. 2011a;58:181–187. doi: 10.1097/QAI.0b013e31822d490a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JS, Psaros C, Batchelder A, Applebaum A, Newville H, Safren S. Clinician-assessed depression and ART adherence in HIV-infected individuals in methadone maintenance treatment. Annals of Behavioral Medicine. 2011b;42:120–126. doi: 10.1007/s12160-011-9268-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore-Felton C, Koopman C. Behavioral mediation of the relationship between psychosocial factors and HIV disease progression. Psychosomatic Medicine. 2008;70:569–574. doi: 10.1097/PSY.0b013e318177353e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Communication Monographs. 2009;76:408–420. [Google Scholar]
- Hicks PL, Mulvey KP, Chander G, Fleishman JA, Josephs JS, Korthuis PT, et al. The impact of illicit drug use and substance abuse treatment on adherence to ART. AIDS Care. 2007;19:1134–1140. doi: 10.1080/09540120701351888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroto DS. Locus of control and learned helplessness. Journal of Experimental Psychology. 1974;102:187–193. [Google Scholar]
- Holzemer WL, Corless IB, Nokes KM, Turner JG, Brown MA, Powell-Cope GM, et al. Predictors of self-reported adherence in persons living with HIV disease. AIDS Patient Care and STDs. 1999;13:185–197. doi: 10.1089/apc.1999.13.185. [DOI] [PubMed] [Google Scholar]
- Horberg MA, Silverberg MJ, Hurley LB, Towner WJ, Klein DB, Bersoff-Matcha S, et al. Effects of depression and selective serotonin reuptake inhibitor use on adherence to highly active antiretroviral therapy and on clinical outcomes in HIV-infected patients. JAIDS. 2008;27:384–390. doi: 10.1097/QAI.0b013e318160d53e. [DOI] [PubMed] [Google Scholar]
- Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women. JAMA. 2001;285:1466–1474. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- Ingersoll K. The impact of psychiatric symptoms, drug use, and medication regimen on non-adherence to HIV treatment. AIDS Care. 2004;16:199–211. doi: 10.1080/09540120410001641048. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Amaral CM, Swetzes C, Jones M, Macy R, Kalichman MO, et al. A simple single-item rating scale to measure medication adherence: Further evidence for convergent validity. Journal of the International Association of Physicians in AIDS Care. 2009;8:367–374. doi: 10.1177/1545109709352884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanter JW, Mulick PS, Busch AM, Berlin KS, Martell CR. The Behavioral Activation for Depression Scale (BADS): Psychometric properties and factor structure. Journal of Psychopathology and Behavioral Assessment. 2007;29:191–202. [Google Scholar]
- Kanter JW, Rusch LC, Busch AM, Sedivy SK. Validation of the Behavioral Activation for Depression Scale (BADS) in a community sample with elevated depressive symptoms. Journal of Psychopathology and Behavioral Assessment. 2009;31:36–42. [Google Scholar]
- Leserman J. Role of depression, stress, and trauma in HIV disease progression. Psychosomatic Medicine. 2008;70:539–545. doi: 10.1097/PSY.0b013e3181777a5f. [DOI] [PubMed] [Google Scholar]
- Leserman J, Petitto JM, Gu H, Gaynes BN, Barroso J, Golden RN, et al. Progression to AIDS, a clinical AIDS condition and mortality: Psychosocial and physiological predictors. Psychological Medicine. 2002;32:1059–1073. doi: 10.1017/s0033291702005949. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM. A behavioral approach to depression. In: Friedman RM, Katz MM, editors. The psychology of depression: Contemporary theory and research. New York: Wiley; 1974. [Google Scholar]
