Abstract
A plasma membrane-bound G protein-coupled receptor, TGR5, that transmits bile acid signaling into a cellular response primarily via the cAMP pathway is expressed in human and rodent cholangiocytes and is localized to multiple, diverse subcellular compartments, including primary cilia. Ciliary-associated TGR5 plays an important role in cholangiocyte physiology and may contribute to a group of liver diseases referred to as the `cholangiociliopathies', which include polycystic liver disease (PLD) and, possibly, cholangiocarcinoma and primary sclerosing cholangitis. Based on our observations that (1) ciliated and nonciliated cholangiocytes respond to TGR5 activation differently (i.e. the level of cAMP increases in nonciliated cholangiocytes but decreases in ciliated cells) and (2) hepatic cysts are derived from cholangiocytes that are characterized by both malformed cilia and increased cAMP levels, we hypothesized that TGR5-mediated cAMP signaling in cystic cholangiocytes contributes to hepatic cystogenesis. Indeed, our studies show that TGR5 is overexpressed and mislocalized in cystic cholangiocytes, and when activated by ligands, results in increased intracellular cAMP levels, cholangiocyte hyperproliferation and cyst growth. Our studies also show that genetic elimination of TGR5 in an animal model of PLD inhibits hepatic cystogenesis. Collectively, these data suggest the involvement of TGR5 in PLD and that TGR5 targeting in cystic cholangiocytes may have therapeutic potential.
Keywords: Cholangiocytes, Cilia, Cholangiociliopathies, Polycystic liver disease, Hepatic cysts
Introduction
In many cell types, bile acids perform signaling functions via a plasma membrane-bound, G protein-coupled receptor, TGR5, which mediates their rapid, transcription-independent actions [1–3]. In the liver, TGR5 is expressed in cholangiocytes, Kupffer cells and sinusoidal endothelial cells [1, 3–7]. Because cholangiocytes show weak or no expression of other bile acid receptors such as nuclear farnesoid X receptor and pregnane X receptor, TGR5 is presumably a major receptor for bile acid signaling in biliary epithelia. In cholangiocytes, TGR5 is localized to the apical plasma membrane and primary cilia (fig. 1). Functionally, TGR5 is linked to the cAMP signaling pathway, and its activation by free taurine-conjugated and glycine-conjugated bile acids induces an increase in intracellular cAMP levels followed by a cell-specific response in freshly isolated and short-term cultured cholangiocytes [7].
Fig 1.
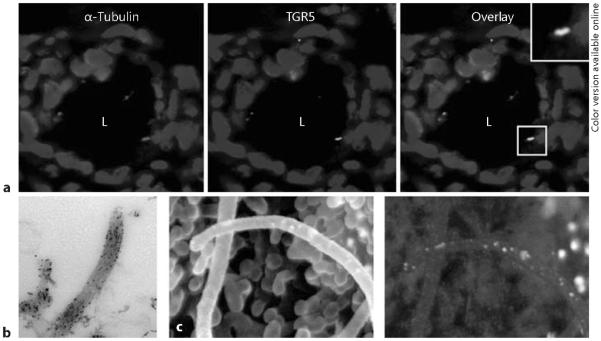
TGR5 is localized to cholangiocyte cilia, a Primary cilia in rat cholangiocytes were visualized with antibodies to a ciliary marker, acetylated α-tubulin (red; color in online version only), and TGR5 (green). Nuclei were stained with DAPI (blue). Colocalization of acetylated α-tubulin and TGR5 is shown in yellow with insets at a higher magnification (`L' – the lumen of intrahepatic bile duct), b Immunogold transmission electron microscopy shows localization of TGR5 to the ciliary membrane in intrahepatic bile ducts isolated from rat liver, c The conventional immunogold scanning electron microscopy of isolated split-open rat intrahepatic bile ducts shows primary cilia and microvilli extending from the cholangiocyte apical plasma membrane. The image generated by backscattered electrons shows numerous white dots revealing the presence of TGR5 on cholangiocyte cilia and the surface of the apical membrane. Images published with permission.
