Abstract
RATIONALE
Repetitive transcranial magnetic stimulation (rTMS) is a technique for noninvasive focal brain stimulation where small intracranial electrical currents are generated by a fluctuating extracranial magnetic field. In clinical epilepsy rTMS has been applied most often interictally to reduce seizure frequency. Less often, rTMS has been used to terminate ongoing seizures, as in instances of epilepsia partialis continua (EPC). Whether ictal rTMS is effective and safe in treatment of EPC has not been extensively studied. Here, we report our recent experience with rTMS in treatment of EPC, as an early step towards evaluating the safety and efficacy of rTMS in the treatment of intractable ongoing focal seizures.
METHODS
Seven patients with EPC due to mixed etiologies were treated with rTMS applied over the seizure. rTMS was delivered in high frequency (20 to 100 Hz) bursts or as prolonged low frequency (1 Hz) trains. EEG was recorded in three of seven.
RESULTS
rTMS resulted in a brief (20 – 30 minutes) pause in seizures in three of seven patients and a lasting (≥ 1 day) pause in two of seven. A literature search identified six additional reports of EPC treated rTMS where seizures were suppressed in three of six. Seizures were not exacerbated by rTMS in any patient. Generally mild side-effects included transient head and limb pain, and limb stiffening during high-frequency rTMS trains.
CONCLUSIONS
Our clinical observations in a small number of patients suggest that rTMS may be safe and effective in suppressing ongoing seizures associated with EPC. However, a controlled trial is needed to assess the safety and anticonvulsive efficacy of rTMS in EPC treatment.
Keywords: transcranial magnetic stimulation, seizure, epilepsia partialis continua
1. Introduction
Repetitive transcranial magnetic stimulation (rTMS) has been applied in several cases of intractable epilepsia partialis continua (EPC) to terminate the ongoing seizures. Here, we present a case series summarizing our recent experience with rTMS in treatment of EPC as an early step towards controlled trials testing of the safety and efficacy of rTMS in the treatment of continuous focal seizures.
rTMS is a noninvasive method for focal cortical stimulation that is based on Faraday’s principle of electromagnetic induction. During rTMS small intracranial electrical currents are repetitively generated by a strong fluctuating extracranial magnetic field [1, 2]. In recent years, the potential for low frequency (≤1 Hz) rTMS to induce a lasting decrease in cortical excitability has been applied to clinical epilepsy where interictal rTMS delivered over a neocortical seizure focus may reduce seizure frequency [3–5]. The precise mechanism for this antiepileptic property is unknown, but likely resembles that of long-term depression (LTD) that can be induced with direct electrical low frequency cortical stimulation.
In contrast to the more common interictal application of rTMS, ictal rTMS may be aimed to interrupt ongoing seizures, rather than to reduce seizure frequency in established epilepsy. The rationale for applying rTMS to suppress seizures in real time relates to its potential to interrupt ongoing neuronal activity, as well as to the potential to change focal cortical excitability (reviewed in [6]). Ictal application of rTMS however has not been extensively studied. Accordingly, we reviewed the published reports and report experience in our laboratory to summarize available efficacy and safety data related to ictal rTMS in cases of EPC.
2. Methods
We reviewed laboratory records to identify patients with refractory EPC who were treated with rTMS at the Berenson-Allen Center of Noninvasive Brain Stimulation (CNBS), and affiliated institutions. All patients presented with EPC of one day’s duration or greater. In all instances, the seizures were refractory to anticonvulsant medications, and a decision to treat the ongoing seizures with rTMS was made by the clinical team. rTMS was delivered by physicians trained in clinical application of rTMS (APL, AR, JMT), after obtaining informed consent. Where available, accompanying video and EEG were reviewed for the present report.
3. Results
3.1 Seizure suppression by rTMS
We treated seven patients with EPC of varying etiologies (Table 1). In each instance, seizures were clinically apparent as repetitive myoclonic or clonic movements involving the face, hand, arm or leg. The stimulating coil was positioned over the scalp region approximating the motor cortex contralateral to the side involved in the clinical movements. EEG was recorded for some or all of the rTMS sessions for three of seven patients.
Table 1.
