Abstract
High-dose cyclophosphamide (Cy) is frequently employed for peripheral blood mobilization of hematopoietic stem cells before high-dose chemotherapy with autologous stem cell transplantation (ASCT) in multiple myeloma (MM). The benefit of mobilization with Cy over filgrastim (granulocyte colony-stimulating factor; G-CSF) alone is unclear. Between 2000 and 2008, 167 patients with newly diagnosed MM underwent single ASCT after melphalan conditioning at our institution. Seventy-three patients were mobilized with G-CSF alone, and 94 patients with Cy plus G-CSF (Cy+G-CSF). We retrospectively analyzed Cy’s impact on both toxicity and efficacy. Mobilization efficiency was augmented by Cy; a mean total of 12 versus 5.8 × 106 CD34+ cells/kg were collected from patients mobilized with Cy+G-CSF versus G-CSF, respectively, (P <0.01), over a mean of 1.6 versus 2.2 days of peripheral blood apheresis (p = 0.001). Mobilization-related toxicity was also, however, augmented by Cy; 14% of Cy+G-CSF patients were hospitalized because of complications versus none receiving G-CSF (P <0.0001). Toxicity, including death, related to ASCT was similar between cohorts. Regarding long-term outcomes, multivariate analysis revealed no difference for Cy+G-CSF versus G-CSF (hazard ratio 0.8 for event-free survival [95% confidence interval {CI} 0.57–1.25] and 0.96 for overall survival [95% CI 0.61–1.54]). In summary, we show that mobilization with Cy increases toxicity without positively impacting long-term outcomes in MM. Our findings place into question Cy’s benefit as a routine component of stem cell mobilization regimens in MM. Randomized trials are needed to elucidate the risks and benefits of Cy more definitively.
Keywords: myeloma, mobilization, cyclophosphamide, autologous
INTRODUCTION
High-dose chemotherapy with autologous peripheral blood hematopoietic stem cell transplantation (ASCT) is a standard treatment approach for fit patients with multiple myeloma (MM) [1]. The optimal mobilization regimen for collection of stem cells via peripheral blood is still a matter of debate. High-dose cyclophosphamide (Cy) causes the release of proteases and the cleavage of key adhesion molecules, such as vascular cell adhesion molecule-1 (VCAM-1) and C-X-C chemokine receptor type 4 (CXCR4), culminating in the release of hematopoietic stem cells into the peripheral blood [2,3]. Cy-based mobilization remains in common use since its inception nearly two decades ago [4], but growth factors such as filgrastim (granulocyte colony-stimulating factor; G-CSF) have also been shown to successfully mobilize stem cells without Cy’s toxicity [5–13]. Newer MM therapies such as lenalidomide impair stem cell collection [14] and the finding that patients treated with lenalidomide can often be mobilized with the addition of Cy [15,16] amplified the necessity for Cy in recent years. Countering that more recently, however, is the introduction of the pricey yet efficacious CXCR4 antagonist plerixafor, which can mobilize even lenalidomide-treated patients [17,18]. Cy’s role in mobilization is therefore murky but the question is important; many MM patients receive not one but two ASCTs as part of their MM care (either back-to-back as tandem ASCT, or early and again later over their years of MM therapy), and so adequate stem cell collection for not one but two ASCTs is generally held as the goal for any mobilization program. Efficient and safe mobilization is critical.
Aside from its capacity to mobilize, Cy’s popularity also stems from its cytotoxicity, which carries the allure of “debulking” MM during stem cell collection. Retrospective studies, however, have shown that Cy does not impact survival [19,20], which suggests that Cy’s theoretical anti-MM effects during mobilization are clinically irrelevant. Published data are still very limited, so we undertook a retrospective analysis of our own consecutively treated, newly diagnosed MM patients with long-term follow-up, who underwent a single ASCT following melphalan conditioning at our institution, after mobilization with either Cy plus G-CSF (Cy+G-CSF) or G-CSF alone.
MATERIALS AND METHODS
Patients and Study Design
We retrospectively interrogated a prospectively maintained clinical database of transplant patients at our institution and reviewed individual charts for adult patients with MM who underwent peripheral blood stem cell collection followed by ASCT at Duke Cancer Institute between 2001 and 2008. We excluded patients who: carried a second, concurrent malignancy (e.g., non-Hodgkin’s lymphoma) or plasma cell leukemia; received either tandem ASCT or their first ASCT not as part of initial therapy (defined as first ASCT ≥2 years after diagnosis of active MM); received plerixafor or etoposide as part of their mobilization regimen; or received regimen other than single-agent melphalan as conditioning. Institutional review board (IRB) approval at Duke University Medical Center was obtained before study initiation.