Recent studies have provided strong evidence for an explicit role for TGR5 in the organs and cells in which this receptor is expressed, and also suggest a possible association with human diseases. In some diseases, TGR5 shows a protective effect or involvement in the improvement of disease, while in other cases TGR5 appears to contribute to disease pathogenesis [2, 8–14]. In this report, we summarize our current knowledge of TGR5's role in a group of liver diseases referred to as the `cholangiociliopathies'.
Cholangiociliopathies
The cholangiopathies are a diverse group of hepatobiliary diseases in which cholangiocytes, the epithelial cells lining bile ducts, are a disease target. Among this group of diseases is a subgroup which we have termed the cholangiociliopathies – i.e. genetic disorders resulting from mutations in genes encoding proteins localized to primary cilia, the sensory organelles extending from the cholangiocyte apical plasma membrane into the bile duct lumen [15–18].
At present, liver diseases associated with several genetic disorders such as autosomal dominant polycystic kidney disease (ADPKD), autosomal recessive polycystic kidney disease (ARPKD), nephronophthisis and Meckel-Gruber syndrome are considered as cholangiociliopathies (fig. 2). Polycystic liver disease (PLD) is the liver manifestation of both ADPKD and ARPKD, and is characterized by development of fluid-filled hepatic cysts arising from cholangiocytes and growing progressively. In contrast, nephronophthisis and Meckel-Gruber syndrome are characterized by congenital hepatic fibrosis, biliary dysgenesis and ductal plate malformations without significant hepatic cystogenesis [18–21].
Fig 2.
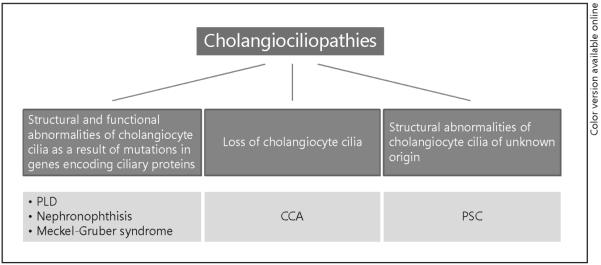
Cholangiociliopathies are hepatobiliary diseases with abnormalities in structure and functions of cholangiocyte cilia.
We recently reported that cholangiocarcinoma (CCA), a highly aggressive tumor derived from cholangiocytes, is characterized by the loss of cilia and by activation of the ciliary-associated Hh (hedgehog) and MAPK (mitogen-activated protein kinase) signaling pathways [22]. In contrast, cholangiocytes in primary sclerosing cholangitis (PSC) possess longer primary cilia compared to healthy cells [unpubl. data]. Based on these observations, we speculate that CCA and PSC represent a novel subclass of cholangiociliopathies (fig. 2).
The most prominent example of cholangiociliopathies is PLD, which occurs in combination with both ADPKD and ARPKD [19, 21, 23, 24]. ADPKD is linked to mutations in either PKD1 or PKD2 genes encoding the proteins polycystin-1 and polycystin-2, respectively. ARPKD is linked to a single gene, PKHD1, which encodes the protein fibrocystin. In healthy cholangiocytes, polycystin-1, polycystin-2 and fibrocystin are localized to primary cilia where they form sensory complexes through which changes in extracellular milieu are transduced into cholangiocyte functional responses [15, 25]. The disruption of these proteins by either spontaneous mutations or experimental manipulations leads to structural and/or functional abnormalities in cholangiocyte cilia subsequently activating or shutting down the associated intracellular signaling mechanisms and resulting in abnormal cell proliferation, disturbed cell-matrix interactions and dysregulation of fluid secretion and absorption, all of which contribute to hepatic cystogenesis [15,26–31].