Summary of EPC cases treated with rTMS in the Berenson-Allen Center for Noninvasive Brain Stimulation, and affiliated institutions.
| Patient | Age (Years) | Etiology | Coil Position | rTMS Intensity | rTMS Frequency | Train Duration | # Trains | Outcome | Adverse Events |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 42 | Hypoglycemia | Seizure Focus | 100% MT | 1 Hz | 1800 sec | 3 | Clinical seizures stopped for 30 min after each train, then resumed | none |
| 2 | 56 | Stroke, Hypoglycemia | Seizure Focus | 100% MT | 1st Session: 1 Hz 2nd Session: 20 Hz, then 1 Hz, |
20 Hz: 2 sec 1 Hz: 1600 sec |
1st Session: 1 (1 Hz) 2nd Session: 40 at 20 Hz, then 1 at 1 Hz |
1st Session: No effect 2nd Session: clinical seizures stopped for 20 min, then resumed |
|
| 3 | 33 | Unknown | Seizure Focus | 100% MT | 6 Hz then 1 Hz | 6 Hz priming then 1 Hz for 1600 sec | 2 | 1st Session 1: Clinical seizures improved during rTMS 2nd Session: Clinical seizures improved during stimulation, stopped for 20 minutes, then resumed |
none |
| 4 | 18 | Unknown | Seizure Focus | 100% MT | 1 Hz | 2000 sec | 1 | Clinical seizures stopped, and were absent for remainder of 2-day inpatient stay* | none |
| 5 | 46 | Resected cortical vascular malformation | Seizure Focus | 90–100% MO | 100 Hz, 1 Hz | 100 Hz: 0.05–1.25 sec 1 Hz: 1600–1800 sec |
1st Session: 15 at100 Hz†, then 1 at1 Hz) 2nd Session: 10 at 1 Hz, daily |
1st Session: Clinical seizures improved after stimulation, however resumed within 3 months 2nd Session: Clinical seizures improved and were suppressed at last follow-up (>4 months after rTMS) |
none |
| 6 [1] | 11 | Rasmussen’s Encephalitis | Seizure Focus | 100% MT | 1 Hz | 1800 sec | 9, daily | Clinical and EEG seizures were improved during stimulation, but returned to baseline within 30 min after each daily session | none |
| 7 | 79 | Stroke | Seizure Focus | 100% MT | 20 Hz, 1 Hz | 20 Hz: 4 sec 1Hz: 30 min | 1st session >20 (20 Hz) 2nd Session 1 (1 Hz) |
1st session: No effect 2nd Session: Clinical seizures involving face arm and leg improved slightly to involve arm and leg only** |
Scalp, arm and leg pain with 20 Hz |
MO: machine output; MT: motor threshold;
100 Hz trains were delivered at successively increasing durations ranging from 0.05 to 1.25 secs;
further follow-up is not available;
patient sedated and intubated within one day of rTMS, thereafter lost to follow-up.
In these seven patients, we report one of three observations during or immediately after rTMS as follows: (1) [n = 2 patients] seizures continued uninterrupted, or (2) [n = 3 patients] seizures paused for 20–30 minutes and then resumed, or (3) [n = 2 patients] seizures stopped and did not resume for the duration of follow-up. In total, ongoing seizures were disrupted after rTMS in five of seven patients. However, in three, the effect was short-lived, lasting only 20–30 minutes after an rTMS train before relapse of clinical seizures, whereas in two others the anticonvulsive effect lasted days or longer.
In two patients who experienced a durable (>1 day) arrest in seizures, follow-up was limited to two days in one, and therefore information about relapse in days after rTMS is not available. However, in one case where extensive follow-up was available, seizures originating near a resected vascular malformation, and characterized by continuous right hand movements of approximately 20 years’ duration were initially disrupted by high frequency (100-Hz) rTMS bursts, and a durable (> 4 months) EPC suppression was achieved with ten daily 30-minute sessions of 1-Hz rTMS delivered with a figure-8 coil over the seizure focus.