Mobilization and Transplant Procedures
Mobilization regimens were chosen purely according to physician discretion. G-CSF patients were mobilized with single agent G-CSF (filgrastim) 10 μg/kg subcutaneously (SC) for 5 days, while patients in the Cy+G-CSF arm received Cy 3–4 gm/m2 intravenously (IV) 4 days before initiation of G-CSF 10 μg/kg/day SC, which was given until stem cell collection. Collection was performed the day the white blood cell count (WBC) recovered to ≥5,000/μl. The minimum required cell collection was 2 × 106 CD34+ cells/kg, while the target collection was 8–10 × 106 CD34+ cells/kg. Within 2 weeks of stem cell collection, on day 1, most patients received a single dose of intravenous melphalan 200 mg/m2. Patients over age 70 or with impaired renal function (defined as a calculated creatinine clearance of <50 ml/min) received dose-reduced melphalan at 140 mg/m2 as standard practice. On day 0, half of the collected cells, with a minimum of 2 × 106 CD34+/kg, were reinfused. Patients received G-CSF support (5 μg/kg/day SC) following cell infusion until engraftment. Maintenance therapy was defined as initiation of therapy at any time post-ASCT without clinical or serological evidence of relapsed/progressive MM. Multi-agent consolidation regimens were almost never employed in this patient cohort.
Engraftment, Toxicity, and Response Assessment
Engraftment was defined by transfusion-independent platelet levels ≥20,000/μl, and G-CSF-independent absolute neutrophil count >500/μl, each for at least three consecutive days. Objective response to therapy was assessed using standard International Myeloma Working Group criteria [21]. Both overall survival (OS) and event-free survival (EFS) were calculated from day of stem cell reinfusion (day 0). OS was defined as death by any cause. Missing OS data in the clinical records were ascertained by accessing the Social Security Death Index. EFS was defined as time to relapse or progression according to standard definitions [21] or death. Treatment-related mortality was defined as death not related to disease relapse within 6 months of ASCT. Toxicity was assessed qualitatively and with a focus on need for hospitalization in order to reduce imprecision that could arise from retrospectively grading mild to moderate toxic events based on chart review.
Statistical Analysis
Patient data through November 1, 2013 were analyzed. Subjects who were free of events then were censored as of that date. We employed chi-square and Fisher’s exact test to compare differences between nominal variables, and Mann–Whitney tests for continuous variables. Correction for multiple testing was not performed in this exploratory analysis. For OS and EFS, we plotted Kaplan–Meier survival curves and used the log-rank test to test differences between the two groups. We fitted a proportional hazards model to test the difference in OS and EFS between the two groups, while controlling for confounding variables. Two-way analysis of variance (ANOVA) was performed to test the impact of mobilization regimen and pretransplant depth of response (dichotomized as very good partial response [VGPR] vs. partial response [PR] or worse) on stem cell collection yield, including testing for interaction. All analyses were performed using GraphPad Prism 5 v5.04 for nominal and categorical variables, and R for Kaplan-Meier time-to-event, ANOVA, and multivariate analyses.
RESULTS
Patient and Treatment Characteristics
One hundred eighty-six patients were initially identified in the database as potential subjects for this study. Nineteen patients were excluded on chart review primarily due to a synchronous malignancy or tandem ASCT. Ultimately 94 subjects were included who had received Cy+G-CSF, whereas 73 had received G-CSF alone. Toxicity data from 17 patients have been reported as part of a prior study by members of our group [13]. There were no significant differences between groups in age, gender, immunoglobulin subtype, or premobilization assessments of International Staging System (ISS) stage [22] (Table I), serum creatinine, or hemoglobin (data not shown). Cy+G-CSF versus G-CSF use varied somewhat by year, ranging without a clear trend from 27.3% of subjects receiving Cy-G-CSF in 2006 to 85.3% in 2003. Table II shows that induction regimens varied widely based on employment of novel agents and need for salvage induction regimens (>1 line of therapy). Although overall response rate (ORR) after induction was similar between groups, VGPR or better was seen in 23% of patients mobilized with Cy+G-CSF in comparison to 41% of those who received G-CSF (P = 0.02). Median time between diagnosis and ASCT was 8.6 months for Cy+G-CSF and 7.7 months for G-CSF (P = 0.23). For ASCT, high-dose melphalan was dose-reduced from the standard 200 mg/m2 in similar numbers of patients from each group and after ASCT, more Cy+G-CSF patients received maintenance therapy.
TABLE I.