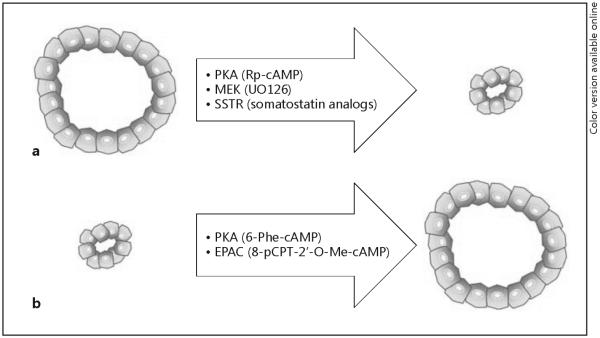
Despite the links of PLD to cholangiocyte cilia, the mechanisms involved in hepatic cyst growth remain obscure. It is evident from our previous work that cAMP signaling is the most important intracellular signaling pathway regulating hepatic cystogenesis [28, 32, 33]. We have demonstrated in a number of laboratory studies and clinical trials that experimental manipulations with cAMP levels in cystic cholangiocytes may induce or halt cyst expansion in vitro and in vivo, depending on the manipulation (fig. 3) [27, 32–35]. Based on these observations, we hypothesized that the connection between cholangiocyte cilia and the cAMP intracellular signaling pathway in PLD occurs via cilia-associated mechanisms of cAMP signaling initiated by activation of G protein-coupled receptors localized to ciliary membrane, and that TGR5 is one such G protein-coupled receptor that could be involved.
Fig 3.
Targeting of the cAMP signaling machinery differentially affects hepatic cystogenesis. a Inhibition of the cAMP downstream effectors PKA and MEK suppressed growth of hepatic cysts in vitro. Somatostatin analogs (i.e. octreotide and pasireotide) target G protein-coupled plasma membrane-bound somatostatin receptors (SSTR) which are the upstream modulators of the cAMP signaling. SSTR activation stimulated cyst retardation in vitro and in vivo in animal models of PLD. b Activation of cAMP machinery (i.e. PKA and EPAK proteins) leads to the opposite effects triggering cyst expansion.
TGR5 Is a Ciliary-Associated Bile Acid Receptor
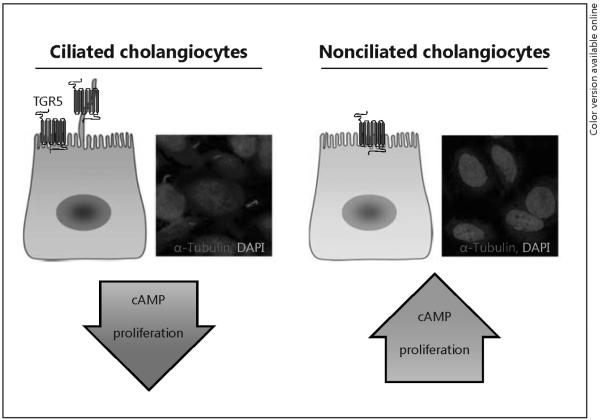
The expression of TGR5 in cholangiocyte cilia suggests a novel ciliary-associated, TGR5-mediated bile acid signaling mechanism in biliary epithelia. This idea stemmed from our work which demonstrated cholangiocyte cilia are chemosensory organelles that can transduce signals from constituents in bile (e.g. bile acids, nucleotides) that influence cholangiocyte functional activities [4, 7, 36]. This putative pathway is likely physiologically important because cholangiocyte cilia are constantly exposed to varying amounts and types of biliary constituents, including bile acids, present in the lumen of the biliary tree. Indeed, our recently published work is consistent with the conclusion that the localization of TGR5 on the apical membrane of ciliated or nonciliated cholangiocytes determines the cholangiocyte functional response to bile acid signaling [7]. Using both ciliated and nonciliated cultured cholangiocytes, we demonstrated that TGR5 agonists induce opposite changes in cAMP levels in cells with and without primary cilia, i.e. the cAMP level was increased in nonciliated cholangiocytes but decreased in ciliated cells in response to the same TGR5 agonists, suggesting that ciliary-dependent, TGR5-mediated signaling negatively regulates intracellular cAMP levels in cholangiocytes (fig. 4). Taking into account that cystic cholangiocytes are characterized by malformed cilia and increased levels of cAMP [28, 29], it seemed plausible to us that, just like nonciliated normal cholangiocytes, cystic cholangiocytes have lost the ciliary-associated TGR5-mediated mechanisms negatively controlling cAMP levels. Indeed, our initial experiments to test this hypothesis demonstrated TGR5 is overexpressed and mislocalized in cystic cholangiocytes, resulting in increased cAMP levels, cell hyperproliferation and cyst growth. Further, in vivo activation of TGR5 in rodents stimulates hepatic cystogenesis while genetic elimination of Gpbar1 inhibits cyst formation. Thus, these data suggest an important role for TGR5 in hepatic cystogenesis, and provide the rationale for considering TGR5 as a potential therapeutic target in PLD.