In the cases where there was no appreciable pause in seizures after rTMS, nevertheless some change in seizure was observed. In one of the two patients, a child with Rasmussen’s encephalitis, seizures were suppressed only for the duration of a 30-minute 1 Hz rTMS train, but resumed immediately after rTMS on each of nine days of daily treatment. Details related to this case were previously published by our group [7]. In the other patient, seizures most likely due to an acute stroke continued without pause, but changed in character immediately after the rTMS trains from a hemiconvulsion involving face arm and leg, to slightly milder seizures involving arm and leg only.
3.2 Adverse events associated with rTMS
In our experience, as in published cases [8–11], side-effects related to rTMS were generally mild and short-lived. The more common reported adverse events were transient head and limb pain, and limb stiffening during high-frequency rTMS. Notably, although seizures may be triggered by interictal rTMS in patients with epilepsy [12], in the available data, no instance of seizure exacerbation such as secondary generalization or increase in severity is identified.
The absence of a proconvulsive side-effect of stimulation is underscored in three instances where EEG was recorded simultaneously with rTMS. In these cases, we reviewed the EEG and found no evidence of subclinical exacerbation such as generalization or spike provocation with either high or low frequency rTMS. Representative EEGs from one patient are shown in Figure 1, and EEG from another paper has been previously published by our group [7].
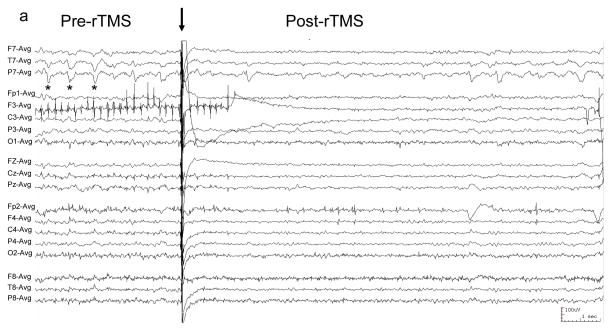
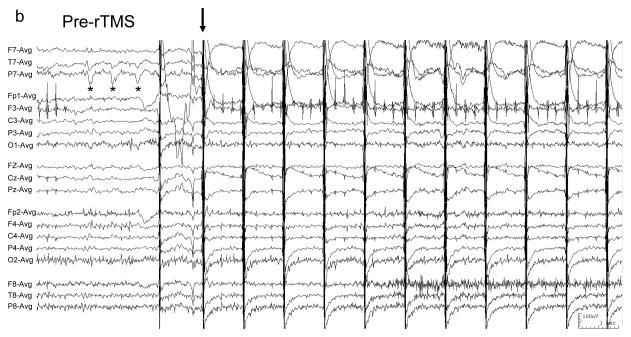
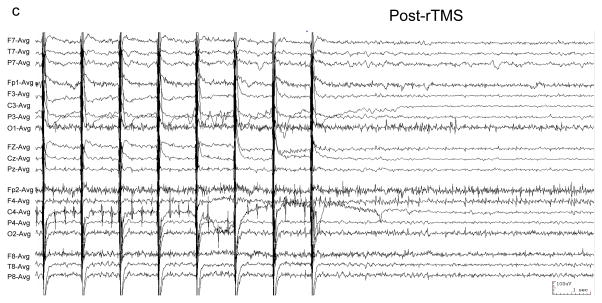
Figure 1. EEG of patient with EPC undergoing rTMS.
EEG was recorded with a conventional 10–20 international scalp electrode configuration, filtered 1–70 Hz, and displayed in a referential montage. “*” demarcates typical left temporal sharp waves. (a) 100 Hz rTMS was at 100% MO with figure-8 coil for 0.05 seconds (arrow). EEG following rTMS shows transiently-reduced sharp wave frequency. (b) Typical sharp waves (*) precede 1 Hz rTMS (arrow), and are absent (c) following rTMS.