Patient and MM Pretransplant Characteristics
| Cy+G-CSF (n = 94) | G-CSF (n = 73) | P-value | |
|---|---|---|---|
| Female gender, % | 35 | 44 | 0.27 |
| Median age at ASCT, years (range) | 59 (34–76) | 57 (33–73) | 0.19 |
| MM subtype, % | 0.63 | ||
| IgG | 62 | 64 | |
| IgA | 22 | 21 | |
| Light chain | 14 | 15 | |
| Other | 1 | 0 | |
| ISS Stage20, % | 0.39 | ||
| I | 48 | 56 | |
| II | 28 | 30 | |
| III | 21 | 14 | |
| Unknown* | 3 | 0 | |
| Abnormal bone marrow cytogenetics or fluorescent in situ hydridization, % | 0.09 | ||
| No | 51 | 55 | |
| Yes | 19 | 29 | |
| Unknowna | 30 | 16 |
Totals of percentages may not equal 100% due to rounding.
ISS = International Staging System.
Unknown subjects were excluded from statistical analysis.
TABLE II.
Data Regarding Therapy
| Cy+G-CSF (n = 94) | G-CSF (n = 73) | P-value | |
|---|---|---|---|
| Pre-ASCT induction regimen, % | |||
| Included thalidomide or lenalidomide | 40 | 38 | 0.87 |
| Included bortezomib | 23 | 48 | 0.001 |
| Multiple regimens (i.e., required salvage) | 27 | 25 | 0.85 |
| Response to induction (pre-ASCT), % | |||
| Overall response rate (PR or better) | 92 | 99 | 0.11 |
| VGPR or better | 23 | 41 | 0.02 |
| CR | 5 | 7 | |
| VGPR | 18 | 34 | |
| PR | 67 | 56 | |
| <PR | 7 | 1 | |
| Unknowna | 1 | 0 | |
| Melphalan dose for ASCT conditioning, % | |||
| 200 mg/m2 | 91 | 84 | 0.32b |
| 140 mg/m2 | 9 | 14 | |
| Received maintenance thalidomide or lenalidomide, % | 14 | 3 | 0.01 |
Totals of percentages may not equal 100% due to rounding.
Counted as no response (<PR) for statistical analysis.
Fisher’s exact test of full versus reduced dose melphalan.
Stem Cell Collection, Toxicity, and Engraftment
Stem cell collection was enhanced by Cy, with mean total collection of 12 versus 5.8 × 106 CD34+ cells/kg from patients mobilized with Cy+G-CSF vs. G-CSF, respectively (P <0.01). Premobilization depth of response did not affect collection ((P = 0.18). Testing for interaction between mobilization regimen and pre-mobilization depth of response was not statistically significant (P = 0.27). Cy+G-CSF also shortened number of days required for apheresis to 1.6 days from 2.2 days for subjects on G-CSF (P = 0.001). Toxicity and engraftment data are tabulated in Table III. During mobilization, mild myalgias were commonly noted in G-CSF-mobilized patients (data not shown) but no serious adverse events were observed. Conversely, many Cy+G-CSF patients experienced toxicity, culminating in a 14% rate of hospitalization. Once patients advanced to ASCT, the incidence of specific toxicities was similar. Our center routinely performs ASCT in an outpatient clinic, and hospitalization rates during ASCT were comparable as was treatment-related mortality between the two groups. Platelet engraftment was slower in G-CSF-mobilized patients, whereas median time until neutrophil engraftment was statistically equivalent.
TABLE III.