Fig 4.
Activation of TGR5 differentially affects cAMP signaling and cell proliferation in ciliated and nonciliated cholangiocytes. In ciliated cholangiocytes, TGR5 agonists decrease cAMP levels and cell proliferation. In contrast, in nonciliated cholangiocytes effects of TGR5 stimulation are opposite and resulting in increased cAMP levels and cell proliferation.
The role of TGR5 in two other potential cholangiociliopathies, i.e. PSC and CCA, remains unclear. Despite the identification of mutations in the GPBAR1 gene, which may reduce or abolish TGR5 function, the contribution of these mutations to PSC is not obvious [14]. However, we do not exclude the involvement of TGR5 in PSC since we have preliminary data showing a decreased expression of TGR5 in human PSC livers. This finding may suggest that PSC cholangiocytes, which are characterized by excessive expression and release of proinflammatory mediators [e.g. IL-6, IL-8, and cytokine monocyte chemoattractant protein-1 (MCP-1 or CCL2)], have abnormalities in the mechanisms of proinflammatory cytokine and chemokine production. These mechanisms are potentially associated with TGR5-mediated bile acid signaling, which is known to inhibit proinflammatory cytokine production in Kupffer cells [5].
The role of TGR5 in CCA is also unclear, but preliminary studies show a high expression of TGR5 in human CCA livers [14]. It was hypothesized that overexpression of TGR5 in CCA may result in increased cholangiocyte proliferation and apoptosis resistance, thus contributing to the progression of disease.
Conclusion
Existing knowledge related to the levels of expression, site of localization and functions of TGR5 in cholangiocytes suggests that this bile acid receptor may contribute to a number of liver diseases known as cholangiociliopathies. It is obvious that GPBAR1, the gene encoding TGR5, is not a causative gene for any of the known cholangiociliopa thies, whereas the expression and function of TGR5 in cholangiocytes with functional and structural abnormalities in primary cilia may contribute to the course of liver diseases. Currently, we are increasing our understanding of the role of TGR5 in PLD, and are beginning to understand the role of this bile acid receptor in PSC and CCA. The outcome of these studies will almost certainly provide further support for TGR5 as an effective therapeutic target.
Footnotes
Disclosure Statement The authors declare that no financial or other conflict of interest exists in relation to the content of the article.
References
- 1.Schaap FG, Trauner M, Jansen PL. Bile acid receptors as targets for drug development. Nat Rev Gastroenterol Hepatol. 2014;11:55–67. doi: 10.1038/nrgastro.2013.151. [DOI] [PubMed] [Google Scholar]
- 2.Pols TW. TGR5 in inflammation and cardiovascular disease. Biochem Soc Trans. 2014;42:244–249. doi: 10.1042/BST20130279. [DOI] [PubMed] [Google Scholar]
- 3.Duboc H, Tache Y, Hofmann AF. The bile acid TGR5 membrane receptor: from basic research to clinical application. Dig Liver Dis. 2014;46:302–312. doi: 10.1016/j.dld.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keitel V, Haussinger D. TGR5 in cholangiocytes. Curr Opin Gastroenterol. 2013;29:299–304. doi: 10.1097/MOG.0b013e32835f3f14. [DOI] [PubMed] [Google Scholar]
- 5.Keitel V, Donner M, Winandy S, Kubitz R, Häussinger D. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem Biophys Res Commun. 2008;372:78–84. doi: 10.1016/j.bbrc.2008.04.171. [DOI] [PubMed] [Google Scholar]
- 6.Keitel V, Cupisti K, Ullmer C, Knoefel WT, Kubitz R, Haussinger D. The membrane-bound bile acid receptor TGR5 is localized in the epithelium of human gallbladders. Hepatology. 2009;50:861–870. doi: 10.1002/hep.23032. [DOI] [PubMed] [Google Scholar]