4. Discussion
We present an overview of our experience where rTMS was applied for EPC treatment. A major limitation of the current study is that data were obtained from only a small and heterogeneous group of patients with EPC have been treated with rTMS. Accordingly, conclusions with respect to anticonvulsive efficacy cannot be made at this point. Patient characteristics, seizure mechanisms and rTMS protocols that are best-associated with EPC suppression will need to be addressed in future studies. Interactions of rTMS with anticonvulsants and other medications that may facilitate or interfere with its anticonvulsive potential will also have to be investigated. However, the transient (up to minutes) seizure suppression in three of seven patients and durable (days to months) seizure suppression in two of seven patients suggest that there may be utility in future controlled trials of rTMS in EPC management. Particularly the lasting response of some patients to low-frequency rTMS trains raises the possibility that repetitive stimulation protocols that lead to reduced cortical excitability, perhaps by induction of LTD-like changes in synaptic strength, may be of benefit in terminating ongoing seizures as well as in preventing their relapse.
The relationship of rTMS to clinical improvement seems causal rather than coincidental in our series. That is, in instances of EPC where seizures were essentially unchanged over periods lasting days to years, we assume that a change that occurs during or immediately after rTMS is directly related to the stimulation, rather than coincidental and due to a chance change in seizure pattern. This is underscored in instances where patients experienced only a short-lived response that was reproduced with multiple rTMS sessions [7].
Our observations are consistent with published reports which also demonstrate relatively inconsistent efficacy of rTMS in treatment of EPC. A literature search, as well as a search of the American Epilepsy Society (AES) online abstract archive identified six additional reports of EPC treated with low frequency (0.5 to 1 Hz) or high frequency (6 to 20 Hz) rTMS trains (Table 2). Clinical seizures were suppressed in three of six reported cases. As in our experience, published cases were of EPC due to mixed etiologies in a heterogeneous group of patients. Consistent patient characteristics which favored seizure suppression by rTMS were not identified in our review of the literature, although the few instances where a clinical benefit lasting days or longer was achieved were associated with low frequency rTMS trains. Similarly, in instances where seizures continued after rTMS, there were no consistent findings with respect to EPC etiology,
Table 2.
Summary of published EPC cases treated with rTMS.
| Patient | Age (Years) | Etiology | Coil Position | rTMS Intensity | rTMS Frequency | Train Duration | # Trains | Outcome | Adverse Events |
|---|---|---|---|---|---|---|---|---|---|
| 1 [8] | 7 | Unknown, focal cortical atrophy on MRI | Seizure Focus | 50% MO | 20 Hz | 2 sec | 15 | Clinical seizures became intermittent and stopped in 24 hrs | none reported |
| 2 [8] | 11 | Unknown, focal cortical atrophy on MRI | Seizure Focus | 128% MT | 20 Hz | 2 sec | 15 | No change in clinical seizures, improved EEG | none reported |
| 3 [11] | 48 | Unknown, Normal MRI | Seizure Focus | 100% MT | 0.5 Hz | 900 sec | 16 (2 trains per session, biweekly, for 4 weeks) | Clinical seizures decreased during rTMS, and decreased further on follow-up | none reported |
| 4 [9] | 31 | Cortical Dysplasia | Seizure Focus | 90% MT | 0.5 Hz | 200 sec | 1 | Clinical seizures stopped, resumed in 2 months, and stopped again with rTMS | none reported |
| 5 [10] | 8 | Neuronal ceroid lipofuscinoscis (probable) | Seizure Focus | 100% MO | 6 Hz than 1 Hz | 6 Hz: 5 sec 1Hz: 600 sec | 3 (1 Hz, one preceded by 4 trains at 6 Hz) | No change | none reported |
| 6 [10] | 16 | Perinatal stroke | Seizure Focus | 76% MO | 6 Hz then 1 Hz | 6 Hz: 5 sec 1Hz: 900 sec | 2 (1 Hz, one preceded by 4 trains at 6 Hz) | No change | mild headache and leg pain |
MO: machine output; MT: motor threshold.
Adverse events in our small series were relatively mild and stopped immediately after stimulation. Encouragingly, seizure exacerbation which is a concerning potential side-effect of rTMS [12], was not seen by our group and was not reported elsewhere. There thus appears to be no evidence that applying rTMS during active seizures will lead to an increase in severity or in generalization of the seizure. Indeed, in cases where rTMS was combined with real-time EEG, provoked spikes or subclinical seizure exacerbation were not seen (Fig 1, and published in [7]). As with efficacy, the safety of rTMS in EPC treatment will have to be the work of future controlled trials.