Data regarding Toxicity, Engraftment, and Post-Transplant Response
| Cy+G-CSF (n = 94) | G-CSF (n = 73) | P-value | |
|---|---|---|---|
| Toxicity related to mobilization (%) | |||
| Febrile neutropenia | 23 | 0 | <0.0001 |
| Hematuria (hemorrhagic cystitis) | 8 | 0 | <0.0001 |
| Toxicity requiring hospitalization (%) | 14 | 0 | <0.0001 |
| Toxicity related to ASCT (%) | |||
| Febrile neutropenia | 70 | 68 | 0.87 |
| Pneumonitis | 14 | 16 | 0.66 |
| Toxicity requiring hospitalization (%) | 31 | 38 | 0.33 |
| Treatment-related mortality (%) | 4 | 1 | 0.39 |
| Median time to neutrophil engraftment, days (range) | 11 (9–15) | 11 (9–23) | 0.4 |
| Median time to platelet engraftment, days (range) | 13(10–21) | 14 (9–31) | 0.007 |
| Response after ASCT, % | |||
| Overall response rate (PR or better) | 94 | 94 | 1 |
| VGPR or better | 44 | 58 | 0.11 |
| CR | 5 | 7 | |
| VGPR | 39 | 51 | |
| PR | 49 | 36 | |
| <PR | 1 | 1 | |
| Unknown or treatment-related mortality | 5 | 5 | |
Response to Transplant and Predictors of Remission and Survival
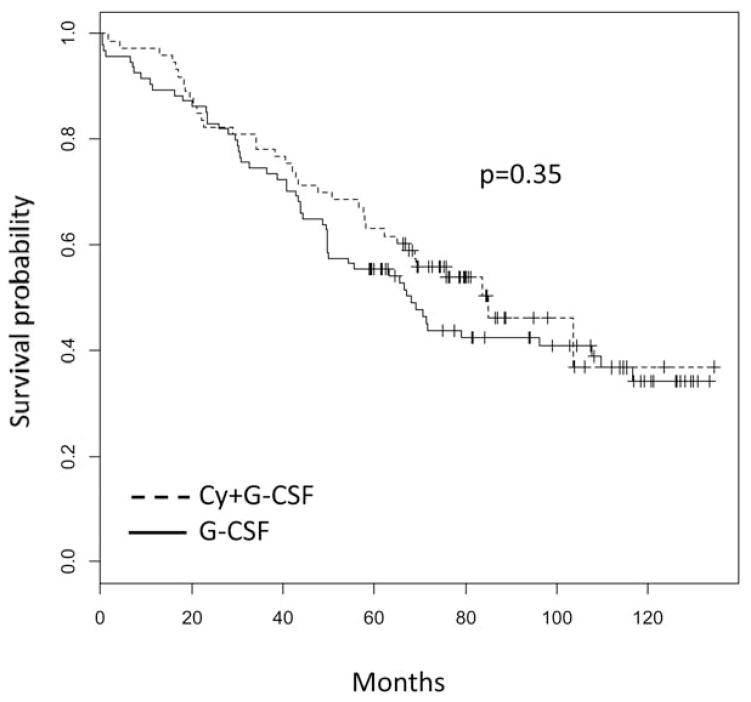
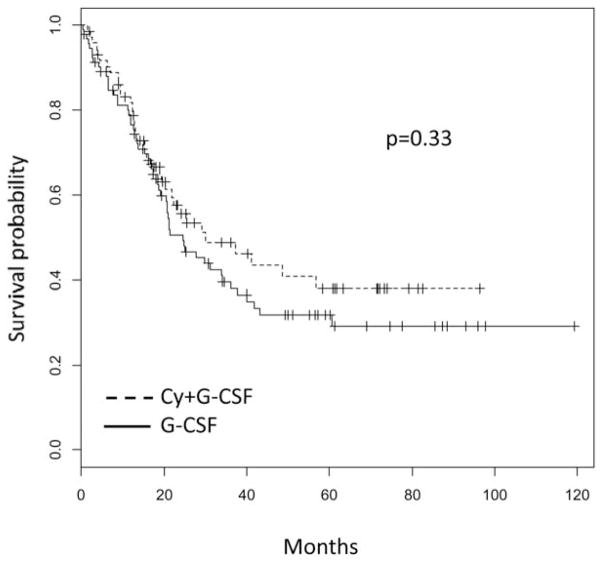
Objective responses post-ASCT are summarized in Table III and were similar between groups, as were OS and EFS (Figs 1 and 2, respectively). Given the variability in induction regimens and premobilization depth of response, we performed multivariate analysis in an effort to dissect the effect of confounders from Cy’s effect on long-term endpoints. As shown in Table IV, the only predictor of longer EFS was the premobilization achievement of VGPR or better, whereas VGPR or better and ISS stage I or II both predicted longer OS. Abnormalities in cytogenetics or fluorescent in situ hybridization (FISH), employment of novel agents with induction, maintenance therapy, and Cy all were all insignificant predictors of outcomes.
Fig. 1.
Overall survival.
Fig. 2.
Event-free survival.
TABLE IV.