- Lewinsohn PM, Graf M. Pleasant activities and depression. Journal of Consulting and Clinical Psychology (JCCP) 1973;41:261–268. doi: 10.1037/h0035142. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Libet J. Pleasant events, activity schedules, and depressions. Journal of Abnormal Psychology. 1972;79:291–295. doi: 10.1037/h0033207. [DOI] [PubMed] [Google Scholar]
- Liu H, Golin CE, Miller LG, Hays RD, Beck CK, Sanandaji S. A comparison study of multiple measures of adherence to HIV protease inhibitors. Annals of Internal Medicine. 2001;134:968–977. doi: 10.7326/0003-4819-134-10-200105150-00011. [DOI] [PubMed] [Google Scholar]
- Magidson JF, Blashill AJ, Safren SA, Wagner GJ. Depressive symptoms, lifestyle structure, and ART adherence among HIV-infected individuals: A longitudinal mediation analysis. AIDS and Behavior. 2014 doi: 10.1007/s10461-014-0802-3. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magidson JF, Seitz-Brown CJ, Safren SA, Daughters SB. Implementing behavioral activation and Life-Steps for depression and HIV medication adherence in a community health center. Cognitive and Behavioral Practice. 2013 doi: 10.1016/j.cbpra.2013.10.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier W, Philipp M. Improving the assessment of severity of depressive states: A reduction of the Hamilton Depression Rating Scale. Pharmacopsychiatry. 1985;18:114–115. [Google Scholar]
- Malebranche DJ, Peterson JL, Fullilove RE, Stackhouse RW. Race and sexual identity: Perceptions about medical culture and healthcare among Black men who have sex with men. Journal of the National Medical Association. 2004;96:97–107. [PMC free article] [PubMed] [Google Scholar]
- Malta M, Strathdee SA, Magnanini MM, Bastos FI. Adherence to antiretroviral therapy for human immunodeficiency virus/acquired immune deficiency syndrome among drug users: A systematic review. Addiction. 2008;103:1242–1257. doi: 10.1111/j.1360-0443.2008.02269.x. [DOI] [PubMed] [Google Scholar]
- Maxwell SE, Cole DA. Bias in cross-sectional analyses of longitudinal mediation. Psychological Methods. 2007;12:23–44. doi: 10.1037/1082-989X.12.1.23. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Konarski JZ, Mancini DA, Fulton KA, Parikh SV, Grigoriadis S, Grupp LA, Kennedy SH. Measuring the severity of depression and remission in primary care: Validation of the HAMD-7 scale. Canadian Medical Association Journal. 2005;17 doi: 10.1503/cmaj.050786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb MD, Harlow LL. Life events and substance use among adolescents: Mediating effects of perceived loss of control and meaninglessness in life. Journal of Personality and Social Psychology. 1986;51:564. doi: 10.1037//0022-3514.51.3.564. [DOI] [PubMed] [Google Scholar]
- Nezu AM, Ronan GF, Meadows EA, McClure KS. Practitioners guide to empirically based measures of depression. New York: Kluwer Academic/Plenum Publishers; 2000. [Google Scholar]
- O’Cleirigh C, Ironson G, Smits JA. Does distress tolerance moderate the impact of major life events on psychosocial variables and behaviors important in the management of HIV? Behavior Therapist. 2007;38:314–323. doi: 10.1016/j.beth.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pablos-Méndez A, Knirsch CA, Barr RG, Lerner BH, Frieden TR. Nonadherence in tuberculosis treatment: Predictors and consequences in New York City. The American Journal of Medicine. 1997;102:164–170. doi: 10.1016/s0002-9343(96)00402-0. [DOI] [PubMed] [Google Scholar]
- Palmer NB, Salcedo J, Miller AL, Winiarski M, Arno P. Psychiatric and social barriers to HIV medication adherence in a triply diagnosed methadone population. AIDS Patient Care and STDs. 2003;17:635–644. doi: 10.1089/108729103771928690. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Roberts KJ. Barriers to and facilitators of HIV-positive patients’ adherence to antiretroviral treatment regimens. AIDS Patient Care and STDs. 2000;14:155–168. doi: 10.1089/108729100317948. [DOI] [PubMed] [Google Scholar]