A natural extension of the work with EPC may be the application of rTMS to other forms of intractable status epilepticus. The above-described suggestion of clinical efficacy and the relatively benign safety profile of rTMS in patient with epilepsy [12] suggest that rTMS warrants consideration, perhaps in cases of primary or secondarily generalized seizures where rTMS may be used to disrupt organized cortical activity. We anticipate that near-future work with patients as well as with animal status epilepticus models will provide more insight into the clinical capacity of this method.
Acknowledgments
AR: The Siegel Family Fund for Epilepsy Research; Citizens United for Epilepsy Research (CURE)
APL: Berenson-Allen Family Foundation, a BBVA Foundation Chair in Translational Medicine, and NIH grants K24 RR018875 and NCRR MO1 RR01032
Footnotes
The authors appreciate the details of a clinical case contributed by Dr. Lara M. Schrader.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106–7. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2:145–56. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- 3.Fregni F, Thome-Souza S, Bermpohl F, Marcolin MA, Herzog A, Pascual-Leone A, Valente KD. Antiepileptic effects of repetitive transcranial magnetic stimulation in patients with cortical malformations: an EEG and clinical study. Stereotact Funct Neurosurg. 2005;83:57–62. doi: 10.1159/000086674. [DOI] [PubMed] [Google Scholar]
- 4.Santiago-Rodriguez E, Cardenas-Morales L, Harmony T, Fernandez-Bouzas A, Porras-Kattz E, Hernandez A. Repetitive transcranial magnetic stimulation decreases the number of seizures in patients with focal neocortical epilepsy. Seizure. 2008 doi: 10.1016/j.seizure.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Fregni F, Thome-Souza S, Nitsche MA, Freedman SD, Valente KD, Pascual-Leone A. A controlled clinical trial of cathodal DC polarization in patients with refractory epilepsy. Epilepsia. 2006;47:335–42. doi: 10.1111/j.1528-1167.2006.00426.x. [DOI] [PubMed] [Google Scholar]
- 6.Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55:187–99. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 7.Rotenberg A, Depositario-Cabacar D, Bae EH, Harini C, Pascual-Leone A, Takeoka M. Transient suppression of seizures by repetitive transcranial magnetic stimulation in a case of Rasmussen’s encephalitis. Epilepsy Behav. 2008;13:260–2. doi: 10.1016/j.yebeh.2007.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graff-Guerrero A, Gonzales-Olvera J, Ruiz-Garcia M, Avila-Ordonez U, Vaugier V, Garcia-Reyna JC. rTMS reduces focal brain hyperperfusion in two patients with EPC. Acta Neurol Scand. 2004;109:290–6. doi: 10.1046/j.1600-0404.2003.00222.x. [DOI] [PubMed] [Google Scholar]
- 9.Misawa S, Kuwabara S, Shibuya K, Mamada K, Hattori T. Low-frequency transcranial magnetic stimulation for epilepsia partialis continua due to cortical dysplasia. J Neurol Sci. 2005;234:37–9. doi: 10.1016/j.jns.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 10.Morales OG, Henry ME, Nobler MS, Wassermann EM, Lisanby SH. Electroconvulsive therapy and repetitive transcranial magnetic stimulation in children and adolescents: a review and report of two cases of epilepsia partialis continua. Child Adolesc Psychiatr Clin N Am. 2005;14:193–210. viii–ix. doi: 10.1016/j.chc.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Schrader LMKL, Nuwer MR, Engel J, Stern JM. [Abst. 2.396] Therapeutic efficacy of low frequency repetitive transcranial magnetic stimulation (LF-rTMS) stereotactically directed at a well-defined epileptogenic region (ER). American Epilepsy Society and American Clinical Neurophysiology Society Joint Annual Meeting; 2005; p. 225. [Google Scholar]
- 12.Bae EH, Schrader LM, Machii K, Alonso-Alonso M, Riviello JJ, Jr, Pascual-Leone A, Rotenberg A. Safety and tolerability of repetitive transcranial magnetic stimulation in patients with epilepsy: a review of the literature. Epilepsy Behav. 2007;10:521–528. doi: 10.1016/j.yebeh.2007.03.004. [DOI] [PubMed] [Google Scholar]