Results of Fitting Proportional Hazards Model to OS and EFS
| OS
|
EFS
|
|||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value | |
| G-CSF | 1 | – | – | 1 | – | – |
| Cy+G-CSF | 0.94 | (0.55, 1.62) | 0.83 | 1.11 | (0.64, 1.92) | 0.72 |
| Age | 1.01 | (0.98, 1.04) | 0.60 | 1.01 | (0.98, 1.04) | 0.54 |
| ISS Stage | ||||||
| I or II | 1 | – | – | 1 | – | – |
| III | 2.03 | (1.04, 3.95) | 0.04 | 2.51 | (1.28, 4.93) | 0.01 |
| Bortezomib with induction | ||||||
| no | 1 | – | – | 1 | – | – |
| yes | 0.92 | (0.49, 1.74) | 0.80 | 0.88 | (0.46, 1.68) | 0.71 |
| Lenalidomide or thalidomide with induction | ||||||
| no | 1 | – | – | 1 | – | – |
| yes | 1.16 | (0.67, 2.03) | 0.59 | 1.25 | (0.71, 2.19) | 0.44 |
| Pre-ASCT VGPR or better | ||||||
| no | 1 | – | – | 1 | – | – |
| yes | 0.53 | (0.29, 0.96) | 0.04 | 0.40 | (0.21, 0.74) | 0.00 |
| Full-dose melphalan conditioning | ||||||
| no | 1 | – | – | 1 | – | – |
| yes | 1.18 | (0.52, 2.67) | 0.69 | 1.43 | (0.63, 3.23) | 0.39 |
| Bone marrow cytogenetics/FISH | ||||||
| normal | 1 | – | – | 1 | – | – |
| abnormal | 0.89 | (0.47, 1.69) | 0.72 | 0.75 | (0.40, 1.41) | 0.37 |
| Year of ASCT | ||||||
| 2001–2003 | 1 | – | – | 1 | – | – |
| 2004–2006 | 0.58 | (0.26, 1.25) | 0.16 | 0.78 | (0.36, 1.67) | 0.52 |
| 2007–2008 | 0.70 | (0.31, 1.58) | 0.38 | 0.67 | (0.30, 1.53) | 0.35 |
ASCT = high-dose chemotherapy with autologous stem cell transplantation; VGPR = very good partial response; ISS = International staging system; CI = confidence interval; FISH = fluorescent in situ hybridization; OS = overall survival; EFS = event-free survival.
DISCUSSION
ASCT remains a standard of care for MM, prolonging remission and in some studies OS as compared with conventional chemotherapy [1,23–25]. Contemporary MM treatment often includes not one but two ASCTs over the course of any given patient’s treatment course. Hence stem cell mobilization strategies are generally aimed at collecting enough stem cells for two ASCTs. Effective mobilization is critical.
Cy’s popularity as a component of mobilization regimens has increased in recent years because of its capacity to augment stem cell collection [4–12], which is especially relevant for patients treated with lenalidomide [14–16]. Additionally Cy theoretically adds an anti-MM effect to mobilization programs. Countering those advantages is Cy’s more severe toxicity profile compared with G-CSF alone. It is hence vital to determine whether Cy’s benefits truly merit the toxicity particularly with the availability of plerixafor, which is expensive but can salvage collection in patients who fail G-CSF mobilization, and without Cy’s toxicity [17.]
In this study, we report our retrospective institutional experience of using Cy+G-CSF vs. G-CSF mobilization in patients with MM undergoing melphalan conditioning and single ASCT for newly diagnosed MM. First, we confirm that Cy enhances mobilization, with Cy+G-CSF mobilized patients yielding more than twice as many CD34+ cells as G-CSF mobilized patients over a smaller number of days of apheresis. However, toxicity related to mobilization was markedly higher in the group that received Cy+G-CSF as well, with 14% of patients requiring hospitalization versus no patients who received G-CSF. ASCT was completed with similar toxicity and efficacy in both groups. Long-term, Cy had no observable effect on EFS or OS in unadjusted and multivariate analyses. In fact, the only predictors of EFS or OS were premobilization achievement of VGPR or better (for both), and ISS stage (for OS alone).
Our findings add important evidence to and are concordant with the limited literature that exists on this topic. Dingli et al., published a similar study [19], updated later [6], in which they saw no change in progression-free survival or OS with Cy+G-CSF vs. G-CSF. A report by Nakasone et al., also demonstrated no benefit to Cy [20]. Directly comparing retrospective studies is of course fraught with challenges, but now multiple studies demonstrating the same effect increase the credibility of this finding as a whole.
Limitations in our report merit discussion and shed light on our findings. First, this is a nonrandomized, retrospective study, and as such it is susceptible to the usual biases and confounders such as selection bias. Second, pre-ASCT and post-ASCT treatment was heterogeneous. Before ASCT, twice the percentage of G-CSF patients received bortezomib when compared with Cy+G-CSF patients. The potential implications of that discrepancy on long-term survival are complex. Given clear data that bortezomib prolongs survival [26]. one would expect the inclusion of bortezomib to benefit OS. However, many patients studied here received initial conventional chemotherapy such as vincristine, doxorubicin, and dexamethasone (VAD), with bortezomib only employed as second-line therapy for inadequate responses; that could easily transform bortezomib usage into a potential predictor of poorer OS. As for Cy, Cy+G-CSF patients tended to have less deep pre-mobilization response, and they also more frequently received post-ASCT maintenance therapy. Conversely, fitter patients are also sometimes preferentially administered Cy because of increased perceived likelihood of tolerating it. Such confounders and biases could be quite mixed in their positive or negative effects on outcome in each patient cohort. It is perhaps therefore unsurprising that employment of neither Cy nor bortezomib predicted EFS or OS even in multivariate analysis. Last, although we were able to comment on whether any cytogenetic or FISH abnormalities were present in most subjects premobilization (a known prognostic factor in and of itself [27]), we lacked information for the majority of patients on the specific nature of those abnormalities, and hence, certain key prognostic markers could not be evaluated as part of this study.