- 7.Masyuk AI, Huang BQ, Radtke BN, Gajdos GB, Splinter PL, Masyuk TV, Gradilone SA, LaRusso NF. Ciliary subcellular localization of TGR5 determines the cholangiocyte functional response to bile acid signaling. Am J Physiol Gastrointest Liver Physiol. 2013;304:G1013–G1024. doi: 10.1152/ajpgi.00383.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yasuda H, Hirata S, Inoue K, Mashima H, Ohnishi H, Yoshiba M. Involvement of membrane-type bile acid receptor M-BAR/TGR5 in bile acid-induced activation of epidermal growth factor receptor and mitogen-activated protein kinases in gastric carcinoma cells. Biochem Biophys Res Commun. 2007;354:154–159. doi: 10.1016/j.bbrc.2006.12.168. [DOI] [PubMed] [Google Scholar]
- 9.Zhong M. TGR5 as a therapeutic target for treating obesity. Curr Top Med Chem. 2010;10:386–396. doi: 10.2174/156802610790980576. [DOI] [PubMed] [Google Scholar]
- 10.Yoneno K, Hisamatsu T, Shimamura K, Kamada N, Ichikawa R, Kitazume MT, Mori M, Uo M, Namikawa Y, Matsuoka K, Sato T, Koganei K, Sugita A, Kanai T, Hibi T. TGR5 signalling inhibits the production of pro-inflammatory cytokines by in vitro differentiated inflammatory and intestinal macrophages in Crohn's disease. Immunology. 2013;139:19–29. doi: 10.1111/imm.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tiwari A, Maiti P. TGR5: an emerging bile acid G-protein-coupled receptor target for the potential treatment of metabolic disorders. Drug Discov Today. 2009;14:523–530. doi: 10.1016/j.drudis.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, Auwerx J, Schoonjans K. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–177. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stepanov V, Stankov K, Mikov M. The bile acid membrane receptor TGR5: a novel pharmacological target in metabolic, inflammatory and neoplastic disorders. J Recept Signal Transduct Res. 2013;33:213–223. doi: 10.3109/10799893.2013.802805. [DOI] [PubMed] [Google Scholar]
- 14.Keitel V, Reich M, Haussinger D. TGR5: pathogenetic role and/or therapeutic target in fibrosing cholangitis? Clin Rev Allergy Immunol. 2014 doi: 10.1007/s12016-014-8443-x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 15.Masyuk AI, Masyuk TV, LaRusso NF. Cholangiocyte primary cilia in liver health and disease. Dev Dyn. 2008;237:2007–2012. doi: 10.1002/dvdy.21530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masyuk T, Masyuk A, LaRusso N. Cholangiociliopathies: genetics, molecular mechanisms and potential therapies. Curr Opin Gastroenterol. 2009;25:265–271. doi: 10.1097/MOG.0b013e328328f4ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torres VE, Harris PC. Strategies targeting c AMP signaling in the treatment of polycystic kidney disease. J Am So Nephrol. 2014;25:18–32. doi: 10.1681/ASN.2013040398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chandok N. Polycystic liver disease: a clinical review. Ann Hepatol. 2012;11:819–826. [PubMed] [Google Scholar]
- 19.Cnossen WR, Drenth JP. Polycystic liver disease: an overview of pathogenesis, clinical manifestations and management. Orphanet J Rare Dis. 2014;9:69. doi: 10.1186/1750-1172-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris PC, Torres VE. Polycystic kidney disease. Annu Rev Med. 2009;60:321–337. doi: 10.1146/annurev.med.60.101707.125712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masyuk T, LaRusso N. Polycystic liver disease: new insights into disease pathogenesis. Hepatology. 2006;43:906–908. doi: 10.1002/hep.21199. [DOI] [PubMed] [Google Scholar]
- 22.Gradilone SA, Radtke BN, Bogert PS, Huang BQ, Gajdos GB, LaRusso NF. HDAC6 inhibition restores ciliary expression and decreases tumor growth. Cancer Res. 2013;73:2259–22570. doi: 10.1158/0008-5472.CAN-12-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strazzabosco M, Somlo S. Polycystic liver diseases: congenital disorders of cholangiocyte signaling. Gastroenterology. 2011;140:1855–1859. 1859.el. doi: 10.1053/j.gastro.2011.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wills ES, Roepman R, Drenth JP. Polycystic liver disease: ductal plate malformation and the primary cilium. Trends Mol Med. 2014;20:261–270. doi: 10.1016/j.molmed.