- Rotter JB. Generalized expectancies for internal versus external control of reinforcement. Psychological Monographs: General and Applied. 1966;80:1–28. [PubMed] [Google Scholar]
- Rotter JB. Some problems and misconceptions related to the construct of internal versus external control of reinforcement. JCCP. 1975;43:56–67. [Google Scholar]
- Ryan GW, Wagner GJ. Pill taking ‘routinization’: A critical factor to understanding episodic medication adherence. AIDS Care. 2003;15:795–806. doi: 10.1080/09540120310001618649. [DOI] [PubMed] [Google Scholar]
- Safren SA, O’Cleirigh CM, Bullis JR, Otto MW, Stein MD, Pollack MH. Cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected injection drug users: A randomized controlled trial. JCCP. 2012;80:404–415. doi: 10.1037/a0028208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlenk EA, Hart LK. Relationship between health locus of control, health value, and social support and compliance of persons with diabetes mellitus. Diabetes Care. 1984;7 doi: 10.2337/diacare.7.6.566. [DOI] [PubMed] [Google Scholar]
- Shah SS, McGowan JP, Smith C, Blum S, Klein RS. Comorbid conditions, treatment, and health maintenance in older persons with human immunodeficiency virus infection in New York City. Clinical Infectious Diseases. 2002;35:1238–1243. doi: 10.1086/343048. [DOI] [PubMed] [Google Scholar]
- Sharpe TT, Lee LM, Nakashima AK, Elam-Evans LD, Fleming PL. Crack cocaine use and adherence to antiretroviral treatment among HIV-infected black women. Journal of Community Health. 2004;29:117–127. doi: 10.1023/b:johe.0000016716.99847.9b. [DOI] [PubMed] [Google Scholar]
- Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA. Self-report measures of antiretroviral therapy adherence: A review with recommendations for HIV research and clinical management. AIDS and Behavior. 2006;10 doi: 10.1007/s10461-006-9078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton AL. Determinants of adherence to medical regimens by hypertensive patients. Journal of Behavioral Medicine. 1987;10:377–394. doi: 10.1007/BF00846477. [DOI] [PubMed] [Google Scholar]
- Tabachnik BG, Fidell LS. Using multivariate statistics. Boston: Pearson; 2007. [Google Scholar]
- Uthman OA, Magidson JF, Safren SA, Nachega JB. Depression and adherence to antiretroviral therapy in low-, middle-, and high-income countries: A systematic review and meta-analysis. Current HIV/AIDS Reports. 2014 doi: 10.1007/s11904-014-0220-1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GJ, Goggin K, Remien RH, Rosen MI, Simoni J, Bangsberg DR, et al. A closer look at depression and its relationship to HIV antiretroviral adherence. Annals of Behavioral Medicine. 2011;42:352–360. doi: 10.1007/s12160-011-9295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GJ, Ryan GW. Relationship between routinization of daily behaviors and medication adherence in HIV-positive drug users. AIDS Patient Care STDs. 2004;18:385–393. doi: 10.1089/1087291041518238. [DOI] [PubMed] [Google Scholar]
- Walkup J, Wei W, Sambamoorthi U, Crystal S. Antidepressant treatment and adherence to combination antiretroviral therapy among patients with AIDS and diagnosed depression. Psychiatric Quarterly. 2008;79:43–53. doi: 10.1007/s11126-007-9055-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallston KA, Wallston BS, DeVellis R. Development of the multidimensional health locus of control (MHLC) scales. Health Education and Behavior. 1978;6:160–170. doi: 10.1177/109019817800600107. [DOI] [PubMed] [Google Scholar]
- Warner EA, Greene GS, Buchsbaum MS, Cooper DS, Robinson BE. Diabetic ketoacidosis associated with cocaine use. Archives of Internal Medicine. 1998;158:1799. doi: 10.1001/archinte.158.16.1799. [DOI] [PubMed] [Google Scholar]