In terms of generalizability, the MM literature is dogged by a constant battle between obsolete therapies and adequate follow-up. Truly discerning the benefit of any regimen is contingent on long-term follow-up of hard endpoints such as duration of remission and overall survival. Median survival in myeloma is now multiple years [28,29], which means that trials will often take years to detect differences in those endpoints and by the time differences become evident, the regimens are obsolete. One strength of our study is that we designed it to strike a balance between those two competing interests. We included only patients transplanted between 2001 and 2008 to capture a population that had largely received novel agents in induction. We excluded more recently transplanted patients so as to mostly avoid those who had received maintenance therapy, which would be a significant confounder, as well as patients with immature follow-up.
Taken together, our retrospective data demonstrate that mobilizing MM patients with Cy in preparation for ASCT increases collection efficiency but has no clear benefit on long-term outcomes, including survival, despite markedly increasing mobilization-associated toxicity. These data add important additional evidence to the currently limited literature that shows little benefit to Cy, particularly nowadays when even patients who are difficult to mobilize using G-CSF alone can be salvaged with plerixafor. Our findings further call into question the need for Cy as a routine component in mobilization strategies. Ultimately, a randomized controlled trial is needed to answer this important question once and for all. Currently, we know of no such study being planned.
CONCLUSIONS
Adding Cy to hematopoietic stem cell mobilization regimens for MM before ASCT enhances stem cell collection but adds toxicity with no clear benefit on long-term outcomes. Prospective, randomized trials are warranted to definitely elucidate risk versus benefit, but for now, the routine employment of Cy should be carefully considered if not avoided in many cases.
References
- 1.Attal M, Harousseau J-L, Stoppa A-M, Sotto J-J, Fuzibet J-G, Rossi J-F, Casassus P, Maisonneuve H, Facon T, Ifrah N, Payen C, Bataille R. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. N Engl J Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 2.Lévesque JP, Takamatsu Y, Nilsson SK, Haylock DN, Simmons PJ. Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood. 2001;98:1289–1297. doi: 10.1182/blood.v98.5.1289. [DOI] [PubMed] [Google Scholar]
- 3.Lévesque J-P, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003;111:187–196. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shpall EJ. The utilization of cytokines in stem cell mobilization strategies. Bone Marrow Transplant. 1999;23 (Suppl 2):S13–S19. doi: 10.1038/sj.bmt.1701669. [DOI] [PubMed] [Google Scholar]
- 5.Narayanasami U, Kanteti R, Morelli J, Klekar A, Al-Olama A, Keating C, O’Connor C, Berkman E, Erban JK, Sprague KA, Miller KB, Schenkein DP. Randomized trial of filgrastim versus chemotherapy and filgrastim mobilization of hematopoietic progenitor cells for rescue in autologous transplantation. Blood. 2001;98:2059–2064. doi: 10.1182/blood.v98.7.2059. [DOI] [PubMed] [Google Scholar]
- 6.Gertz MA, Kumar SK, Lacy MQ, Dispenzieri A, Hayman SR, Buadi FK, Dingli D, Gastineau DA, Winters JL, Litzow MR. Comparison of high-dose CY and growth factor with growth factor alone for mobilization of stem cells for transplantation in patients with multiple myeloma. Bone Marrow Transplant. 2009;43:619–625. doi: 10.1038/bmt.2008.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koc ON, Gerson SL, Cooper BW, Laughlin M, Meyerson H, Kutteh L, Fox RM, Szekely EM, Tainer N, Lazarus HM. Randomized cross-over trial of progenitor-cell mobilization: high-dose cyclophosphamide plus granulocyte colony-stimulating factor (G-CSF) versus granulocyte-macrophage colony-stimulating factor plus G-CSF. J Clin Oncol. 2000;18:1824–1830. doi: 10.1200/JCO.2000.18.9.1824. [DOI] [PubMed] [Google Scholar]