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Masyuk AI, Masyuk TV, Splinter PL, Huang BQ, Stroope AJ, LaRusso NF. Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2+ and cAMP signaling. Gastroenterology. 2006;131:911–920. doi: 10.1053/j.gastro.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banales JM, Masyuk TV, Bogert PS, Huang BQ, Gradilone SA, Lee SO, Stroope AJ, Masyuk AI, Medina JF, LaRusso NF. Hepatic cystogenesis is associated with abnormal expression and location of ion transporters and water channels in an animal model of autosomal recessive polycystic kidney disease. Am J Pathol. 2008;173:1637–1646. doi: 10.2353/ajpath.2008.080125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banales JM, Masyuk TV, Gradilone SA, Masyuk AI, Medina JF, LaRusso NF. The cAMP effectors Epac and protein kinase a (PKA) are involved in the hepatic cystogenesis of an animal model of autosomal recessive polycystic kidney disease (ARPKD) Hepatology. 2009;49:160–174. doi: 10.1002/hep.22636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masyuk TV, Huang BQ, Ward CJ, Masyuk AI, Yuan D, Splinter PL, Punyashthiti R, Ritman EL, Torres VE, Harris PC, LaRusso NF. Defects in cholangiocyte fibrocystin expression and ciliary structure in the PCK rat. Gastroenterology. 2003;125:1303–1310. doi: 10.1016/j.gastro.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Masyuk TV, Huang BQ, Masyuk AI, Ritman EL, Torres VE, Wang X, Harris PC, Larusso NF. Biliary dysgenesis in the PCK rat, an orthologous model of autosomal recessive polycystic kidney disease. Am J Pathol. 2004;165:1719–1730. doi: 10.1016/S0002-9440(10)63427-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masyuk AI, Gradilone SA, Banales JM, Huang BQ, Masyuk TV, Lee SO, Splinter PL, Stroope AJ, Larusso NF. Cholangiocyte primary cilia are chemosensory organelles that detect biliary nucleotides via P2Y12 purinergic receptors. Am J Physiol Gastrointest Liver Physiol. 2008;295:G725–G734. doi: 10.1152/ajpgi.90265.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masyuk TV, Lee SO, Radtke BN, Stroope AJ, Huang B, Banales JM, Masyuk AI, Splinter PL, Gradilone SA, Gajdos GB, LaRusso NF. Centrosomal abnormalities characterize human and rodent cystic cholangiocytes and are associated with Cdc25A overexpression. Am J Pathol. 2014;184:110–121. doi: 10.1016/j.ajpath.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masyuk TV, Masyuk AI, Torres VE, Harris PC, LaRusso NF. Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3′,5′-cyclic monophosphate. Gastroenterology. 2007;132:1104–1116. doi: 10.1053/j.gastro.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 33.Masyuk TV, Radtke BN, Stroope AJ, Banales JM, Gradilone SA, Huang B, Masyuk AI, Hogan MC, Torres VE, Larusso NF. Pasireotide is more effective than octreotide in reducing hepatorenal cystogenesis in rodents with polycystic kidney and liver diseases. Hepatology. 2013;58:409–421. doi: 10.1002/hep.26140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hogan MC, Masyuk TV, Page L, Holmes DR, 3rd, Li X, Bergstralh EJ, Irazabal MV, Kim B, King BF, Glockner JF, LaRusso NF, Torres VE. Somatostatin analog therapy for severe polycystic liver disease: results after 2 years. Nephrol Dial Transplant. 2012;27:3532–3539. doi: 10.1093/ndt/gfs152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hogan MC, Masyuk TV, Page LJ, Kubly VJ, Bergstralh EJ, Li X, Kim B, King BF, Glockner J, Holmes DR, 3rd, Rossetti S, Harris PC, LaRusso NF, Torres VE. Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease. J Am Soc Nephrol. 2010;21:1052–1061. doi: 10.1681/ASN.2009121291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keitel V, Häussinger D. Perspective: TGR5 (Gpbar-1) in liver physiology and disease. Clin Res Hepatol Gastroenterol. 2012;36:412–419. doi: 10.1016/j.clinre.2012.03.008. [DOI] [PubMed] [Google Scholar]