- 8.Alegre A, Tomas JF, Martinez-Chamorro C, Gil-Fernandez JJ, Fernandez-Villalta MJ, Arranz R, Diaz MA, Granda A, Bernardo MR, Escudero A, Lopez-Lorenzo JL, Fernandez-Ranada JM. Comparison of peripheral blood progenitor cell mobilization in patients with multiple myeloma: high-dose cyclophosphamide plus GM-CSF vs G-CSF alone. Bone Marrow Transplant. 1997;20:211–217. doi: 10.1038/sj.bmt.1700867. [DOI] [PubMed] [Google Scholar]
- 9.Fitoussi O, Perreau V, Boiron JM, Bouzigon E, Cony-Makhoul P, Pigneux A, Agape P, Nicolini F, Dazey B, Reiffers J, Salmi R, Marit G. A comparison of toxicity following two different doses of cyclophosphamide for mobilization of peripheral blood progenitor cells in 116 multiple myeloma patients. Bone Marrow Transplant. 2001;27:837–842. doi: 10.1038/sj.bmt.1702879. [DOI] [PubMed] [Google Scholar]
- 10.Pusic I, Jiang SY, Landua S, Uy GL, Rettig MP, Cashen AF, Westervelt P, Vij R, Abboud CN, Stockerl-Goldstein KE, Sempek DS, Smith AL, DiPersio JF. Impact of mobilization and remobilization strategies on achieving sufficient stem cell yields for autologous transplantation. Biol Blood Marrow Transplant. 2008;14:1045–1056. doi: 10.1016/j.bbmt.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Arora M, Burns LJ, Barker JN, Miller JS, Defor TE, Olujohungbe AB, Weisdorf DJ. Randomized comparison of granulocyte colony-stimulating factor versus granulocyte-macrophage colony-stimulating factor plus intensive chemotherapy for peripheral blood stem cell mobilization and autologous transplantation in multiple myeloma. Biol Blood Marrow Transplant. 2004;10:395–404. doi: 10.1016/j.bbmt.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Desikan KR, Barlogie B, Jagannath S, Vesole DH, Siegel D, Fassas A, Munshi N, Singhal S, Mehta J, Tindle S, Nelson J, Bracy D, Mattox S, Tricot G. Comparable engraftment kinetics following peripheral-blood stem-cell infusion mobilized with granulocyte colony-stimulating factor with or without cyclophosphamide in multiple myeloma. J Clin Oncol. 1998;16:1547–1553. doi: 10.1200/JCO.1998.16.4.1547. [DOI] [PubMed] [Google Scholar]
- 13.Sung AD, Grima DT, Bernard LM, Brown S, Carrum G, Holmberg L, Horwitz ME, Liesveld JL, Kanda J, McClune B, Shaughnessy P, Tricot GJ, Chao NJ. Outcomes and costs of autologous stem cell mobilization with chemotherapy plus G-CSF vs G-CSF alone. Bone Marrow Transplant. 2013;48:1444–1449. doi: 10.1038/bmt.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Gastineau DA, Litzow MR, Fonseca R, Roy V, Rajkumar SV, Gertz MA. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia. 2007;21:2035–2042. doi: 10.1038/sj.leu.2404801. [DOI] [PubMed] [Google Scholar]
- 15.Mark T, Stern J, Furst JR, Jayabalan D, Zafar F, LaRow A, Pearse RN, Harpel J, Shore T, Schuster MW, Leonard JP, Christos PJ, Coleman M, Niesvizky R. Stem cell mobilization with cyclophosphamide overcomes the suppressive effect of lenalidomide therapy on stem cell collection in multiple myeloma. Biol Blood Marrow Transplant. 2008;14:795–798. doi: 10.1016/j.bbmt.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Popat U, Saliba R, Thandi R, Hosing C, Qazilbash M, Anderlini P, Shpall E, McMannis J, Korbling M, Alousi A, Andersson B, Nieto Y, Kebriaei P, Khouri I, de Lima M, Weber D, Thomas S, Wang M, Jones R, Champlin R, Giralt S. Impairment of filgrastim-induced stem cell mobilization after prior lenalidomide in patients with multiple myeloma. Biol Blood Marrow Transplant. 2009;15:718–723. doi: 10.1016/j.bbmt.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costa LJ, Miller AN, Alexander ET, Hogan KR, Shabbir M, Schaub C, Stuart RK. Growth factor and patient-adapted use of plerixafor is superior to CY and growth factor for autologous hematopoietic stem cells mobilization. Bone Marrow Transplant. 2011;46:523–528. doi: 10.1038/bmt.2010.170. [DOI] [PubMed] [Google Scholar]
- 18.DiPersio JF, Stadtmauer EA, Nademanee A, Micallef IN, Stiff PJ, Kaufman JL, Maziarz RT, Hosing C, Fruehauf S, Horwitz M, Cooper D, Bridger G, Calandra G. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113:5720–5726. doi: 10.1182/blood-2008-08-174946. [DOI] [PubMed] [Google Scholar]
- 19.Dingli D, Nowakowski GS, Dispenzieri A, Lacy MQ, Hayman S, Litzow MR, Gastineau DA, Gertz MA. Cyclophosphamide mobilization does not improve outcome in patients receiving stem cell transplantation for multiple myeloma. Clin Lymphoma Myeloma. 2006;6:384–388. doi: 10.3816/CLM.2006.n.014. [DOI] [PubMed] [Google Scholar]
- 20.Nakasone H, Kanda Y, Ueda T, Matsumoto K, Shimizu N, Minami J, Sakai R, Hagihara M, Yokota A, Oshima K, Tsukada Y, Tachibana T, Nakaseko C, Fujisawa S, Yano S, Fujita H, Takahashi S, Kanamori H, Okamoto S. Retrospective comparison of mobilization methods for autologous stem cell transplantation in multiple myeloma. Am J Hematol. 2009;84:809–814. doi: 10.1002/ajh.21552. [DOI] [PubMed] [Google Scholar]
- 21.Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R, Blade J, Richardson P, Orlowski R, Siegel D, Jagannath S, Facon T, Avet-Loiseau H, Lonial S, Palumbo A, Zonder J, Ludwig H, Vesole D, Sezer O, Munshi NC, San Miguel J. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117:4691–4695. doi: 10.1182/blood-2010-10-299487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Blade J, Boccadoro M, Child JA, Avet-Loiseau H, Kyle RA, Lahuerta JJ, Ludwig H, Morgan G, Powles R, Shimizu K, Shustik C, Sonneveld P, Tosi P, Turesson I, Westin J. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 23.Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, Brown J, Drayson MT, Selby PJ. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 24.Fermand JP, Katsahian S, Divine M, Leblond V, Dreyfus F, Macro M, Arnulf B, Royer B, Mariette X, Pertuiset E, Belanger C, Janvier M, Chevret S, Brouet JC, Ravaud P. High-dose therapy and autologous blood stem-cell transplantation compared with conventional treatment in myeloma patients aged 55 to 65 years: long-term results of a randomized control trial from the Group Myelome-Autogreffe. J Clin Oncol. 2005;23:9227–9233. doi: 10.1200/JCO.2005.03.0551. [DOI] [PubMed] [Google Scholar]
- 25.Fermand JP, Ravaud P, Chevret S, Divine M, Leblond V, Belanger C, Macro M, Pertuiset E, Dreyfus F, Mariette X, Boccacio C, Brouet JC. High-dose therapy and autologous peripheral blood stem cell transplantation in multiple myeloma: up-front or rescue treatment? Results of a multicenter sequential randomized clinical trial. Blood. 1998;92:3131–3136. [PubMed] [Google Scholar]
- 26.Sonneveld P, Goldschmidt H, Rosiñol L, Bladé J, Lahuerta JJ, Cavo M, Tacchetti P, Zamagni E, Attal M, Lokhorst HM, Desai A, Cakana A, Liu K, van de Velde H, Esseltine D-L, Moreau P. Bortezomib-based versus nonbortezomib-based induction treatment before autologous stem-cell transplantation in patients with previously untreated multiple myeloma: a meta-analysis of phase iii randomized, controlled trials. J Clin Oncol. 2013;31:3279–3287. doi: 10.1200/JCO.2012.48.4626. [DOI] [PubMed] [Google Scholar]
- 27.Waheed S, Shaughnessy JD, van Rhee F, Alsayed Y, Nair B, Anaissie E, Szymonifka J, Hoering A, Crowley J, Barlogie B. International staging system and metaphase cytogenetic abnormalities in the era of gene expression profiling data in multiple myeloma treated with total therapy 2 and 3 protocols. Cancer. 2010;117:1001–1009. doi: 10.1002/cncr.25535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar SK, Dingli D, Lacy MQ, Dispenzieri A, Hayman SR, Buadi FK, Rajkumar SV, Litzow MR, Gertz MA. Autologous stem cell transplantation in patients of 70 years and older with multiple myeloma: Results from a matched pair analysis. Am J Hematol. 2008;83:614–617. doi: 10.1002/ajh.21191. [DOI] [PubMed] [Google Scholar]
- 29.Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S, Kapoor P, Dingli D, Hayman SR, Leung N, Lust J, McCurdy A, Russell SJ, Zeldenrust SR, Kyle RA, Rajkumar SV. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2013;28:1122–1128. doi: 10.1038/leu.2013.313. [DOI] [PMC free article] [PubMed] [Google Scholar]